Research Article, J Clin Exp Radiol Vol: 7 Issue: 1
Histological Quantification of Defined Thrombi with Spectral CT Analysis: An in vitro-Study using Ovine Blood-Samples
Nico Munnich1*, Julia Rozanka1, Ana Moya2, Stephanie Ritter1 and Stefan Rohde1
1Department of Radiology and Neuroradiology, Klinikum Dortmund, Beurhausstraße 40, 44137 Dortmund, Germany
2Department of Business Intelligence & Data Science, International School of Management, Institute of Biomathematics and Statistics, Technical University, Dortmund, Germany
*Corresponding Author: Nico Munnich,
Department of Radiology and Neuroradiology,
Klinikum Dortmund, Beurhausstraße 40 44137 Dortmund, Germany
Tel: +49 173 6577423
E-mail: Nico.Muennich@klinikumdo.de
Received date: 21 April, 2024, Manuscript No. JCER-24-132711;
Editor assigned date: 23 April, 2024, Pre QC No. JCER-24-132711 (PQ);
Reviewed date: 07 May, 2024, QC No. JCER-24-132711;
Revised date: 17 May, 2024, Manuscript No. JCER-24-132711 (R);
Published date: 24 May, 2024, DOI: 10.4172/JCER.1000159
Citation: Nico Munnich, Julia Rozanka, Ana Moya, Stephanie Ritter, Stefan Rohde (2024) Histological Quantification of Defined Thrombi with Spectral CT Analysis: An in vitro-Study Using Ovine Blood-Samples. J Clin Exp Radiol 7:1.
Abstract
Objectives: The histological composition of thrombi is a critical factor in the management of patients with acute stroke as it influences pharmacological and interventional treatment strategies. Mechanical characteristics of the occluding thrombi primarily depend on the proportion between fibrin and Red Blood Cells (RBC). Spectral CT imaging with dual-energy technology enables tissue differentiation depending on the keV specific absorption. We sought to evaluate the feasibility and the potential accuracy of spectral CT in the imaging of thrombus composition in an in-vitro Model using blood samples with defined fibrin/RBC-ratios.
Methods: Five types of defined ovine blood thrombi with a gradually increasing amount of RBC (0%-100%) were scanned with spectral CT imaging using keV-values between 40 and 140 keV. Subsequently, the specific absorption curve was established for each thrombus and statistically correlated using a Kolmogorov-Smirnov test.
Results: The absorption curves showed a statistically significant difference for thrombi with moderate to high levels of RBC (30%-100% RBC fraction, p-value<0.05) with differentiability at keV-levels above 80 keV. Thrombi with an RBC content of more than 40% could be differentiated best.
Conclusion: Spectral CT-imaging can differentiate thrombi with a defined proportion of RBC and fibrin. Discrimination of the histological composition is best achieved in thrombi with an RBC-content of more than 40% and at high keV-levels with a threshold above 80keV. The available data allow for potential further development of this CT-technique in future thrombus.
Keywords: Thrombus histology/composition; Thrombus imaging; Spectral CT; Dual-energy CT; Ischemic stroke; Clot imaging
Introduction
The acute embolic occlusion of a vessel supplying the brain is the leading cause of a stroke in the vast majority of cases [1]. The thrombotic material can embolize into the vessels, e.g., in atrial fibrillation with thrombus formation in the left atrium. More rarely, coagulation within the vessel occurs in the context of atherosclerotic plaques [2].
While in cellular coagulation, erythrocytes bound primarily to the thrombus, causing a high RBC-density of the so-called “red” thrombus, thrombi formed from plasmatic coagulation by activated fibrin cross-linking does not show an increased RBC-density. These fibrin-rich thrombi have much higher mechanical stability than RBCrich thrombi [3].
Differentiation into fibrin or RBC-rich thrombi is usually performed by histopathological methods [4]. An imaging-supported histological differentiation of thrombi in the hyperacute management of stroke patients prior to thrombectomy has not been possible so far [5].
The development of dual-energy technology enables tissue differentiation depending on the keV specific absorption. Technically, the manufacturers have relied on different mechanisms to achieve this:
• A dual-source supported technology with two tubes and a potential 3-material resolution.
• A dual-layer detector set-up, and
• A KV-switch between 70 keV and 120 keV during the scan.
The latter results in a spectrum of different keV strengths that allow a 2-material resolution [6].
In vitro thrombus differentiation using spectral CT technology has been shown as a proof of concept for Siemens dual-source and Philips dual-layer technology [7], but not for GE`s KV-switch technology.
Experiments using a dual-layer CT for thrombus imaging showed a specific absorption that depends on the fibrin content of the thrombi. In addition, fibrin seems to have a higher contrast agent affinity compared to RBC [[8]. However, an in-vitro-recording of the entire absorption spectrum of defined thrombi with the correlation of the determined curves based on the RBC content has not yet been carried out. The objective of this study was to prove if (1) discrimination of defined fibrin and erythrocyte-rich thrombi using a spectral CT with KV-switch technology is feasible, and (2) to determine the potential accuracy of the method.
Materials and Methods
Study design
In vivo, the histology of thrombi is heterogeneous and may vary in size and morphology. Therefore, the study was planned under in-vitro conditions using artificially produced (=defined) thrombi. Imaging was performed with spectral CT with KV-switch technology (CT Revolution, GE Healthcare). The exact amount of fibrin and RBC in each thrombus was known and the histological composition was used as a gold standard for the correlation with the measured absorption spectrum.
Thrombus material
The thrombi were provided by Neuravi Ltd. (Galway, Ireland) with a predetermined ratio of fibrin and RBC. Ovine blood was selected because it has been shown to be best suited for coagulation system studies and is physiologically similar to human coagulation [9]. Seven samples were used for each type of thrombus using a special procedure to extract thrombus material from ovine venous blood. Thrombotic coagulation factors were combined with blood components to obtain thrombi with defined fibrin and erythrocyte [10].
Five different thrombus types with increasing RBC percentage were used for this study (Figure 1):
• <5% RBC (2,5% + 2,5); Fibrin <95% (minimal)
• 10%-40% RBC (25% + 15); Fibrin 60%-90% (low)
• 30%-60% RBC (45% + 15); Fibrin 40%-70% (moderate)
• 60%-85% RBC (72,5% + 12,5); Fibrin 15%-40% (high)
• 90%-100% RBC (95% + 5); Fibrin 0%-10% (maximal)
The average length of the thrombi was 5 cm; the diameter was between 4 mm and 6 mm. After fabrication and delivery, the thrombi were stored at 5°C, and all scanning procedures were performed within 2 days. The entire period between production and scans was 5 days.
CT scan and image analysis
Thrombi were first transferred in Iso-osmolar sodium chloride solution. Labeled 5ml syringes were used for transport, intermediate storage, and as scanning medium. Each of the seven samples was scanned three times, a total of 21 scans per thrombus type. The scans were performed on a high-resolution Spectral CT (CT Revolution, GE Healthcare Waukesha, WI, USA). The scans were conducted with the following parameters: 275 mAs, 140 kVp, 40-140 keV, 0.675 mm slice thickness, 2,5mm increment, pitch 0.516, 0.8s rotation time, iterative reconstruction algorithm).
The scans were uploaded into the AW-server software. A GSI (Gemstone spectral imaging) tool was used for spectral image evaluation. For this purpose, a defined ROI circle was placed centrally in the thrombus (Figure 2). To be able to make a comparable statement about the spectral bandwidth between the thrombi, the average size of the ROI was 8 mm2 and at least 7 mm2. The Hounsfield Units (HU) were measured using a curve in steps of 5 keV in a range of 40 to 140 keV. All thrombi were histologically processed following CT imaging.
Statistical analysis
For each type of thrombus, the mean HU-value, as well as the standard deviation, was calculated based on the twenty-one scans. The average absorption for each keV-level between 40 and 140 keV (5 keV steps) was statistically recorded and presented as a curve. Since the function of the curves was known, they could be pairwise compared by using the Kolmogorov-Smirnov Two-Sample Test. The Kolmogorov-Smirnov quantifies the distance or (dissimilarity between a) The empirical distribution function of a sample and the cumulative distribution function of a reference distribution, or b) Between the empirical distribution functions of two samples. In this study, the latter approach was applied.
The null hypothesis is (H0): The samples are drawn from the same distribution; the distribution considered under the null hypothesis is a continuous distribution but is otherwise unrestricted. The data were evaluated by a statistician (A.M.) using the statistics software R. A p-value below 0.05 was considered statistically significant.
Results
Figure 3 shows the results of spectral CT imaging of the different thrombi. All spectral HU curves showed an exponential drop with a steep slope between 40 keV and 75 keV. The curves flattened out at 80 keV, with higher RBC concentrations of >60%, assuming a more horizontal orientation earlier than for those with lower RBC content. With an increasing keV level, the measured density decreased along the curve. The difference (ΔHU) between the curves was highest at maximum keV-levels. At 140 keV, it was 8.7 HU for the absorption curves with high and maximum RBC concentrations (60%-85%) pairwise analysis of the individual HU-values (Table 1). The curves with minimal and low RBC-content were similar and showed no statistical significance. Pairwise analysis of the individual HU-values. The curves with minimal and low RBC-content were similar and showed no statistical significance. The curves from thrombi with moderate, high and maximum RBC-content showed good discrimination with a p-level<0.05 for all curves in total. For thrombi with an RBC content of 60% and above, statistical testing resulted in highest significance levels (p<0.001), thus indicating that an acceptable accuracy for the histological discrimination might be achieved for thrombi with an RBC content of more than 40% and at keV-levels above 80keV.
| Thrombus |  |
p-values and frequency distribution | Significance level |
|---|---|---|---|
| X1: 0%-5% RBC vs. X2:10%-40% RBC | 0,243 | p (min): 0,18009 (10,5%) p (max): 0,30408 (89,5%) | Not significant |
| X2: 10%-40% RBC vs. X3: 30%-60% RBC | 0,395 | p (min): 0,02113 (30%) p (max): 0,04747 (70%) | P*(<0,05) |
| X3: 30%-60% RBC vs. X4: 60%-85% RBC | 0,706 | p (min): 0,00002 (81,9%) p (max): 0,00009 (18,1%) | p***(<0,001) |
| X4: 60%-85% RBC vs. X5: 90%-100% RBC | 0,465 | p (min): 0,00315 (30%) p (int): 0,00855 (33,3%) p (max): 0,02113 (44,8%) | P**(<0,01) |
| Note: D^+ and the percentage of rejected null hypothesis, H0, i.e. *p-value is less than 0.05. **p-value is the level of statistical significance | |||
Table 1: The results of the comparisons have been summarized as the mean distance or dissimilarity.
Discussion
The KV-switch technology can determine the absorption at each KeV value, whereby significant absorption curves are generated for different types of thrombus. Retrospectively, the RBC percentage can be deduced. Our data prove that differentiation of the RBC fraction is possible using spectral curve analysis. Thrombi with an RBC-content of more than 40% could be differentiated best when using high keV-levels above 80. A limitation exists only in the differentiation of thrombi with minimal (0%-5%) and little (10%-30%) RBCcontent. Comparable experiments on dual-source systems (Siemens) have demonstrated similar results for high keV [7]. However, the observation of the absorption curves and the analysis of the entire keV spectrum, as performed in this study, seem to facilitate the differentiation of the thrombi.
Compared to conventional CT analysis, spectral CT imaging provides further information on the nature of the thrombus. The socalled “Hyperdense Artery Sign” (HAS) in native CT imaging does not allow any conclusion upon the composition of the thrombus and can be misinterpreted by an increased hematocrit value or atherosclerotic plaques. A correlation between the etiology of the thrombus and the histological composition had not yet been determined [11-13]. Data from contrast-enhanced CT scans showed that especially fibrin containing thrombi have an increased contrast agent uptake [8]. However, these studies are less helpful in a real-life scenario, since the stroke-CT is initially performed without contrast. A repeated scan after CTA and CTP would mean an unnecessary loss of time in stroke management. Alternatively, MRI imaging may be possible, in which susceptibility-weighted sequences are also used for thrombus imaging. The blooming effect in correlation to the proportion of RBC serves as a marker for thrombus differentiation [5,14,15]. However, CT imaging has become the initial diagnosis of stroke, and in most centers, MRI plays only a minor role.
A better understanding of the composition of a thrombus may be essential for the further development of stroke therapy. Both pharmacological and interventional treatment strategies might benefit from histological information about thrombus composition [16,17]. Early knowledge about thrombus properties influences the therapeutical decision on IV-lysis, anticoagulation, or antiplatelet treatment in acute stroke patients. Moreover, it might affect the choice of devices and periprocedural anticoagulation in patients that will undergo interventional stroke iso-osmolar [18,19].
Mechanical thrombectomy is the therapy of choice for stroke patients with vascular occlusions of the internal carotid artery and proximal intracranial vessel occlusions [20,21]. The number of thrombectomies has increased continuously in recent years [22]. Vessel elongation and atherosclerotic plaques make it more difficult for the interventionist to access the vessel occlusion. After the thrombus has passed with a micro catheter, a stent-retriever is released, and the thrombus is then removed with a retrieval maneuver under aspiration through the guiding catheter or a distal access catheter. Alternatively, isolated aspiration by hand or via the penumbra system is possible [23]. Statistically, a short intervention time with as few maneuvers as possible is decisive for an excellent clinical patient outcome [24], thus, the first-pass maneuver is the aim of the intervention. Neurointerventionalists know that the success of thrombectomy maneuvers depends not only on the size and the location of the thrombus but in no small part on its mechanical properties: “Red” thrombi with a high amount of RBC usually are easier to extract than organized fibrin-rich thrombi with little RBC content [25]. By knowing the nature of the thrombus, the best material or stent traction system for the individual patient could be selected in advance of the intervention to maximize the chances of rapid vessel reopening.
Although the composition of ovine blood largely corresponds to that of human blood, a transmission of the spectral absorption curves in in-vivo experiments still needs to be verified [9]. In addition, the composition of thrombi is not uniform in reality. Layers with fibrin and RBC can form, as well as serpentine or mixed forms [4] (Figure 4).
An isolated measurement in a defined area is therefore complicated, and errors in determining the ROI or identifying the thrombus carry the risk of misinterpretation. In small vessels (M2, M3) where the area is sometimes only a few square millimeters, a measurement with a ROI and subsequent generation and interpretation of a spectral absorption curve is challenging. A low RBC concentration between 0% and 40% cannot be reliably differentiated, although further studies with lower concentration differences and larger sample size could possibly show better results. Predicting the mechanical properties mixed thrombiwhose composition consists of approximately equal amounts of fibrin and RBC-will not be easy despite the specific absorption curve.
Conclusion
Our data prove that a histological differentiation of defined thrombi is feasible using spectral CT with the KV-switch technique. The accuracy of discrimination was best in thrombi with an RBC content of more than 40% and when using high keV-levels above 80. The results allow for potential further development of the CTtechnique for future studies, in which stroke treatment might take into account the histological nature of the thrombus.
Acknowledgment
We thank Christoph Spieler from GE for technical support and advice during the examination and evaluation of the thrombi. We thank Mr. Ray McCarthy of Neuravi Ltd. (Galway, Ireland) for the production of the thrombi. We also thank Thomas Reuter from the department of pathology for the histological processing of the thrombi.
References
- MEYER JS, Muramatsu K, Shirai T (1996) Cerebral embolism as a cause of stroke and transient ischemic attack. Echocardiography 13(5):513-518.
[Google Scholar] [PubMed]
- Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, et al. (2014) Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One 11;9(2):e88882.
[Google Scholar] [PubMed] [Crossref]
- Gorog DA, Fayad ZA, Fuster V (2017) Arterial thrombus stability: Does it matter and can we detect it?. J Am Coll Cardiol 70(16):2036-2047.
[Google Scholar] [PubMed] [Crossref]
- Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, et al. (2006) Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 37(8):2086-2093.
[Google Scholar] [PubMed] [Crossref]
- Luthman AS, Bouchez L, Botta D, Vargas MI, Machi P, et al. (2020) Imaging clot characteristics in stroke and its possible implication on treatment. Clinical neuroradiol 30:27-35.
[Google Scholar] [PubMed] [Crossref]
- Patino M, Prochowski A, Agrawal MD, Simeone FJ, Gupta R, et al.(2016) Material separation using dual-energy CT: Current and emerging applications. Radiographics 36(4):1087-1105.
[Google Scholar] [PubMed] [Crossref]
- Brinjikji W, Michalak G, Kadirvel R, Dai D, Gilvarry M, et.al. (2017) Utility of single-energy and dual-energy computed tomography in clot characterization: An in-vitro study. Interv Neuroradiology 23(3):279-284.
[Google Scholar] [PubMed] [Crossref]
- Borggrefe J, Kottlors J, Mirza M, Neuhaus VF. (2018) Differentiation of clot composition using conventional and dual-energy computed tomography. Clin neuroradio 28:515-522.
[Google Scholar] [PubMed] [Crossref]
- Siller-Matula JM, Plasenzotti R, Spiel A, Quehenberger P, Jilma B (2008) Interspecies differences in coagulation profile. Thromb Haemost 100(09):397-404.
[Google Scholar] [PubMed] [Crossref]
- Duffy S, Farrell M, McArdle K, Thornton J, Vale D, et al. (2017) Novel methodology to replicate clot analogs with diverse composition in acute ischemic stroke. J Neurointerv Surg 1;9(5):486-491.
[Google Scholar] [PubMed] [Crossref]
- Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, et al. (2017) Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: A systematic review. J Neurointerv Surg 9(6):529-534.
[Google Scholar] [PubMed] [Crossref]
- Krajíčková D, Krajina A, Šteiner I, Vyšata O, Herzig R, et al. (2018) Fibrin clot architecture in acute ischemic stroke treated with mechanical thrombectomy with stent-retrievers-cohort study. Circ J. 82(3):866-873.
[Google Scholar] [PubMed] [Crossref]
- Mair G, Boyd EV, Chappell FM, von Kummer R, Lindley RI,yet al. (2015). Sensitivity and specificity of the hyperdense artery sign for arterial obstruction in acute ischemic stroke. Stroke 46(1):102-107.
[Google Scholar] [PubMed] [Crossref]
- Bourcier R, Pautre R, Mirza M, Castets C, Darcourt J,et al. (2019) MRI quantitative T2* mapping to predict dominant composition of in vitro thrombus. AJNR Am J Neuroradio 40(1):59-64.
[Google Scholar] [PubMed] [Crossref]
- Weisstanner C, Gratz PP, Schroth G, Verma RK, Köchl A et al.(2014) Thrombus imaging in acute stroke: Correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success. Eur radiol 24:1735-1741.
[Google Scholar] [PubMed] [Crossref]
- Alberts MJ. Hyperacute (1997) stroke therapy with tissue plasminogen activator. Am J Cardiol 80(4):29D-34D.
[Google Scholar] [PubMed] [Crossref]
- Hacke W (2007) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 358:1327.
[Google Scholar] [PubMed] [Crossref]
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, et al. (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 12;372(11):1019-1030.
[Google Scholar] [PubMed] [Crossref]
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, et al. (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378(1):11-21.
[Google Scholar] [PubMed] [Crossref]
- Berkhemer OA, Fransen PS, Beumer D, Van Den Berg LA, Lingsma HF, et al. (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372(1):11-20.
[Google Scholar] [PubMed] [Crossref]
- Lindsay E, Nogueira RG, Jadhav AP, Haussen DC (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct: N Engl J Med 1: 1;54(4):583-584.
[Google Scholar] [PubMed] [Crossref]
- Saber H, Navi BB, Grotta JC, Kamel H, Bambhroliya A, et al. (2019) Real-world treatment trends in endovascular stroke therapy. Stroke 50(3):683-689.
[Google Scholar] [PubMed] [Crossref]
- Silva GS, Nogueira RG (2020) Endovascular treatment of acute ischemic stroke. Continuum (MinneapMinn) 26(2):310-331.
[Google Scholar] [PubMed] [Crossref]
- García-Tornel Á, Requena M, Rubiera M, Muchada M, Pagola et al. (2019) When to stop: detrimental effect of device passes in acute ischemic stroke secondary to large vessel occlusion. Stroke 50(7):1781-1788.
[Google Scholar] [PubMed] [Crossref]
- Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, et al. (2018) Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra 8(1):39-49.
[Google Scholar] [PubMed] [Crossref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 


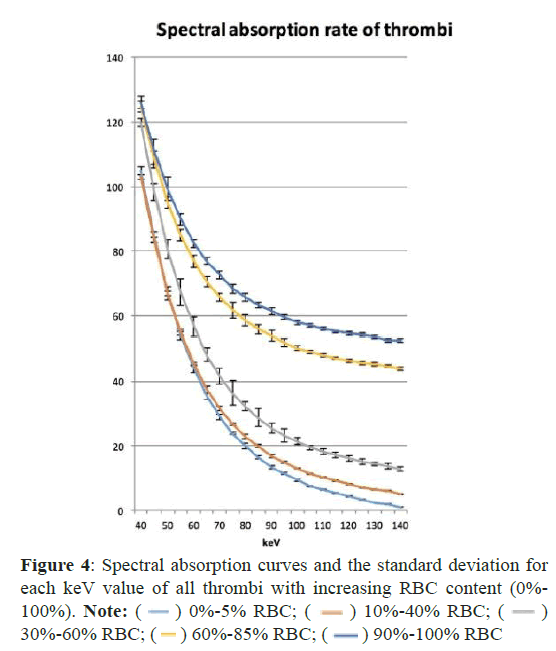
 ) 0%-5% RBC; (
) 0%-5% RBC; ( ) 10%-40% RBC; (
) 10%-40% RBC; ( )
30%-60% RBC; (
)
30%-60% RBC; ( ) 60%-85% RBC; (
) 60%-85% RBC; ( ) 90%-100% RBC
) 90%-100% RBC