Research Article, Vector Biol J Vol: 7 Issue: 5
Determination of the Efficacy of Entomopathogenic Fungus (Metarhizium anisopliae) for the Control of Culex quinquefasciatus, a Filariasis Vector
Fatima Muhammad Yayajo1*, Ismail Muhammad1, Muhammed Tanko Mahmoud2and Bala Abubakar3
1Department of Zoology, Gombe State University, Gombe, Gombe State, Nigeria
2Department of Biomedical and Pharmaceutical Technology, Federal Polytechnic, Mubi, Adamawa state, Nigeria
3Department of Life Sciences, Modibbo Adama University, Yola, Adamawa State, Nigeria
*Corresponding Author: Fatima Muhammad Yayajo
Department of Zoology,
Gombe State University,
Gombe,
Gombe State,
Nigeria,
Tel: +2347069951809;
Email: muhammadismail5609@gsu.edu.ng
Received date: 28 May, 2022, Manuscript No. VBJ-22-65206; Editor assigned date: 31 May, 2022, PreQC No. VBJ-22-65206 (PQ); Reviewed date: 14 June, 2022, QC No. VBJ-22-65206; Revised date: 27 July, 2022, Manuscript No. VBJ-22-65206 (R); Published date: 03 August, 2022, DOI: 10.4172/2473-4810.1000252.
Citation: Yayajo FM, Muhammad I, Mahmoud MT, Abubakar B (2022) Determination of the efficacy of Entomopathogenic Fungus (Metarhizium anisopliae) for the Control of Culex quinquefasciatus, a Filariasis Vector. Vector Biol J 7:5.
Abstract
Several mosquito species serve as vectors for many parasitic diseases. Control measures of such vector borne diseases rely majorly on the use of chemical insecticide, which in turn has an adverse effect on both the environment and public health. There is an increase need for an eco-friendly approach to counteract the negative impact of the conventional chemical insecticide. In this study, the efficacy entomopathogenic fungi Metarhizium anisopliae was tested against the larvae of Culex quinquefasciatus under laboratory condition. Fourth instar larvae of Culex quinquefasciatus were treated against different concentration (107, 108, 109 and 1010 conidia/ml) of Metarhizium anisopliae, each treatment containing 20 larvae with three replicates and a control. The experiment was allowed to run for a period of 120 hours. Mortality was recorded at an interval of 24 hours. Probit analysis was used to determine LC50, LC90, LC95 and LC99. For all the selected concentration, highest mortality of 42 (70,0%), 48 (80.0%), 51 (85.0%) and 57 (95.0%) were recorded after 120 hour. 1.79 × 103, 3.96 × 104, 4.97 × 105, 7.58 × 108 conidia/ml were the LC50, LC90, LC95 and LC99 recorded respectively. Metarhizium anisopliae has the potential to be used as biocontrol agent for Culex quinquefasciatus and is suitable candidate for further research.
Keywords: Biological control; Entomopathogenic fungi; M. anisopliae; Culex quinquefasciatus
Introduction
Several mosquito species are known to serve as vectors for several parasitic diseases such as malaria, filariasis, yellow fever, Japanese encephalitis, dengue, and Zika virus [1]. Malaria and filariasis are among the most common prevalent parasitic disease globally [2], as of 2011, 1.3 billion were at risk of the filariasis and also about one hundred and twenty million people were infected with disease globally [3]. Presently conventional control measures of several mosquito borne infection like malaria and lymphatic filariasis heavenly rely on vector control which in turn depends heavily on the use of chemical insecticide like parathyroid and personal preventives which involves the use of Insecticide Treated Nets (ITN) and Indoor Residual Spraying (IRS) [4,5].
The adverse consequences of synthetic chemical insecticides in the environment are a serious and major public health problem. Over the last fifty years, many problems have been resulted due to the misuse of synthetic insecticides in agriculture and public health programs, such as insecticide resistance, environmental pollution, toxic hazards to human and other non-target organisms [6], coupled with accumulation of these chemicals in the food chain and environmental pollution [7], these necessitates exploring eco-friendly and biological control methods [8]. Therefore in past few years’ interest in mosquito control using entomopathogenic fungi is evident, mainly due to continuous and increasing levels of insecticide resistance and increase in global risk of mosquito borne diseases [9]. The use of entomopathogenic fungi has so many advantages over conventional insecticide and pesticide, in that, having target selectivity, environmental compatibility, economic variability, novel mode of action, safer to environment and beneficial organisms as well as rational approach at a long run [10].
Entomopathogenic fungi which are normally found in soil and are widespread in temperate agro-ecosystems and semi-natural habitats are a very heterogeneous group of insect pathogens, there nearly 700 species belonging to approximately 100 orders [11]. Entomopathogenic fungi have the ability to target the larval stage of the pest and vector right from the breeding site which is more advantageous and attractive targets for the fact that insects like mosquitoes usually breed in stagnant water and thus, it is easy to deal with them in such habitat (breeding site) [12,13]. The genus Metarhizium is pathogenic to a large number of insect species thus, are among the natural enemies of pests and Vectors. It causes a disease known as ‘green muscardine’ in insect hosts because of the green colour of its conidial cells [14]. Entomopathogenic fungal metabolites could be an alternative source for mosquito larvicides because they constitute a potential source of bioactive compounds and generally free from harmful effects [15]. For example, M. anisopliae has a large host-range, including arachnids and five orders of insects, comprising over 200 species. Despite the fact that, mosquitoes are not listed as natural hosts for M. anisopliae some strains have shown to be virulent against mosquito larvae. Spores (conidia) of M. anisopliae have been known for some time to be infectious to adults and emerging pupae of some insects. Therefore, the aim of the present study was to isolate and determine the efficacy of Metarhizium anisopliae in the control of larval stage of Culex quinquefasciatus.
Materials and Methods
Study area
The study was conducted at Abubakar Tafawa Balewa University Bauchi (ATBU), Yelwa Campus (Longitude 9.792o East and Latitude 10.279o North) Bauchi Local Government Area, Bauchi State, Nigeria.
Mosquito Culex quinquefasciatus collection and breeding
One hundred and ninety two (192) blood fed mosquitoes were collected from their resting site in some selected houses of Gombe metropolis using aspirators. The collected mosquito species were placed in collecting cubs and transported to the insectary unit of biological sciences laboratory of Gombe state university for confirmation and identification. 109 (56.77%) were identified to be Culex quinquefasciatus and released in to two separate breeding cages for rearing and breeding, while in the cages they were fed with 10% sugar solution. Five (5) egg cups each containing 500 ml dechlorinated water were placed in each for eggs laying. Pieces of filter paper were placed on to the water and allowed to float for easy recovery of the eggs laid. The eggs were collected from the filter paper the following morning and transferred in to containers (45 × 20 cm) containing 500 ml of unchloronated water. No any food was provided to the containers until the first instar appears, then they were fed with 10% yeast and filtering was conducted once the water was dirty. The larvae were monitored until third and fourth instar larvae emerged, and were ready for the experimentation.
Fungal culture and conidial production
An inoculum of Metarhizium anisopliae was obtained from the biological science department Abubakar Tafawa Balewa University, Bauchi. Method of was adopted, but slight modification in culturing of Metarhizium anisopliae. In this, briefly the Metarhizium anisopliae were cultured on Potato Dextrose Agar (PDA) medium in an aseptic cupboard fumes and incubated at 28°C for 3 days. The conidia were harvested by scraping the surface of 3 days old culture suspended in distilled water. The mixture was stirred with a magnetic stirrer for 10 minute. The conidial concentration was determined using a hemocytometer which was used to count the number of conidia under a compound microscope. The conidial suspension was further diluted with 0.05% Tween 80 solution, until it reaches a desired concentration with a countable number of spores. After having the established concentration of conidia, suspensions were diluted with distilled water to the concentrations of 1 × 107, 108, 109, and 1010 conidia/ml. Only distilled water and 0.01% of poly sorbent added in the control treatment.
Bioassays
Laboratory bioassay was done following the methods of world health organisation with some modifications. Conidia of M. anisopliae were tested larvae of C. quinquefasciatus by adding fungal suspension to plastic cups containing water with 20 larvae of C. quinquefaciatus. Each plastic cup was inoculated with 1 ml of fungal suspensions of varying concentration (107, 108, 109, and 1010 conidia/ ml). Control treatments were prepared by adding of 20 ml of distilled water and 0.01% poly sorbent only. Each assay was replicated three times. Larvae were fed with yeast powder and their mortality was observed in a 24 h interval for 5 days.
Mortality determination
A glass rod was used to determine whether the larvae are dead or not. The rod was dipped into the container and bring very close to suspected dead larvae (which usually lie flat on the water surface). For the larvae that was still alive responded rapidly by either bending or moving away from the rods. On the other hand, for the dead larvae no matter how close the rod was brought, there was no any respond.
Mycosis test
For this test, three petri dishes were set up; two containing distilled water and the other one with 70% alcohol. Firstly all dead Culex quinquefasciatus larvae were placed in to the distilled water one by one, later in to ethanol and then into the distilled water again in order to kill the fungus on the surface of the larvae. After which microscopic examination of treated Culex quinquefasciatus larvae was conducted. Any sign of fungal growth on the body surface the larvae indicated the actual number of insect that died from fungal infestation.
Statistical analysis
All data generated were entered in to an excel software, and later transferred into a Minitab software for the actual analysis. Probit regression analysis was used to determine the relationship between concentration and mortality of Culex quinquefasciatus in given treatment. The LC50, LC90, and LC99 values were calculated with 95% confidence limit.
Results
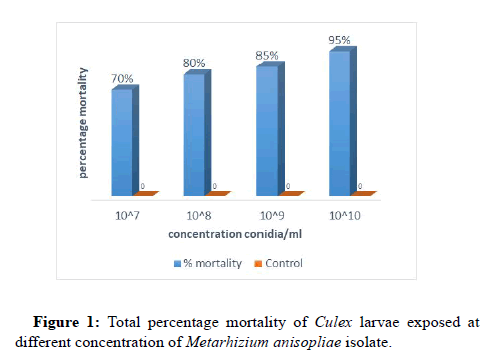
Result from this study shows that M. anisopliae isolate tested against fourth instars larvae of C. quinquefasciatus has pathogenic effect in all the treatment, but there was no mortality recorded in all the controls. However, pathogenicity varied according to concentration of spores and period of exposure. For the four concentrations; 107, 108, 109, and 1010 conidia/ml of the fungal isolate tested, it was observed that, mortality increased with increased in the time of exposure and also increased as the conidia concentration increased (Figure 1). Highest mortality of 57 (95%) was recorded at the highest concentration (1010) of conidia/ml applied. Consequently, other lower concentrations of 107, 108 and 109 conidia/ml recorded a Culex larval mortality of 42 (70%), 48(80%) and 51 (85%) respectively.
Mortality for the efficacy of Metarhizium anisopliae was assessed against Culex quinquefasciatus larvae based on the exposure period at various conidia concentration (107, 108 and 109 1010conidia/ml). The result revealed that, mortality increases as the exposure time also increases as shown in Table 1 below.
| Concentration | Time/hour | ||||
|---|---|---|---|---|---|
| (Conidiag/ml) | 24 h | 48 h | 72 h | 96 h | 120 h |
| Mortality (%) | Mortality (%) | Mortality (%) | Mortality (%) | Mortality (%) | |
| 107 | 0 | 3 (5.0) | 15 (20) | 26 (43.3) | 42 (70.0) |
| 108 | 0 | 6 (10.0) | 17 (28.3) | 30 (50.0) | 48 (80.0) |
| 109 | 0 | 09 (15.0) | 18 (30.0) | 32 (53.3) | 51 (85.0) |
| 1010 | 3 (5.0) | 12 (20.0) | 26 (43.3) | 41 (68.33) | 57 (95.0) |
| Control | 0 | 0 | 0 | 0 | 0 |
Table 1: Percentage mortality (%) of mosquito larvae Culex quinquefasciatus exposed at different time and concentrations of Metarhizium anisopliae.
At the concentration of 107, 108, 109, in 24 hours of exposure time there was no any mortality but at 1010 concentration 3 (5%) larval mortality was recorded. After 48hours of post exposure, mortality increases to 3 (5%), 6 (10), 9 (15) and 12 (20%) at 107, 108, 109, 1010 Conidiag/ml respectively. 15 (20), 17 (28.3), 18 (30) and 26 (43.3%) mortality were observed after 72 hours of exposure time using 107, 108, 109, 1010 Conidiag/ml. 26 (43.3), 30 (50), 32 (53.3) and 41 (68.33%) after 96 hours of exposure to the concentration of 107, 108, 109, 1010 Conidiag/ml while at 120 hours of exposure to the same concentration 42 (70), 48 (80), 51 (85) and 57 (95%) mortality were respectively recorded.c
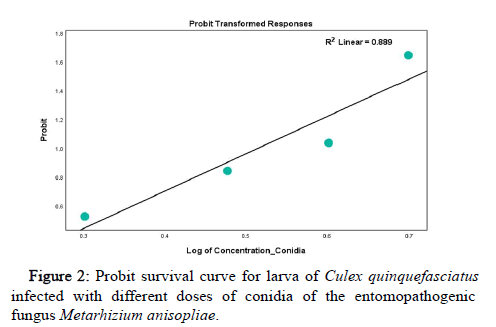
The survival data of biological vector, Culex quinquefasciatus, closely fitted the probit distribution model which showed a significant increase in the mortality rate of C. quinquefasciatus following exposure to different doses of conidia. It can be indicated from this result that survival rates were inversely related to the exposure of dose. That is, the higher the dose of conidia to the test organism, the increase in the mortality rate, While the lowest mortality rate was observed in the lowest concentration of conidia (107) and the higher mortality rate was found in the higher concentration of conidia (1010), as shown in Figure 2 below.
The result of the probit modelling analysis (Table 2) revealed the lethal concentrations that cause 50% (LC50), 90% (LC90), 95% (LC95) and 99% (LC99) mortality of Culex quinquefasciatus exposed to different doses of conidia of the entomopathogenic fungus Metarhizium anisopliae as shown in Table 3, 1.79 × 103 conidia/ml (95% CI was 0.23 to 2.54), 3.96 × 104 conidia/ml (95% CI was 2.80 to 25.51), 4.97 × 105 conidia/ml (95% CI was 3.37 to 82.92), 7.58 × 108 conidia/ml (95% CI was 4.46 to 810.92) were reported as the LC50, LC90, LC95 and LC99 respectively.
| LC | Estimate | Lower bound | Upper bound |
|---|---|---|---|
| LC50 | 1.79 | 0.23 | 2.54 |
| LC 90 | 3.96 | 2.8 | 25.51 |
| LC95 | 4.97 | 3.37 | 82.92 |
| LC99 | 7.58 | 4.46 | 810.92 |
Table 2: Estimation of Lethal Concentrations (LC) of Culex quinquefasciatus exposed to different concentration of conidia.
| Specie | Response | R2 | Pearson Goodness of fit Chi square | Sig. level | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Culex quinquefasciatus | y=3.71 + 0.52x | 0.89 | 10.17 | 0.02* | 2.69 | 4.72 |
*Represent significant (P < 0.05). Since the significance level is less than 0.05, there is significance between the observed and the expected value.
Table 3: Probit transformed response for Culex quinquefasciatus following exposure to varying doses of conidia of Metarhizium anisopliae.
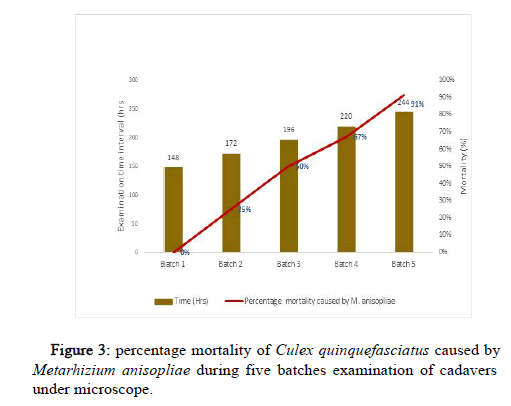
Mycosis results showed that the hypae of the fungal conidia start to appear after 172 hours post treatment with 25% C. quinquefasciatus. Highest (91%) conidial appearance was observed at 244 hours of post treatment. Conidial appearance on the surface of 67% and 50% for mosquito larvae after 220 and 196 hours of post treatment respectively. There was no conidial growth on the surface of C. quinquefasciatus after 148 hours of post treatment as shown in Figure 3 below.
Discussion
The present study evaluates the bio-efficacy of the fungus, M. anisopliae against larval stage of C. quinquefasciatus under laboratory conditions. The findings from the study revealed a very promising outcome, where M. anisopliae demonstrated high level of pathogenic activity against C. quinquefasciatus larva. This is not surprising, as previous studies revealed that Spores and metabolites of entomopathogenic fungi have been reported as larvicides/biocontrol agents against larval stage of different mosquitoes species [16], coupled with the fact that M. anisopliae is among the best natural enemy of several biological vector [17]. Therefore M. anisopliae could serve as a better replacement present chemical insecticide and pesticide in a future. Findings from this study are in agreement to the findings of who reported that, M. anisopliae causes significant mortality in different mosquito including C. quinquefasciatus [18]. The larvicidal activity of M. anisopliae is dose dependent, this findings is in concordance with findings who equally reported positive correlation between larval mortality and concentration of the entomopathogenic fungi (M. anisopliae), for that high level of larval mortalaity of 95% was reported from 1 × 1010 conidia/ml which was the highest concentration [19,20]. This result is the very similar to the findings of who equally reported reported high larval mortality of 91% at a conidial concentration of 1 × 108 Conidia/ml [21]. This clearly demonstrates the impact of concentration on larvicidal actitivity of the Entomopathogenic fungi. In addition, larvicidal activity of M. anisopliae against C. quinquefasciatus is directly proportional to the time of exposure that is larval mortality increases as sharply from 24hours up to 120hours, this finding is in agreement with findings of who also reported similar condition [22]. Therefore the longer the exposure time of lavae to the M. anisopliae isolate more the metabolite and other active component and toxins such as Destruxin, Bavericin, and Efrapeptins are secreted by M. anisopliae thereby penetrate deeply and establish themselves and eventually kill the larvae [23].
Irrespective of time, the lethal concentration that will kill at least 50% (LC50) recorded in this study was 1.79 × 103 conidia/ml with the range of 0.23-2.54 conidia/ml. Considering this low value of LC50 and a very lower boundary range of 0.23 confirmed the effectiveness of M. anisopliae as good biological control agent. This result is by far lower than 3.9 x 108 reported as LC50 conidia/ml of Metarhizium anisopliae against mosquito larvae after 24 by [24]. Mycosis result of this study confirmed the actual effectiveness of M. anisopliae as more 90% of the larval mortality in was attributed to M. anisopliae isolate due to the development hypae of the fungal conidia on the dead C. quinquefasciatus larvae 244hours after treatment.
Conclusions
M. anisopliae significantly demonstrate high level of efficacy in the control of larval stage of C. quinquefasciatus, as 95% of larval mortality was reported at higher conidia/ml concentration of 1 × 1010. Likewise other lower concentration demonstrates relatively good larvicidal activity even at 24 hour of larval exposure. Therefore in a near future entomopathogenic can favourably compete and even replace conventional synthetic insecticide.
References
- Kamalakannan S, Naik KG, Kovendan K, Balachandar V, Chauhan A (2022) Sources of Potential Fungi Generated Biogenic Nanoparticles for the Control of Diseases Transmitting Mosquitoes : A Review. Appl NanoBioScience 11:3523–3536.
- Scholte E, Njiru BN, Smallegange RC, Takken W, Knols BGJ (2003) Metarhizium anisopliae. Malar J 2:1–8.
- Singh G, Prakash S (2011) Studies on Fungal Cultural Filtrates against Adult Culex quinquefasciatus (Diptera : Culicidae) a Vector of Filariasis. J Parasitol Res 2011:147373.
- Howard AFV, Guessan RN, Koenraadt CJM, Asidi A, Farenhorst M, et al. (2010). The entomopathogenic fungus Beauveria bassiana reduces instantaneous blood feeding in wild multi-insecticide-resistant Culex quinquefasciatus mosquitoes in Benin, West Africa. Parasit Vectors 3:1–11.
- Paula AR, Carolino AT, Paula CO, Samuels RI (2011) The combination of the entomopathogenic fungus Metarhizium anisopliae with the insecticide Imidacloprid increases virulence against the dengue vector Aedes aegypti (Diptera : Culicidae). Parasit Vectors 4:1–8.
- Bilal H, Hassan SA, Khan IA (2012) Isolation and efficacy of entomopathogenic fungus (Metarhizium anisoplia) for the control of Aedes albopictus Skuse larvae : suspected dengue vector in Pakistan. Asian Pac J Trop Biomed 2:298–300.
- Kidanu S, Hagos L (2020) Entomopathogenic Fungi as a Biological Pest Management Option : A Review. Int J Res Stud Agric Sci 6:1–10.
- Hamama HM, Zyaan OH, Abu OA, Saleh DI, Elakkad HA, et al. (2022) Saudi Journal of Biological Sciences Virulence of entomopathogenic fungi against Culex pipiens : Impact on biomolecules availability and life table parameters. Saudi J Biol Sci 29:385–393.
[Crossref][Googlescholar] [Indexed]
- Khan S, Majumder DR, Sharif S, Shaikh Z (2017) Fungi: Mosquito LarvicidE. Int J Curr Res 9:50955–50961.
- Chaudhari SJ (2020) Role of Entomopathogenic Fungi in Insect Pests Control of Field Crops. Agri Mirror 1:7–11.
- Meyling NV, Thorup-kristensen K, Eilenberg J (2011) Biological Control Below- and aboveground abundance and distribution of fungal entomopathogens in experimental conventional and organic cropping systems. Biological Control 59:180–186.
- Muhammad I, Shuaibu AB, Rejoice A, Muhaamd I, AMM, et al. (2007) Journal Of Pure And Applied Science (Bti) and It’s Mode Of Action In The Control Of Larval Stage Of Malaria Vector : A Review. J Pure Appl Sci 8:1–11.
- Wahab AA, Ayanwola BJ, Adeyemo AT, Egbo OH, Afolabi BA, Adeyemo AT (2021) Larvicidal Efficacy of Entomopathogenic Fungi Isolated from the Farmland. J Med Sci Clin Res 9:224–232.
- Rai D, Updhyay V, Mehra P, Rana M, Pandey AK (2014) Potential of entomopathogenic fungi as biopesticides. Indian J Sci Technol 2:7–13.
- Vyas N, Dua KK, Prakash S (2015) Entomology, Ornithology and Herpetology Larvicidal Activity of Metabolites of Metarhizium anisopliae against Aedes and Culex Mosquitoes. Entomol Ornithol Herpetol 4:1-3.
- Podder D, Ghosh SK (2019) A new application of Trichoderma asperellum as an anopheline larvicide for eco-friendly management in medical science. Sci Rep 9:1–15.
[Crossref] [Googlescholar][Indexed]
- Khan S, Guo L, Maimaiti Y, Mijit M, Qiu D (2015) Entomopathogenic fungi as biocontrol agents Entomopathogenic Fungi as Microbial Biocontrol Agent. Mol Plant Breed 3:63–79.
- Vivekanandhan P, Swathy K, Murugan AC (2022) Insecticidal Efficacy of Metarhizium anisopliae Derived Chemical Constituents against Disease-Vector Mosquitoes. J Fungi 8:1–12.
[Crossref] [Googlescholar] [Indexed]
- Sani I, Suleiman M (2017) Larvicidal Activity of Entomopathogenic Fungus, Paecilomyces Specie against Fourth Instar Larvae of the Mosquito Culex quinquefasciatus (Culicidae:Diptera) Larvicidal Activity of Entomopathogenic Fungus, Paecilomyces Specie Against. Umaru Musa Yar’adua Univ J 2.
- Salwa SR, Gary WB (2020) Control of human filarial vector, Culex Quinquefasciatus (Diptera: Culicidae) through combination of the Entomopathogenic Fungus, Metarhizium Anisopliae and nanoparticles of zinc oxide and aluminum oxide. J Egypt Soc Parasitol 50:221–227.
- Abrar A, Sarwar S, Abbas M, Chaudhry H, Ghani N, et al. (2021) Identification of locally isolated entomopathogenic Fusarium species from the soil of Changa Manga Forest, Pakistan and evaluation of their larvicidal efficacy against Aedes aegypti. Braz J Biol 6:e246230.
[Crossref] [Googlescholar][Indexed]
- Annon MR (2021) Pathogenicity of Metabolites of Nematophagous Fungus Paecilomyces Lilacinus Against the Larvae of Anopheles Stephensi And Culex Quinquefasciatus. Sys Rev Pharm 12:40–44.
- Deka B, Baruah C, Babu A (2021) Entomopathogenic microorganisms : their role in insect pest management. Egypt J Biol Pest Control 31:1–8.
- Benserradj O, Mihoubi I (2014) Original Research Article Larvicidal activity of entomopathogenic fungi Metarhizium anisopliae against mosquito larvae in Algeria. Int J Curr Microbiol 3:54–62.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi