The Middle East: A mine for orphan drugs development
Tony Zbeidy
Orphan-Europe, France
: Androl Gynecol: Curr Res
Abstract
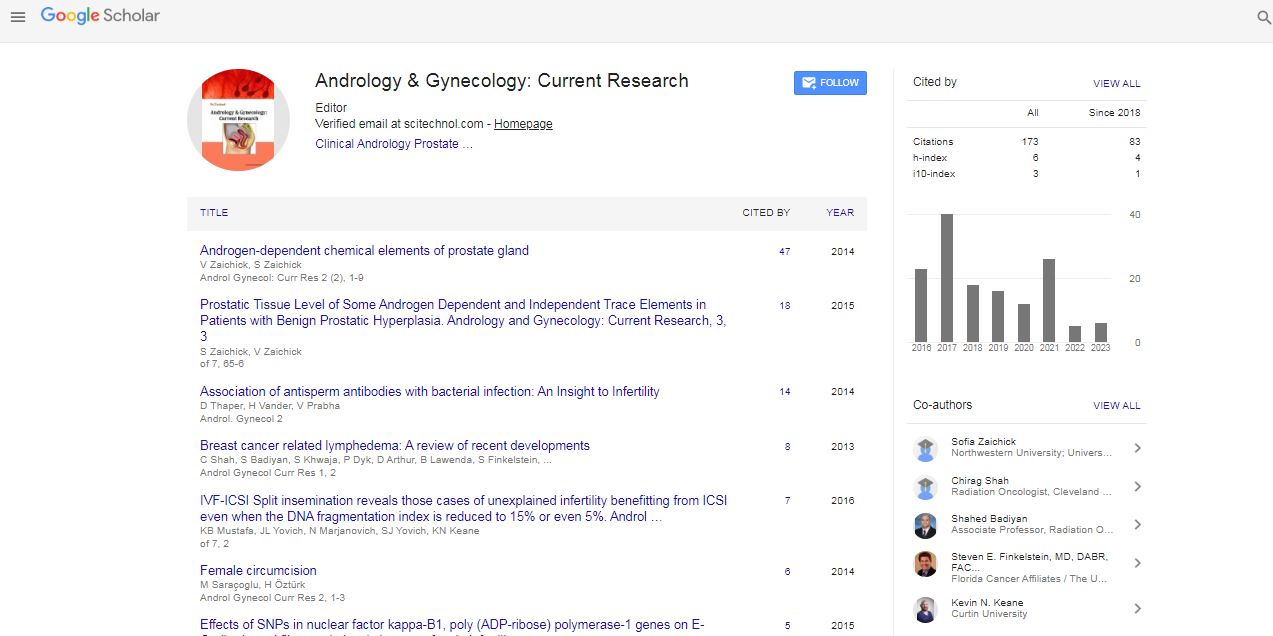
The Middle East and North Africa region, commonly known as MENA, is nowadays the space for geopolitical conflicts and wars. Despite this fact, the MENA region proved most resistant to the recent economic crisis, especially in the rare disorders domain, continuously presenting a relatively steady market growth. Such situation makes of MENA a high potential space for investments in Orphan Drug development. But what makes MENA of particular interest for the development of Orphan Drugs? And what did many governments in the MENA region, which indirectly boosted this potential higher than ever? MENA is a unique region presenting a high consanguinity rate. Knowing that most rare disorders are recessive and that the incidence of recessive disorders correlates with the level of consanguinity, MENA became a mine for patient recruitment and Orphan Drugs clinical development. Moreover, the global awareness around rare disorders drove the governments in the region to put in place national screening programs over the past ten years. Solid from 16 years’ experience in the Rare Disorders Business in MENA and witness of the regional metamorphosis over time, our presentation will illustrate the business rationale dividing MENA target territories into six clusters, reporting regional consanguinity rates and detailing the principal screening programs put in place in the region. Thus, this presentation is intended an open window for rare disorder drug developers to Market Access the MENA region from its different angles being clinical, business, or both.
Biography
Tony Zbeidy is a globe trotter French-Lebanese national and a US Graduate Medical Doctor who initially studied in Lebanon and obtained a BS in Biology. He travelled to US, obtained an MD and moved to UK training and working in Romford, Harold Wood and Basildon Hospitals. In 2001, he moved to France and joined Sanofi (Aventis at that time) as Medical Information Officer; in 2003, joined Orphan-Europe as Medical Advisor and 3 years later, established OrphanEurope FZ LLC subsidiary in Dubai Healthcare City in 2006 as GM MENA, representing 1/3rd of Orphan-Europe total turnover in 2015 and growing. He has a distinguished achievement, having established alone the Middle East Metabolic Group (MEMG) and is organizing its annual meeting since 2004. In 2011, major pharma companies acting in the field of rare disorders such as Genzyme, Biomarin, Nutricia, Merck-Serono, SOBI, Nestle, Synageva, etc, joined in and in 2015, it represented 250 selected metabolic and genetic specialists from the MENA region.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi