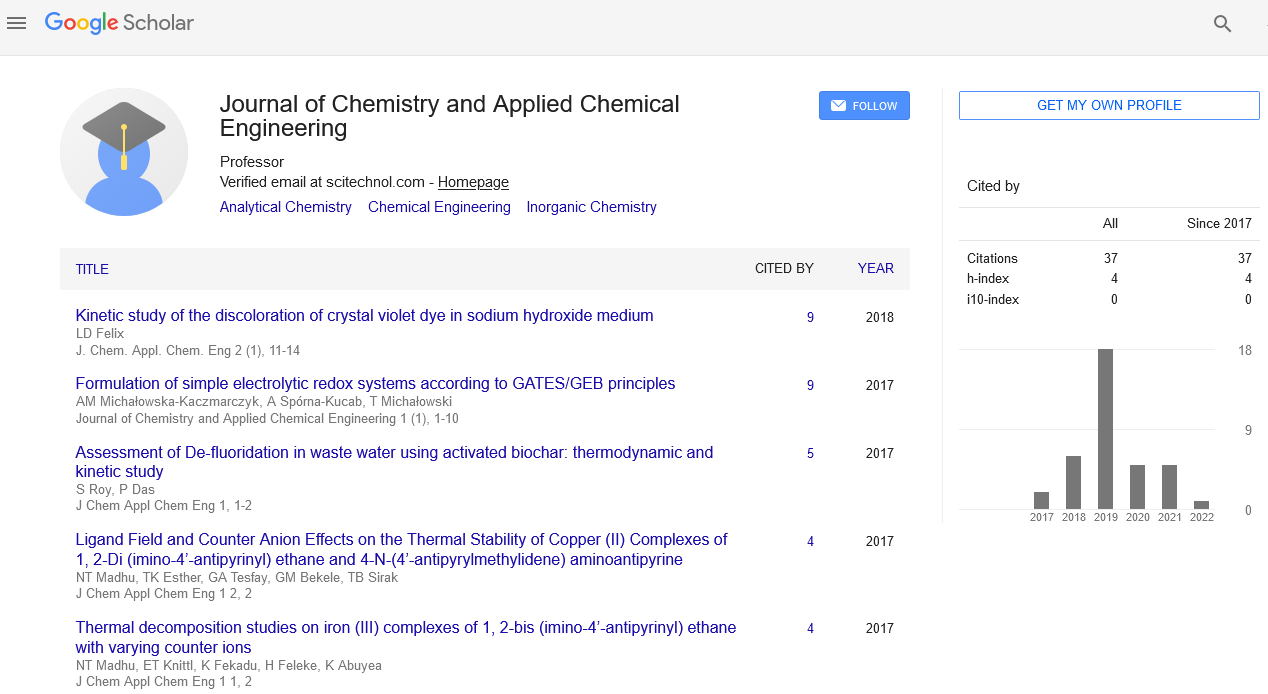
Soft sensor methodology for the precise spatial and temporal prediction of thermodynamic states inside chemical reactor
Guenther Holzer, Fabian ZAPF, Elisabeth Rammerstorfer, Erich Jeitler and Thomas Wallek
Graz University of Technology, Austria
Prozess Optimal CAP GmbH, Austria
: J Chem Appl Chem Eng
Abstract
Inside a continuous chemical reactor, in which phase transitions occur during the reaction, thermodynamic conditions as well as compositions of the process stream are subject to significant spatial and temporal changes. Given that measured information about thermodynamic state variables is only available for the inlet and outlet stream of the reactor, it is currently not possible to determine a spatial distribution of thermodynamic states within the reactor on the basis of measured information via software sensors. As, in particular, exothermic chemical reactions often show temperature peaks which are likely to destroy catalysts and components or adversely affect their service life, this limited information about internal reactor states poses a considerable risk with regard to product quality and process reliability. Therefore, this paper proposes a novel approach in the area of sensor technology which is based on rigorous thermodynamic models and enables a more detailed prediction of the reaction progress in view of avoiding undesired temperature peaks, based on measured process parameters of the input and output streams only. As a reference model, a production plant for ethyl tert-butyl ether (ETBE) as part of the gasoline line of a refinery is chosen. The liquid educts of the reaction are fed into a cooled tubular reactor in a counter flow arrangement, and the products also leave the reactor in a liquid state. Although the exothermic nature of the reaction leads to partial evaporation inside the reactor, the reaction solely occurs in the liquid phase. The influence of the phase equilibrium on the kinetics has already been described in literature. Via a user kinetic extension, the kinetics for ETBE and related side reactions is implemented into the commercial flow-sheet based simulation program KBC PetroSIM®, which allows for static and dynamic simulations. Consequently, differential material balances, bulk models for the phase equilibrium calculation (activity coefficients and/or equations of state) and the reaction kinetics are combined into one system of equations for the entire reactor section. This has the advantage that the convergence speed of the mathematical solver is considerably higher, compared to finite element reactor modeling approaches where the systems of equations must be solved for each mesh cell. Furthermore, the combination of a user kinetic model with a commercial process simulation tool opens the access to a broad variety of thermodynamic property models, i.e., activity coefficients and equations of state, which makes it possible to model reactions for practically all kinds of highly non-ideal systems. Because the kinetic model for the reference reaction is based on activities, the liquid phase fugacities in the course of vapor-liquid equilibrium calculations are described by the NRTL activity coefficient model, where missing binary parameters are estimated using the modified UNIFAC (Dortmund) group contribution approach. The formation of a vapor phase has a significant influence on the hydrodynamics inside the reactor because, for reasons of continuity, the flow rate increases and, consequently, the residence time of the reactants in the liquid phase decreases significantly. The core of the CAT-SAVE approach is the rigorous local calculation of phase equilibria. As the reaction yield increases, also the concentration of the product increases. If due to the exothermic nature of the reaction the local formation of a vapor phase occurs, the concentrations of the reactants and products (and, consequently, the activities) in both phases change. Because in the ETBE reaction the product represents the high-boiling component in the mixture, a partial evaporation implies an enrichment of the product in the liquid phase, which adversely affects the reaction rate. If, on the other hand, the concentration of the product increases, the reaction rate decreases. The resulting output of the model is a real-time visualization of the key process parameters, including the precise spatial and temporal prediction of temperatures inside the reactor and on the walls. Based on this information, destructive temperature peaks as well as undesired by-product formation can be avoided, and plant throughputs can be optimized precisely, taking into account material life and catalyst life cycle. For the reference plant at hand, the throughput-related catalyst life cycle could by increased by up to 10%. The method can easily be transferred to other processes.
Biography
Guenther Holzer has completed his master in Chemical Engineering from Technical University of Graz, Austria. He is the CEO of the Prozess Optimal CAP GmbH Company, a premier company for process optimization since 2007. He is leading the R&D in this company and has published several papers in reputed journals.
E-mail: holzer@prozess-otpimal.at
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi