Expert Review, Res J Opt Photonics Vol: 7 Issue: 3
Understanding Clinical Laser Selection and Laser-Tissue Interactions in Glaucoma
Ryan Lamrani1,2*, Brian Simon1,3, Anne Nguyen1,4, and Michael S. Berlin1,5
1Department of Ophthalmology, Glaucoma Institute of Beverly Hills, Los Angeles, CA, USA
2Department of Ophthalmology, Eastern Virginia Medical School, Norfolk, VA, USA
3Department of Ophthalmology, Loyola Medicine MacNeal Hospital, Berwyn, IL, USA
4Department of Ophthalmology, Charles R. Drew University of Science and Medicine, Los Angeles, CA, USA
5Department of Ophthalmology, UCLA Stein Eye Institute, Los Angeles, CA, USA
*Corresponding Author: Ryan Lamrani,
Department of Ophthalmology,
Glaucoma Institute of Beverly Hills, 330W Brambleton Avenue, APT
714Norfolk, VA 23510, USA
Tel: +1347-972-0361
E-mail: amranr@evms.edu
Received date: 16 June, 2023, Manuscript No. RJOP-23-102742;
Editor assigned date: 19 June, 2023, PreQC No. RJOP-23-102742 (PQ);
Reviewed date: 03 July, 2023, QC No. RJOP-23-102742;
Revised date: 13 July, 2023, Manuscript No. RJOP-23-102742 (R);
Published date: 20 July, 2023, DOI: 10.4172/RJOP.1000050
Citation: Lamrani R, Simon B, Nguyen A, Berlin MS(2023) Understanding Clinical Laser Selection and Laser-Tissue Interactions in Glaucoma. Res J Opt Photonics 7:3
Abstract
Importance: It is critical for the ophthalmic surgeon to understand lasers and their interactions with biological tissue in order to best select the device and technique specific to the treatments’ goals.
Objective: To describe the various types of lasers and how they differ in their mechanisms of action and interaction and subsequent clinical utility.
Methods: This review addresses lasers historical development, optical properties, laser-tissue interactions, diagnostic advantages, clinical applications, post-operative results and complications which are associated with each of the various lasers.
Findings: Light therapy in ophthalmology has enabled remarkable advances in the therapeutics of many conditions including refractive surgery, diabetic retinopathy, cataract and glaucoma. Due to their specific laser-tissue interactions each laser enables specific therapeutic interventions with some lasers created for application requiring scarring (retinal repair), some to move tissue physically (Nd:YAG capsulotomy), and some to non-thermally break chemical bonds to sculpt tissue without creating scar (PRK, LASIK, and ELT). They have proven increasingly useful in their application in ophthalmic surgery by providing safer and more effective surgical outcomes.
Conclusion and Relevance: This historical review demonstrates the evolution of our understanding of lasers which has stimulated the development of laser technologies enabling new surgical procedures with improved safety and efficacy. This evolution and the various applications of lasers and laser techniques have redefined glaucoma therapy to the benefit of patients.
Keywords: Glaucoma; Laser; Photothermal; Photodissociative; Photodisruptive; SLT; Excimer Laser Trabeculostomy (ELT); DSLT; Micropulse; MP-CPC; Cyclophotocoagulation; CPC; Endocyclophotocoagulation, ECPC; Historical perspective
Abbervations
Laser; SLT: Selective Laser Trabeculoplasty; Endoscopic; Surgery; Trabculostomy; FLAC: Femtosecond Laser Assisted Cataract Surgery; LASIK: Laser Assisted In Situ Keratomileusis; PRK: Photorefractive Keratectomy; ECPC: Endoscopic Cyclophotocoagulation; MP CPC: Micropulse Cyclophotocoagulation; TSCPC: Transscleral Cyclophotocoagulation; DSLT: Direct Selective Laser Trabeculoplasty
Introduction
The management of glaucoma has been significantly affected by the evolution and advancement of laser technologies and the innovative applications of these technologies. A variety of lasers, delivery systems and procedures are the current standard of care for both the diagnosis and the treatment of glaucoma. This review addresses these technologies’ applications, their historical development, optical properties, laser-tissue interactions, diagnostic advantages, post- operative results and complications which are associated with each of the various lasers. Our goal is to provide insights as to how these devices, all under the umbrella of “laser” actually differ in their mechanisms of action and interaction and subsequent clinical utility [1].
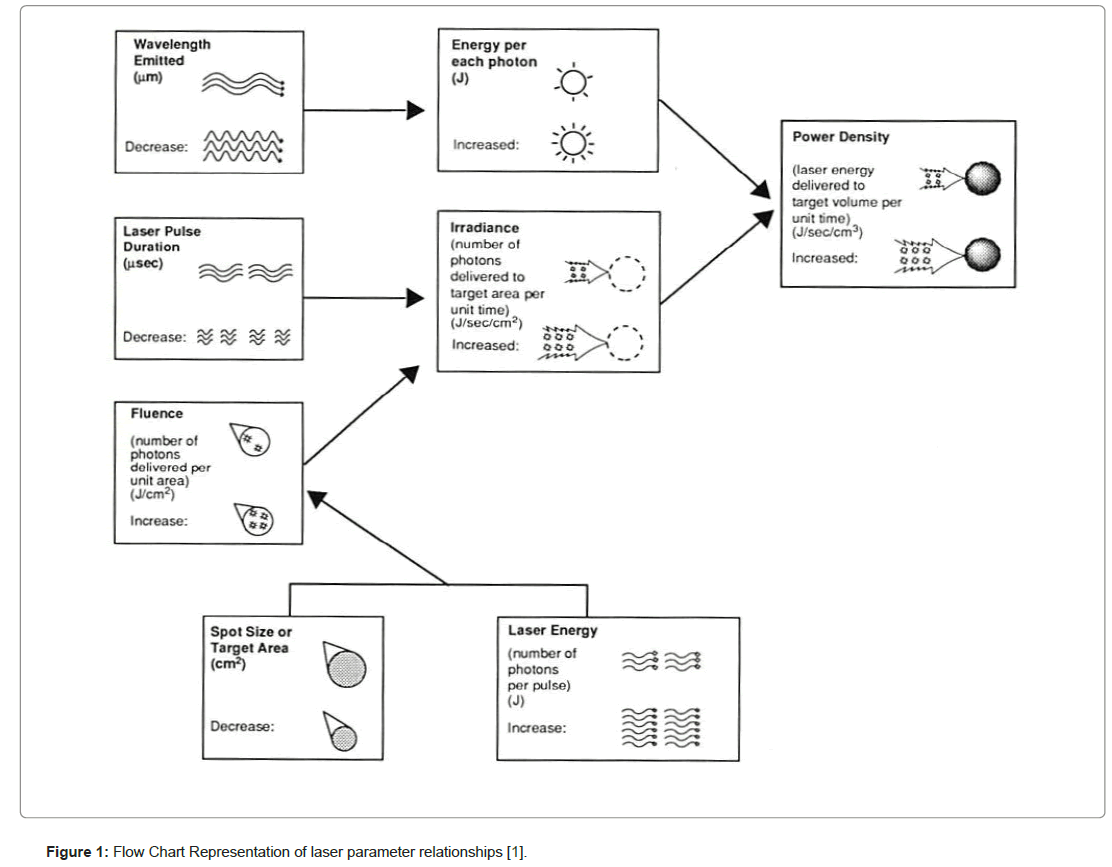
“Laser” is an acronym for “light amplification by the stimulated emission of radiation.” Every laser in clinical use interacts with tissues in specific ways. The wavelength of the radiation, and consequently the energy of the photons in the beam (which is inversely proportional to the wavelength), determines to what extent the laser energy is absorbed, and therefore which tissue components, called chromophores, are potential targets. The number of photons delivered to the target area, the irradiance, as well as the speed of photon delivery, the pulse duration, and the distance into the target in which photon absorption occurs, the absorption depth, are key elements to understanding whether the laser-tissue interaction will be mainly (photo) thermal, (photo) mechanical or (photo) chemical (Figure 1).
Figure 1: Flow Chart Representation of laser parameter relationships [1].
Current laser applications in glaucoma therapy have evolved with the development of laser technologies as well as surgical techniques and include non-invasive ab-interno and ab-externo and invasive ab- interno and ab-externo procedures (Table 1).
| Laser | Wavelength (nm) | E/photon (J) | Output: Pulsed (& duration) or Continuous wave |
|---|---|---|---|
| PHOTOTHERMAL | |||
| Pulsed dye | 666 |
2.98 x 10-19 | Pulsed (1-20 Hz) |
| Holmium: YAG | 2,100 |
9.46 x 10-20 | Pulsed (1-20 Hz) |
| Frequency Doubled Q-Switched Nd:YAG | 532 |
3.74 x 10-19 | Continuous Wave |
| PHOTODISRUPTIVE | |||
| Q-switched Nd:YAG | 1,064 |
1.87 x 10-19 | Nanosec. Pulse (0.01-5 x 104Hz) |
| Neodymium:YLF | 1,053 |
1.89 x 10-19 | Picosec. Pulse (up to 1000Hz) |
| PHOTODISSOCIATIVE | |||
| Argon Fluoride Excimer | 193 |
1.03 x 10-18 | Pulsed, 5-25 ns at 1-1000 Hz |
| Xenon Chloride Excimer | 308 |
6.45 x 10-19 | Pulsed, 1-80 ns at 1-500 Hz |
| Erbium:YAG | 2,940 |
6.76 x 10-20 | Pulsed (1-25 Hz) |
Table 1: Characteristics of various types of lasers.
Laser-tissue interactions
When considering the use of lasers for ophthalmic procedures, it is important to understand the photothermal, photoacoustic and photochemical effects that can occur due to the specific laser/tissue interactions of each application. Lasers are devices which enable highly concentrated beams of light to be focused onto small spatial regions, allowing precise tissue targeting. This precision can be valuable in microsurgical procedures by providing a predictable and reproducible effect on target tissues while minimizing trauma to adjacent ocular structures [2].
When a laser beam contacts a tissue, the energy is partially or totally absorbed by the tissue, transmitted through the tissue, scattered through the tissue or reflected away from it. The lightinteractive properties of the target tissue are a function of the target chromophores, the light absorbing (often pigment-containing) regions of molecules within the tissue. The limbal sclera which is the target tissue in a number of glaucoma surgeries is composed of proteins, predominantly collagen as well as glucose, aminoglycans, inorganic salts and water [3]. Absorption characteristics of a tissue depend upon its molecular composition, with each molecule having its own wavelength-dependent absorption profile. The chromophores in the cornea and sclera highly absorb ultraviolet radiation and their high-water content also allows near-infrared radiation to be absorbed.
For example, laser sclerostomy essentially requires that the organic polymers of the sclera are fragmented and ejected in a gaseous state. This tissue phase change may be brought about by either a) Photo vaporization, where molecular fragmentation is achieved thermally, b) Photo disruption, where molecular fragmentation is achieved mechanically, or by c) Photo dissociation where non-thermal molecular decomposition is achieved chemically: In the case of thermal lasers, once the local temperature becomes elevated enough to vaporize water, removal of tissue can ensue. However, local heat diffuses out of the focal region into the surrounding tissue, which can result in significant undesired effects and adjacent tissue damage [4].
Photo vaporization: Laser-tissue interactions of visible and long-pulsed infrared lasers are predominantly thermal. These lasers create a sclerostomy by inducing a focal temperature rise in the target tissue. Induced rise in focal temperature denatures the proteins like scleral collagen and glycosaminoglycans. The clinical uses of thermal lasers in ophthalmology include cyclo photo coagulation (thermal destruction of the ciliary body), thermal laser iridotomy and iridoplasty, treatment of retinal tears and detachments (thermal annealing of retina to sclera), choroidal neovascular diseases (thermal destruction of capillaries), macular edemas (annealing of vascular leakage), and destruction of various intraocular tumors such as retinoblastomas, melanomas, retinal angiomas etc.
Photo disruption: In the case of photodisruptive lasers, high energies delivered over very short durations ionize tissues, resulting in plasma formation which yields rapid volume expansion and mechanical shock wave propagation. This photoacoustic energy can photodisrupt tissue, although the spatial dispersion of the unrestricted shock wave cannot always be controlled with precision. Examples include Nd:YAG (1064 nm) posterior capsulotomy and iridotomy. Additional examples include femtosecond lasers with pulse durations in the femtosecond range (10-15 sec) and wavelengths in the visible and near infrared range (532-1700 nm), used for LASIK flap creation and Femtosecond Laser Assisted Cataract Surgery (FLAC).
Photo dissociation: Alternatively, photo dissociative response from the laser/tissue interactions can be harnessed surgically to remove tissue with extreme precision and with minimal or no undesired thermal damage to adjacent tissue (excimer lasers). These lasers use ultraviolet radiation to break molecular bonds directly with high energy short pulse durations; thus, ablation is essentially non-thermal sublimation. In ophthalmology, excimer lasers are used clinically for cornea refractive surgery Laser Assisted In Situ Keratomileusis (LASIK) and glaucoma surgery Excimer Laser Trabeculostomy (ELT) [5].
Methods of Literature Search
This review was compiled using articles identified by searching the PubMed. The following search terms were used: lasers and glaucoma, lasers and ophthalmology, glaucoma and photothermal, glaucoma and photo dissociative, and glaucoma and photo disruptive. Criteria for inclusion included the relevance, clinical importance, and scientific importance of articles to the subject of this paper. Articles cited in the reference lists of other articles were reviewed and included when considered appropriate. All articles with English abstracts were reviewed, and foreign-language articles were included when an English abstract was available and contained sufficient information for use in this paper. A select number of articles have been included before 1990 for historical purposes.
Historical perspectives
G. Meyer-Schwickerath first experimented in 1949 with focusing natural sunlight through a telescope and a series of mirrors to treat retinal disease. The evolution of this concept arose from his observation in 1945 of macular holes in a patient who had gazed at the sun during a solar eclipse. Although the concept was promising clinically, the effects were unpredictable due to inability to predict solar radiation [6]. After experimenting with carbon arc lamps, Meyer-Schwickerath collaborated with Hans Littman to develop the (non-laser) Xenon Arc photocoagulator (which emits wavelengths between 400 and 1600 nm). By the early 1950’s, he had performed the first successful ophthalmic “light therapy” with a xenon arc lamp to enable the first controlled retinal photocoagulation [7].
The advent of the invention of lasers by Theodore Maiman in 1960 enabled controllable and predictable sources of coherent light, Laser therapies have subsequently progressed including the development of a variety of gas lasers (Xenon Arc, Argon laser, CO2 laser, Excimer lasers) and solid state lasers (Ruby Laser, Nd:YAG, Nd:YLF, Ho:YAG, Er:YAG). The Table below discusses these developments (Table 2).
| Year of Laser/Device Invention (Inventor, if known) | Light Therapy & Laser Types (Wavelength) | Early Documented Ophthalmic Use (Year) | Example Procedure(s) | Description and Subsequent Clinical Use |
|---|---|---|---|---|
| Early 1950’s | Xenon Arc Photocoagulator (broadband: 400-1600 nm) | G Meyer Schwickerath (1956) [8] |
|
|
| 1960 (Maiman) | Ruby Laser (694 nm) | Campbell [12] |
|
|
| 1964 (Bridges) [15] | Argon Gas Laser (488 nm +514 nm) | L’Esperance [16] |
|
|
| (Geusic & Smith) [21] | Nd:YAG Solid State Laser (1064 nm) | Beckman and Sugar (1973) [22] |
|
|
| 1964 (Patel) [27] | CO2 Gas Laser (10,600 nm) | Beckman and Fuller [28]. |
|
|
| 1970 (Basov) | Excimer Laser (193 nm, 248 nm, and 308 nm) | MacDonald (1988) [31] |
|
|
| 1975 (Zharikov) [38] | Er:YAG (Solid State Laser (2940 nm) | Peyman et al. [39] |
|
|
| Hall [42] | Diode Semiconductor Lasers (various wavelengths: 810 to 1064 nm) | Uram [43] |
|
|
| 1962 (Johnson & Soden) [48] | Ho:YAG Solid State Laser (2100 nm) | Iwach et al [49]. |
|
|
| 1973 (Bates) [51] | Frequency doubled Nd:YAG Solid State Laser (532 nm) | Latina and Park [52] |
|
|
Table 2: Historical Perspectives-chronology of laser uses in ophthalmology.
Laser Selection for Specific Clinical Indication
Photodisruptive lasers
Photodisruptive lasers like the solid-state Q-switched Nd:YAG (1064 nm) consist of directing a high irradiance beam to a very small area of tissue such that it ionized: the electrons are stripped off their atoms and plasma is formed. The Q-switched mode enables short pulse, high energy bursts which enable plasma formation [13,14]. This plasma kickstarts extremely rapid volume expansion that creates a mechanical shock wave which is a supersonic pressure wave resulting in mechanical tissue alterations [15-23]. A notable issue with photodisruption is the significant and potentially undesired tissue trauma to adjacent tissues [24-53]. Selective Laser Trabeculoplasty (SLT) is an innovative photodisruptive modification of photothermal Argon Laser Trabeculoplasty (ALT).
Transcorneal Ab-interno SLT
In an effort to reduce the permanent structural damage that results from thermal lasers, Latina et al., created the Selective Laser Trabeculoplasty (SLT) laser. SLT uses a frequency doubled Q-switched Nd:YAG (532 nm) laser to enable short pulsed (3 ns) high energy photons to be delivered to intracellular chromophores which result in improved aqueous humor outflow. The SLT uses a Q-switched 532 nm Nd:YAG laser with a spot size of 400 μm. The scale of photodisruption differs from that of Q-switched 1064 nm Nd:YAG lasers in that only a select few pigmented cells absorb the SLT laser energy: the heat produced and the mechanical shock wave are thereby minimized [54].
An advantage of SLT compared to thermal ALT is its potential for repeatability. When SLT is successful initially, later repeated SLT if ever necessary may enable a further 20% reduction in IOP. In contrast, thermal ALT results in fibroblast proliferation and scar [54-59].
Transscleral Ab-externo SLT
In contrast to ab-interno SLT which requires a goniolens to visualize the trabecular meshwork and is performed one application at a time, Belkin et al., devised an innovative ab-externo approach called Direct Selective Laser Trabeculoplasty (DSLT) in which multiple SLT spots occur simultaneously. The approach consists of focusing the laser on the perilimbal sclera, which is a highly scattering tissue that reflects about 60% of 532 nm light emitted by the Q-switch Nd:YAG laser, and only transmits about 10% of the light through its full thickness (0.8 mm). Belkin et al., calculated that DSLT has a peak transmission of 6.6% and a limbus transmission of 11.2% to the trabecular meshwork. Given the scattering and diffusing properties of light and its ab-externo approach, the DSLT requires the delivery of 0.82-1.1 mJ of energy to the trabecular meshwork to reach the equivalent energy delivered by the ab-interno traditional SLT approach [60,61].
Photodissociative lasers
The laser/tissue interaction associated with photo dissociative lasers is predominantly photochemical. Excimer (a contraction of “excited dimer”) lasers use a combination of a noble gas and a halogen gas, which under high pressure and electrical stimulation emit laser light in the ultraviolet range. For example, the argon-fluorine excimer (193 nm) is used for cornea refractive surgery (ab-externo: PRK and LASIK), the krypton-fluoride (248 nm) (not clinically useful due to potential mutagenicity) and the xenon-chloride excimer (308 nm) is used for glaucoma surgery (ab-interno: ELT; ab-interno and abexterno: sclerostomy) [62]. The energy delivered by the individual photons from these UV lasers is sufficient to break the bonds between carbon atoms and between carbon and nitrogen atoms. They are very precise due to their short absorption path lengths, thereby enabling very accurate surgery.
When adequate organic tissue bonds are broken, the organic molecular fragments are ejected as gas from the target tissue surface. The absorbed energy which would be expected to dissipate thermally by inducing molecular vibration is instead used in breaking bonds directly. The ejection of the organic material from the target tissue surface dissipates the potential heat energy, enabling the photodissociative tissue ablation to occur in an essentially nonthermal manner.
The main advantage of photodissociative lasers is their precision and predictability regarding the amount of tissue removed: at a constant fluence, there is a consistent depth of tissue that is removed with each pulse. When the organic molecular fragments are ejected as a gas from the targeted tissue surface, the momentum imparted through the remaining tissue creates a mechanical wave like previously seen with photodisruptive lasers. However, with photodissociative lasers this photoacoustic shock wave is significantly less intense [63,64].
Ab-interno excimer laser
The initial medical application of these lasers in ophthalmology was for refractive surgery. They enable a method of precise, nonthermal and smooth etching of the corneal surface. Excimer lasers also offer several advantages in the surgical treatment of glaucoma by enabling precise non-thermal channel creation to bypass the outflow obstruction known to be localized in most open angle glaucoma’s to the juxtacanalicular trabecular meshwork and inner wall of Schlemm’s canal. The precision of these lasers enables avoiding trauma to the outer wall of Schlemm’s canal minimizing inflammatory responses while concurrently improving outflow. An additional advantage when used within the anterior chamber is that the production of gas from the product of ablation also dilates Schlemm’s canal in a manner similar to canaloplasty. However, in order to deliver ultraviolet energy within the eye in contrast to delivering ultraviolet to the corneal surface, since the cornea absorbs ultraviolet energy, treating glaucoma with an excimer laser requires an intraocular delivery system. There are no intraocular delivery systems amenable to 193 nm energy, thus 308 nm energy is the wavelength of choice for these ab-interno ELT procedures [65]. ELT is not SLT, DSLT, ALT, or MP CPC. In contrast to these other laser treatments for glaucoma, ELT is a MIGS procedure which removes tissue non-thermally creating channels between the anterior chamber and Schlemm’s canal identical to stent based MIGS, however without stents, and the channels created, because the laser/tissue interaction is essentially non-thermal, do not heal closed ensuring long term patency.
Discussion
In a 2020, 8-study meta-analysis, Durr et al., [66] positively reviewed the safety profile of Excimer Laser Trabeculotomy (ELT) and its efficacy in lowering IOP. In a cited 2010 randomized control study, Babighian et al., compared the IOP lowering effects of ELT and 180-degrees SLT in 30 eyes over a period of 2 years. Baseline IOP was 25.0 ± 1.9 mmHg and 23.9 ± 0.9 for ELT and SLT respectively and dropped to 17.6 ± 2.2 mmHg (30% reduction) in the ELT group and 19.1 ± 1.8 mmHg (21% reduction) in the SLT group, with similar medication reduction compared to baseline. Mild reflux bleeding was noticed in 12/15 eyes in the ELT group as well as small hyphemas in 80% of patients but all complications resolved within 5 days [67].
In a 2020 retrospective interventional case series of 105 eyes, Lozic et al., compared the efficacy of cataract excision (CE) + ELT to CE + trabectome to CE alone in lowering IOP. The researchers concluded that the mean survival time for the ELT + CE group (20.6 ± 1.0 months) was improved compared to the other groups (13.2 ± 0.4 months in CE alone, and 12.9 ± 0.6 months for CE + Trabectome) [68].
Two other retrospective case series include that of Töteberg- Harms et al., who followed the evolution of IOP in 24 eyes after ELT + CE surgery. IOP reduction was 8.8 ± 5.3 mmHg (37.7% reduction) at one year; mean medication use decreased from 2.3 ± 1.3 to 1.5 ± 1.4. The 6-month retrospective study by Moreno Valladares et al. shows similar results with a decrease of IOP to 17.8 mmHg from 21.2 mmHg and a drop in medication use from 1.8 meds to 0.7 meds [69,70].
Complications were rare in the studies evaluated and most were minor in nature and resolved spontaneously within days of the surgery [66].
Photothermal lasers
Infrared, near infrared, and visible lasers are used mainly to enable thermal laser/tissue interactions. These lasers induce a focal increase in temperature at the target tissue. Examples of photothermal lasers include argon gas lasers (488 and 515 nm), frequency doubled Nd:YAG (532 nm) which is a solid state laser and is often misnamed “argon laser” as the laser/tissue interactions are similar to those of an argon laser. Other examples include pulsed dye lasers which are liquid lasers (664 nm), continuous-wave solid state Nd:YAG lasers (1064 nm), solid-state holmium:YAG (2100 nm) lasers, and molecular gas carbon dioxide (10,600 nm) lasers. Photocoagulation occurs as the scleral collagen aminoglycans are denatured by focal temperature elevations resulting in tissue contraction and concurrent vaporization of water and ejection of adjacent tissue. The presence of oxygen causes burning and results in deposition of char. While photothermal lasers enable an effective cautery result, the creation of large zones of thermal damage around the focal point results in a significant volume of necrotic tissue that often produces an undesirable healing response [71,72]. Photothermal lasers are used for ciliary body destructive procedures such as cyclophotocoagulation. These include abinterno Endoscopic Cyclophotocoagulation (ECPC) and ab-externo cyclophotocoagulation and Micropulse Cyclophotocoagulation (MP CPC), among others [54].
Ab-interno ECPC
Multiple variables affect transscleral procedures. These include appropriate localization of the ciliary body, variations in scleral thickness, pigmentation, and optical fiber application including pressure exerted on the sclera by the fiber [73]. Since the ciliary body anatomy and the scleral thickness vary between individuals, Transscleral Cyclophotocoagulation (TSCPC) laser procedures necessitate that the laser spot size be large [74]. While both TSCPC and Endoscopic Cyclophotocoagulation (ECPC) use an 810 nm diode laser, ECPC is an advantageous innovation over TSCPC due to direct visualization of the target tissue (ciliary body) [75]. ECPC has demonstrated safety and efficacy, however precision of pressure control is not attainable and therefore these procedures are usually reserved for late-stage intractable glaucomas [76].
Ab-externo MP CPC
Traditional transscleral CPC lowers intraocular pressure through the destruction of the ciliary processes, the MP CPC procedure is not as destructive. The procedure similarly uses an 810 nm diode laser or a 577 nm laser. The innovation is in using MP CPC is the concept of repetitive short pulses (energy is applied 31.5% of the time) enabling less temperature elevation. The exact mechanism of action is currently not fully understood, therefore there is no definitive single procedure and many variations have been attempted. This is currently a procedure in evolution [77,78].
Conclusion
Light therapy in ophthalmology has enabled remarkable advances in the therapeutics of many conditions including refractive surgery, diabetic retinopathy, cataract, and glaucoma. Techniques have evolved from the harnessing of sunlight through magnifying glasses, focusing incoherent and multi-wavelength xenon-arc photothermal light to managing coherent light from lasers’ optical, mechanical and chemical effects on tissues. Due to their specific laser/tissue interactions, each laser enables specific therapeutic interventions, with some lasers created for application requiring scarring (retinal repair), some to move tissue physically (Nd:YAG capsulotomy), and some to non-thermally break chemical bonds to sculpt tissue without creating scar (PRK, LASIK, and ELT). These laser procedures have proven increasingly useful in their application in ophthalmic surgery by providing safer and more effective surgical outcomes.
To enable and to better utilize these remarkably advantageous surgical tools, it is important for the ophthalmic surgeon to understand lasers and laser-tissue interactions. Application of this knowledge will ensure selection of the most suitable device and technique specific to the treatments’ goals. This historical review demonstrates the evolution of our understanding of lasers which has stimulated the development of laser technologies enabling new surgical procedures with improved safety and efficacy. This evolution and the various applications of lasers and laser techniques have redefined glaucoma therapy to the benefit of patients. Understanding these applications enables better decision making to individualize patient care and maximize treatment outcomes.
References
- Berlin MS, Ahn R (1993) Laser sclerostomy: the state of the art. Ophthalmol Clin North Am. Philadelphia, PA: WB Saunders Company 6:415-424.
- Berlin MS, Ahn RJ (1996) Current options in laser sclerotomy. The Glaucomas: Glaucoma Therapy. St. Louis, Missouri: Mosby, Inc 1591-604.
- Vogel A, Dlugos C, Nuffer R, Birngruber R (1991) Optical properties of human sclera, and their consequences for transscleral laser applications. Lasers Surg Med 11(4):331-340.
- Cummins L, Nauenberg M (1983) Thermal effects of laser radiation in biological tissue. Biophys J 42(1):99-102.
- Berlin M (1988) Excimer Laser Applications in Glaucoma Surgery. New Techniques in Glaucoma Surgery, Ophthalmology Clinics of North America 1: 255-263.
- Sen M (1989). Xenon Arc Photocoagulation. Acta Ophthalmologica, 67: 32-43.
- Ober MD, Hariprasad SM (2009) Retinal lasers: past, present, and future. Retinal physician 1(1):2009.
- Meyer-Schwickerath G (1956) Erfahrungen mit der Lichtkoagulation der Netzhaut und der Iris. Doc Ophthalmol 10:91-131.
- Meyer-Schwickerath G (1950) Koagulation der Netzhaut mit Sonnenlicht. Ber Dtsch Ophthalmol Ges 55:256-259.
- BURNS RP (1965) Improvements in Technique of Photocoagulation of the Iris: 1. Higher Magnification as an Aid in Focusing the Light Beam 2. Combination of Photocoagulation With Discission. Arch Ophthal 74(3):306-9.
- Hogan MJ, Schwartz A (1960) Experimental photocoagulation of the iris of guinea pigs. Am. J. Ophth 49:629.
- Campbel CJ, Rittler MC, Koester CJ (1963) Optical maser as retinal coagulator. An evaluation. Trans Amer Acad Ophthal Otolaryng 67:58-67.
- Krasnov MM (1973) Laseropuncture of anterior chamber angle in glaucoma. Am J Ophthalmol 75(4):674-8.
- Krasnov MM (1974) Q-switched laser goniopuncture. Arch Ophthal 92(1):37-41.
- Bridges WB (1964) Laser oscillation in singly ionized argon in the visible spectrum. Appl Phys Lett 4(7):128-30.
- L'Esperance Jr FA (1969) The treatment of ophthalmic vascular disease by argon laser photocoagulation. Transactions-American Academy of Ophthalmology and Otolaryngology. Trans Am Acad Ophthalmol Otolaryngol 73(6):1077-1096.
- Khuri CH (1973) Argon laser iridectomies. Am J Ophthalmol. 76(4):490-493.
[Crossref]
- Lieberman MF (1983) Suture lysis by laser and goniolens. Am J Ophthalmol 95(2):257-258.
- Wise JB, Witter SL (1979) Argon laser therapy for open-angle glaucoma: a pilot study. Arch Ophthal 97(2):319-322.
- Moulin F, Haut J (1983) Results of argon laser treatment of 100 eyes with open-angle glaucoma (trabeculoplasty, trabeculoretraction). J Fr Ophtalmol 6(8-9):661-670.
- Geusic JE, Marcos HM, Van Uitert L (1964) Laser oscillations in Nd‐doped yttrium aluminum, yttrium gallium and gadolinium garnets. Appl Phys Lett 4(10):182-184.
- Beckman H, Sugar HS (1973) Neodymium laser cyclocoagulation. Appl Phys Lett 90(1):27-28.
- March WF, Gherezghiher T, Koss MC, Nordquist RE (1984) Experimental YAG laser sclerostomy. Arch Ophthal 102(12):1834-1836.
- March WF, Gherezghiher T, Koss MC, Shaver RP, Heath WD et al. (1985) Histologic study of a neodymium-YAG laser sclerostomy. Arch Ophthal 103(6):860-863.
- March WF, LaFuente H, Rol P (1987) Improved goniolens for YAG sclerostomy. Ophthalmic Surg Lasers Imaging Retina 18(7):513.
- Fankhauser F, Dürr U, England C, England C, Kwasniewska S et al. (1992) Optical principles related to optimizing sclerostomy procedures. Ophthalmic Surg Lasers Imaging Retina 23(11):752-761.
- Patel CK (1964) Continuous-wave laser action on vibrational-rotational transitions of C O 2. Physical review 136(5A):A1187.
- Beckman H, Fuller TA (1979) Carbon dioxide laser scleral dissection and filtering procedure for glaucoma. Am J Ophthalmol 88(1):73-77.
- Stefan C, Batras M, De Simone A, Hosseini-Ramhormozi J (2015) Current options for surgical treatment of glaucoma. Rom J Ophthalmol 59(3):194-201.
- Duan X, He Z, Zhang X (2010) A comparative study of the effects of ab externo superpulse carbon dioxide laser-assisted trabeculectomy with conventional trabeculectomy in rabbits. Photomed Laser Surg 28(1):109-113.
- McDonald MB, Beuerman R, Falzoni W, Rivera L, Kaufman HE (1987) Refractive surgery with the excimer laser. Am J Ophthalmol 103(3).
- Seiler T, Bende T, Wollensak J, Trokel S (1988) Excimer laser keratectomy for correction of astigmatism. Am J Ophthalmol 105(2):117-124.
- Hendrich C, Siebert WE (1997) Mutagenic effects of the excimer laser using a fibroblast transformation assay. Arthroscopy: The Journal of Arthroscopic & Related Surgery 13(2):151-155.
- Berlin MS, Rajacich G, Duffy M, Grundfest W, Goldenberg T (1987) Excimer laser photoablation in glaucoma filtering surgery. Am J Ophthalmol 103(5):713-714.
- Pasta J (2013) Laser therapy in ophthalmology. Lasers for Medical Applications: Diagnostics, Therapy and Surgery. 395-458.
- Vogel M, Lauritzen K, Quentin CD (1996) Targetted ablation of the trabecular meshwork with excimer laser in primary open-angle glaucoma. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 93(5):565-568.
- Berlin MS, Töteberg-Harms M, Kim E, Vuong I, Giers U (2013) Excimer laser trabeculostomy (ELT): an effective MIGS procedure for open-angle glaucoma. InSurgical innovations in glaucoma 31:85-95.
- Zharikov EV, Zhekov VI, Kulevskii LA, Murina TM, Osiko VV et al. (1975) Stimulated emission from Er3+ ions in yttrium aluminum garnet crystals at λ= 2.94 μ. Soviet Journal of Quantum Electronics 4(8):1039.
- Peyman GA, Badaro RM, Khoobehi B (1989) Corneal ablation in rabbits using an infrared (2.9-μm) erbium: YAG laser. Ophthalmology 96(8):1160-1170.
- Wetzel W, Scheu M (1993) Laser sclerostomy ab externo using mid infrared lasers. Ophthalmic Surg Lasers Imaging Retina 24(1):6-9.
- Spiegel D, Wetzel W, Birngruber R (1998) Ab externo erbium YAG laser sclerostomy versus conventional trabeculectomy. Treatment of glaucoma patients. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 95(8):537-41.
- Hall RN, Fenner GE, Kingsley JD, Soltys TJ, Carlson RO (1962) Coherent light emission from GaAs junctions. Phys Rev Lett 9(9):366.
- Uram M (1992) Ophthalmic laser microendoscope endophotocoagulation. Ophthalmology 99(12):1829-1832.
- Seibold LK, SooHoo JR, Kahook MY (2015) Endoscopic cyclophotocoagulation. Middle East Afr J Ophthalmol 22(1):18.
- Pankratov MM (1990) Pulsed delivery of laser energy in experimental thermal retinal photocoagulation. InLaser-Tissue Interaction (Vol. 1202, pp. 205-213). SPIE.
- Ingvoldstad DD, Krishna R, Willoughby L (2005) Micropulse diode laser trabeculoplasty versus argon laser trabeculoplasty in the treatment of open angle glaucoma. Invest Ophthalmol Vis Sci 46(13):123.
- Abramowitz B, Chadha N, Kouchouk A, Alhabshan R, Belyea DA et al. (2018) Selective laser trabeculoplasty vs micropulse laser trabeculoplasty in open-angle glaucoma. Clin Ophthalmol 30:1599-604.
- Johnson LF, Soden RR (1962) Optical maser characteristics of Nd3+ in SrMoO4. J Appl Phys 33(2):757.
- Iwach AG, Hoskins Jr HD, Drake MV, Dickens CJ (1993) Subconjunctival THC: YAG (“holmium”) laser thermal sclerostomy ab externo: a one-year report. Ophthalmology 100(3):356-366.
- Onda E, Ando H, Jikihara S, Kitazawa Y (1997) Holmium YAG laser sclerostomy ab externo for refractory glaucoma. Int Ophthalmol 20:309-314.
- Bates HE (1973) Burst-mode frequency-doubled YAG: Nd 3+ laser for time-sequenced high-speed photography and holography. Appl Opt 12(6):1172-1178.
- Latina MA, Park C (1995) Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res 60(4):359-371.
- Namazi N, Fleckner M, Feld JR, Lin CP, Puliafito CA (1993) Effects of cavitation bubbles created by pulsed laser ablation in the anterior-chamber. Invest Ophthalmol Vis Sci 34(4):1070-1070.
- Berlin MS, Ahn R (1995) Perspectives on new laser techniques in managing glaucoma. Ophthalmol Clin North Am 8:341-363.
- Ansari E (2021) 10-year outcomes of first-line Selective Laser Trabeculoplasty (SLT) for Primary Open-Angle Glaucoma (POAG). Graefes Arch Clin Exp Ophthalmol 259:1597-1604.
- Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V et al. (2019) Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet 393(10180):1505-1516.
- Lai JS, Chua JK, Tham CC, Lam DS (2004) Five‐year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Exp Ophthalmol 32(4):368-372.
- Song J (2016) Complications of selective laser trabeculoplasty: a review. Clin Ophthalmol 14:137-143.
- Sun CQ, Chen TA, Deiner MS, Ou Y (2021) Clinical outcomes of micropulse laser trabeculoplasty compared to selective laser trabeculoplasty at one year in open-angle glaucoma. Clin Ophthalmol 22:243-251.
- Geffen N, Ofir S, Belkin A, Segev F, Barkana Y et al. (2017) Transscleral selective laser trabeculoplasty without a gonioscopy lens. J Glaucoma 26(3):201-217.
- Sacks ZS, Dobkin-Bekman M, Geffen N, Goldenfeld M, Belkin M (2020) Non-contact direct selective laser trabeculoplasty: light propagation analysis. Biomed Opt Express 11(6):2889-2904.
- Mullerstolzenburg N, vonhaebler M, Erb C, Buchwald HJ (1991) Abinterno excimer laser sclerostomy in albino rabbits-influence of a topically applied uv absorber. Invest Ophthalmol Vis Sci 32(4): 860-860.
- Lin CP, Stern D, Puliafito CA (1990) High-speed photography of Er: YAG laser ablation in fluid. Implication for laser vitreous surgery. Invest Ophthalmol Vis Sci 31(12):2546-2550.
- Loertscher H, Shi WQ, Grundfest WS (1992) Tissue ablation through water with erbium: YAG lasers. IEEE Trans Biomed Eng 39(1):86-8.
- Berlin MS (1990) Photoablation: The basis of photochemical laser interactions. In Ophthalmic Laser. 101.
- Durr GM, Töteberg-Harms M, Lewis R, Fea A, Marolo P et al. (2020) Current review of Excimer laser Trabeculostomy. Eye Vis 7:1-9.
- Babighian S, Caretti L, Tavolato M, Cian R, Galan A (2010) Excimer laser trabeculotomy vs 180 degrees selective laser trabeculoplasty in primary open-angle glaucoma. A 2-year randomized, controlled trial. Eye Lond Engl 24: 632–638.
- Jozic L, Magner J, Funk J, Töteberg-Harms M (2020) Success of combined cataract extraction plus excimer laser trabeculotomy exceeds that of combined ab interno trabeculectomy with the trabectome or cataract extraction alone. Int Ophthalmol 40:529-537.
- Moreno Valladares AM, Amorós NP, López FG (2018) Excimer laser trabeculostomy: trabecular MIGS without implant. Revista Española de Glaucoma e Hipertensión Ocular 8(2):19-27.
- Töteberg-Harms M, Ciechanowski PP, Hirn C, Funk J (2011) One-year results after combined cataract surgery and excimer laser trabeculotomy for elevated intraocular pressure. Der Ophthalmologe 108:733-738.
- Hart WM. Adler’s Physiology of the Eye: Clinical Application. St. Louis, Mo: Mosby-Year Book.
- Walsh Jr JT, Flotte TJ, Deutsch TF (1989) Er: YAG laser ablation of tissue: effect of pulse duration and tissue type on thermal damage. Lasers Surg Med 9(4):314-26.
- Mora JS, Iwach AG, Gaffney MM, Wong PC, Ngungen N et al. (1997) Endoscopic diode laser cyclophotocoagulation with a limbal approach. Ophthalmic Surg Lasers Imaging Retina 28(2):118-123.
- Palmer DJ, Cohen J, Torczynski E, Deutch TA (1997) Transscleral diode laser cyclophotocoagulation on autopsy eyes with abnormally thinned sclera. Ophthalmic Surg Lasers Imaging Retina 28(6):495-500.
- Lin S (2002) Endoscopic cyclophotocoagulation. Br J Ophthalmol 86(12):1434-1438.
- Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA (1997) Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol 124(6):787-796.
- Ma A, Stephanie WY, Wong JK (2019) Micropulse laser for the treatment of glaucoma: a literature review. Surv Ophthalmol 64(4):486-497.
- Zaarour K, Abdelmassih Y, Arej N, Cherfan G, Tomey KF et al. (2019) Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma 28(3):270-275.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi