Research Article, J Physiother Rehabil Vol: 3 Issue: 2
Triggered Electrical Stimulation Cueing to Address Hypofunctioning Peroneus Muscle in the Treatment of Chronic Ankle Instability: Two Repeated Single Case Designs
Cheryl J Hickey1*, Deborah L Walker1, Kelly R Hosey1 and Paul A Ullucci2
1Department of Physical Therapy, California State University, Fresno, CA, USA
2Department of Physical Therapy, Sacred Heart University, Fairfield, CT, USA
*Corresponding Author : Cheryl J Hickey
Department of Physical Therapy, California State University, Fresno, CA, USA
Tel: +5592877928
E-mail: cherylba@mail.fresnostate.edu
Received: March 20, 2018 Accepted: April 15, 2019 Published: April 25, 2019
Citation: Hickey CJ, Walker DL, Hosey KR, Ullucci PA (2019) Triggered Electrical Stimulation Cueing to Address Hypofunctioning Peroneus Muscle in the Treatment of Chronic Ankle Instability: Two Repeated Single Case Designs. J Physiother Rehabil 3:2.
Abstract
Background: Approximately two million ankle sprains occur in the United States annually. Lateral ankle sprains (LAS) account for 85% of all ankle sprains, 74% of those individuals report continued symptoms following the initial insult. Persistent pain and instability following ankle sprain is indicative of chronic ankle instability (CAI). There are many interventions to address hypofunctioning muscle including electrical stimulation (ES) and it is known that peripheral ES changes muscle behavior. The purpose of this pilot study was to investigate the effect of electrical stimulation of the peroneal muscles combined with 3 exercises for the treatment of CAI.
Methods: Two participants with CAI were recruited for this repeated single case design. The Cumberland Ankle Instability Tool (CAIT), the modified Star Excursion Balance Test (mSEBT), and center of pressure (CoP) was measured. A single limb hop to stabilization protocol and single limb stance activities were combined with manual triggered electrical stimulation for six sessions.
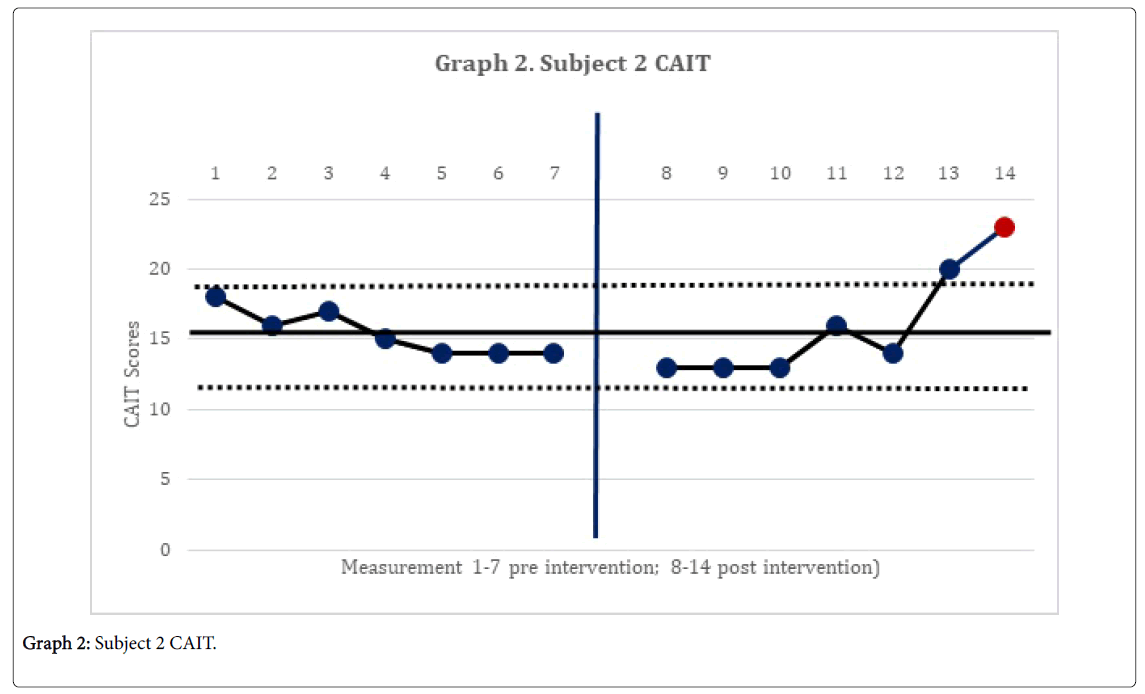
Results: Improvements in CAIT scores for both patients were observed. There was no statistically significant change in mSEBT scores. Center of Pressure measurement improvements varied between subjects. For subject 1, there was no significant change in CoP in eyes open and eyes closed conditions. For subject 2, there was a significant change in the anterior/posterior CoP data points in both conditions.
Discussion: In individuals with CAI, the addition of electrical stimulation to a single limb stance and hop stabilization protocol for hypofunctioning peroneal muscles led to positive CAI outcomes as measured by improved CAIT scores and improved CoP measurement in one subject.
Keywords: Electrical stimulation; Peroneus muscle; Ankle instability
Introduction
Approximately two million ankle sprains occur in the United States annually [1,2]. Lateral ankle sprains (LAS) account for 85% of all ankle sprains [3]. As high as 74% of individuals who experience a LAS report continued symptoms following the initial insult [4]. These symptoms include giving way, joint instability, pain, swelling, loss of function, and/or repeated ankle sprains [4]. Persistent pain and instability is indicative of chronic ankle instability (CAI) [4,5].
Attributes of CAI are referred to as mechanical ankle instability (MAI) and functional ankle instability (FAI) [4-15]. Mechanical ankle instability includes anatomical changes such as pathological laxity, impaired joint arthrokinematics, development of degenerative joint disease, and/or synovial changes affecting the static stabilization of the ankle joint, which result in decreased ankle stability [15].
Functional ankle instability includes impairments in ankle proprioception, cutaneous sensation, nerve-conduction velocity, neuromuscular response times, postural control, and strength [15]. These impairments are associated with altered anticipatory and reactive neurological feedback loops due to damaged mechanoreceptors and muscle spindles [16]. Damaged mechanoreceptors in addition to muscle inhibition or hypofunction, result in incorrect proprioceptive information that can cause further instability at the ankle due to altered alpha and gamma motor neuron firing [17-19]. The hypofunction, may prevent the injured ankle from affectively activating its dynamic lateral ankle stabilizers (peroneals) to appropriately stabilize the ankle during function and sport.
Measures that examine dynamic balance and postural control deficits associated with CAI include, the SEBT (Star Excursion Balance Test), CoP (center of pressure) measures and most recently, wobble board performance changes [20-22]. The research on the SEBT indicates deficits in limb reach during dynamic balance, in CAI subjects compared to non-injured limbs [20]. Center of pressure velocity measures also indicates changes in the mediolateral and anteroposterior direction for CAI subjects [21]. Most recently, computerized wobble board testing in a study by Fusco et al., showed that it may be capable of detecting balance impairment in subject with unilateral CAI [22].
In the absence of external lateral ankle support an intervention to address the hypofunctioning peroneal muscle is the key to successful rehabilitation for CAI, regardless of the measure used because of its contribution to the underlying chronic pathology [23]. The treatment of CAI must give attention to the mechanism of hypofunctioning. To date, treatment for CAI has proven inconsistent in duration, intervention protocol and outcome [24].
Electrical stimulation (ES) has been used for acute LAS to address function, edema, and pain with inconclusive results [25]. Only one study was found that evaluated the effect of ES on CAI [26]. This study showed an improvement in CoP excursion after using sensory level ES to increase postural control following a drop landing.
Although there is support for combined ES and exercise [27], the underlying physiological mechanism and specific protocols are not always clearly understood. Electrical stimulation effects include central nervous system (CNS) and peripheral nervous system (PNS) mechanisms and these effects maybe optimal when applied to the decreased activity in neurologic pathways following injury. This decreased activity reduces the excitability of CNS circuits and weakens pathways between the brain and muscle [25] which likely perpetuates a hypofunctioning muscle following injury. However, increases in CNS activity via ES, may restores the correct muscle recruitment timing of these pathway functions [10,27-29].
There is also virtually no research showing the effects of a combined balance program with triggered ES to address neurological deficits related to hypofunctioning peroneal muscles [11,12]. The purpose of this pilot study was to investigate the effect of electrical stimulation of the peroneal muscles combined with 3 exercises in the treatment of CAI.
Methods
Two participants were recruited for this repeated single case design and informed consent was give as part of the IRB process. One male, 24 years of age and one female, 25 years of age; both of average height and normal BMI. The male subject had a history of 3 significant ankle sprains while playing football to the tested side. The female subject had a history of multiple bilateral sprains during her time as a competitive soccer player. Both subjects had full anatomical ROM with greater ligamentous laxity noted on the tested side. Neither subject sought formal medical treatment of their sprains. Most importantly, both subjects met the selection criteria for patients with CAI as per the International Ankle Consortium position statement (Appendix 1) which includes instability scores on the CAIT and FAI (for the full positions statement criterial, which was used in this study the reader is encouraged to reference the detailed statement published in the Journal of Orthopedic and Sports Physical Therapy) [25]. Exclusion criteria were based on self-reported information. Participants were excluded if they had contraindication to electrical stimulation, an abnormal nervous system, or acute illness.
Participants completed the CAIT and performed the mSEBT [6,13,30,31]. A maximum of six attempts per reach direction were averaged and used to calculate normalized mSEBT scores. Center of pressure was measured with a Kistler force plate (Type 9260AA, Kistler Instruments Inc., Amherst, NY). The methods to obtain this data were similar to Mettler et al. [32]. The force plate data was sampled at 200 Hz and filtered at 6 Hz using a Butterworth low-pass filter. The protocol used was similar to one described by McKeon et al. [33] involving single-limb hops to stabilization and single-limb stance activities.
In addition to the exercise protocol, the participants received manual triggered electrical stimulation. A biphasic pulsatile current was used with parameters that included a pulse width of 250 usec, and a frequency of 25 pps, and the intensity was adjusted to give a visible muscle contraction producing ankle eversion (14-25 mA). During the dynamic exercises the electrical stimulation was triggered immediately before take-off from start position and maintained until the subject returned to start position and then released.
The protocol included single leg hop with 10 repetitions each, anterior/posterior (AP) and lateral/medial (LM) and anteromedial/ posterolateral directions. Single limb stance (SLS) with 3 repetitions each with the following progression: eyes closed arms out on noncompliant surface, progressing from 30 to 90 seconds and then potential progression to foam and foam with ball toss. The last progression included SLS eyes closed with arms across the chest for 30 seconds on compliant surface up to 90 seconds (protocol adapted from McKeon et al., see Appendix 2) [33].
Results
The 2-standard deviation band method yielded improvement in CAIT scores for both subjects. This represented a significant improvement in perceived instability (Graphs 1 and 2). There was no statistically significant change in mSEBT for either subject. For subject 1, there was no significant change in the intervention phase of the CoP AP and ML amplitude, for both eyes open, and eyes closed conditions. However, for subject 2, there was a significant change in the AP CoP data in both the eye open, and eyes closed conditions, of the intervention phase (Graphs 3 and 4), indicating more movement in the subject’s CoP for the AP directions.
Discussion
The subject’s significant improvement in CAIT scores suggests improvement in the perceived instability. Subject 2 demonstrated a significant increase in CoP mean amplitude in the AP directions and greater deviations in all four directions with increases in sway. This may indicate the emergence of new strategies in order to maintain postural control [14]. This finding is consistent with Sefton et al., [34] who found greater variability in static balance CoP measures following 6 weeks of balance training.
There were no significant changes in mSEBT measurements for either subject. This may be due to a number of factors. The first of which is that the time of day influences on dynamic postural control measurements [35]. These measurements tend to improve in the morning when compared to late afternoon or evening [35]. In this study, measurements were taken when the subjects had availability, which varied throughout the day [35]. Additionally the choice of exercise may have negatively impacted mSEBT measurements because they were more static in nature. The addition of dynamic balance exercise would be recommended as a protocol modification. The addition of a HEP would be recommended because it has been used in previous studies successfully to reinforce movement learning in the hypofunctioning muscle [36].
Conclusion
Arthrogenic muscle inhibition, which is present in individuals with CAI [37], with marked decreases in alpha motor neuron excitability, contributes to hypofuncting muscle, (in this case, of the peroneus longus). Because of this inhibition/hypofunction, [6,7,37] the addition of ES in these subjects, may be what lead to positive CAI outcomes. Electrical stimulation increases the excitability of the motor neuron pools observed in the peroneus longus and increases the effectiveness of the altered descending corticospinal pathways, which is something that the patient cannot do voluntarily, without the electrical cueing [8].
References
- Kosik KB, McCann RS, Terada M, Gribble PA (2017) Therapeutic interventions for improving self-reported function in patients with chronic ankle instability: A systematic review. Br J Sports Med 51: 105-112.
- Han K, Ricard MD, Fellingham GW (2009) Effects of a 4-week exercise program on balance using elastic tubing as a perturbation force for individuals with a history of ankle sprains. J Orthop Sports Phys Ther 39: 246-255.
- Ferran NA, Maffulli N (2006) Epidemiology of sprains of the lateral ankle ligament complex. Foot Ankle Clin 11: 659-662.
- Konradsen L, Bech L, Ehrenbjerg M, Nickelsen T (2002) Seven years followâ€Âup after ankle inversion trauma. Scand J Med Sci Sports 12: 129-135.
- Hertel J (2002) Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train 37: 364-375.
- Gutierrez GM, Kaminski TW, Douex AT (2009) Neuromuscular control and ankle instability. PM R 1: 359-365.
- McVey ED, Palmieri RM, Docherty CL, Zinder SM, Ingersoll CD (2005) Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int 26: 1055-1061.
- Nelson RM, Hayes KW, Currier DP (1999) Clinical electrotherapy, 3rd edition. Pearson.
- Gregory CM, Bickel CS (2005) Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther 85: 358-364.
- Pionnier R, Découfour N, Barbier F, Popineau C, Simoneau-Buessinger E (2016) A new approach of the star excursion balance test to assess dynamic postural control in people complaining from chronic ankle instability. Gait Posture 45: 97-102.
- Hall EA, Docherty CL, Simon J, Kingma JJ, Klossner JC (2015) Strength-training protocols to improve deficits in participants with chronic ankle instability: A randomized controlled trial. J Athl Train 50: 36-44.
- Martin RL, Davenport TE, Paulseth S, Wukich DK, Godges JJ (2013) Ankle stability and movement coordination impairments: Ankle ligament sprains. J Orthop Sports Phys Ther 43: A1-A40.
- Pietrosimone BG, Gribble PA (2012) Chronic ankle instability and corticomotor excitability of the fibularis longus muscle. J Athl Train 47: 621-626.
- Palmieri RM, Ingersoll CD, Stone MB, Krause BA (2002) Center-of-pressure parameters used in the assessment of postural control. J Sport Rehab 11: 51-66.
- Holmes A, Delahunt E (2009) Treatment of common deficits associated with chronic ankle instability. Sports Med 39: 207-224.
- Hertel J (2008) Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med 27: 353-370.
- Riemann BL, Lephart SM (2002) The sensorimotor system, part II: The role of proprioception in motor control and functional joint stability. J Athl Train 37: 80-84.
- Riemann BL, Lephart SM (2002) The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train 37: 71-79.
- Freeman MA (1965) Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br 47: 669-677.
- Olmsted LC, Carcia CR, Hertel J, Shultz SJ (2002) Efficacy of the star excursion balance tests in detecting reach deficits in subjects with chronic ankle instability. J Athl Train 37: 501-506.
- Wikstrom EA, Fournier KA, McKeon PO (2010) Postural control differs between those with and without chronic ankle instability. Gait Posture 32: 82-86.
- Fusco A, Giancotti GF, Fuchs F, Wagner H, Varalda C, et al. (2019) Wobble board balance assessment in subjects with chronic ankle instability. Gait Posture 68: 352-356.
- Knapp D, Lee SY, Chinn L, Saliba SA, Hertel J (2011) Differential ability of selected postural-control measures in the prediction of chronic ankle instability status. J Athl Train 46: 257-262.
- Hale SA, Fergus A, Axmacher R, Kiser K (2014) Bilateral improvements in lower extremity function after unilateral balance training in individuals with chronic ankle instability. J Athl Train 49: 181-191.
- Gribble PA, Delahunt E, Bleakley C, Caulfield B, Docherty CL (2013) Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the international ankle consortium. J Orthop Sports Phys Ther 43: 585-591.
- Docherty CL, Gansneder BM, Arnold BL, Hurwitz SR (2006) Development and reliability of the ankle instability instrument. J Athl Train 41: 154-158.
- Kim KM, Croy T, Hertel J, Saliba S (2010) Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: A systematic review. J Orthop Sports Phys Ther 40: 383-391.
- Hiller CE, Refshauge KM, Bundy AC, Herbert RD, Kilbreath SL (2006) The cumberland ankle instability tool: A report of validity and reliability testing. Arch Phys Med Rehabil 87: 1235-1241.
- Wright CJ, Arnold BL, Ross SE, Linens SW (2014) Recalibration and validation of the cumberland ankle instability tool cutoff score for individuals with chronic ankle instability. Arch Phys Med Rehabil 95: 1853-1859.
- Bellew JW, Michlovitz SL, Nolan Jr TP (2016) Michlovitz's modalities for therapeutic intervention, 6th edition. FA Davis.
- Bergquist A, Clair J, Lagerquist O, Mang C, Okuma Y, et al. (2011) Neuromuscular electrical stimulation: implications of the electrically evoked sensory volley. Eur J Appl Physiol 111: 2409-2426.
- Mettler A, Chinn L, Saliba SA, McKeon PO, Hertel J (2015) Balance training and center-of-pressure location in participants with chronic ankle instability. J Athl Train 50: 343-349.
- McKeon PO, Ingersoll CD, Kerrigan DC, Saliba E, Bennett BC, et al. (2008) Balance training improves function and postural control in those with chronic ankle instability. Med Sci Sports Exerc 40: 1810-1819.
- Sefton JM, Yarar C, Hicks-Little CA, Berry JW, Cordova ML (2011) Six weeks of balance training improves sensorimotor function in individuals with chronic ankle instability. J Orthop Sports Phys Ther 41: 81-89.
- Gribble PA, Tucker WS, White PA (2007) Time-of-day influences on static and dynamic postural control. J Athl Train 42: 35-41.
- Walker DL, Hickey CJ, Tregoning MB (2017) The effect of electrical stimulation versus sham cueing on scapular position during exercise in patients with scapular dyskinesis. Int J Sports Phys Ther 12: 425-436.
- Hopkins JT, Ingersoll CD (2000) Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehab 9: 135-159.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi