Expert Review, Dent Health Curr Res Vol: 7 Issue: 8
Treatment Of Oral Leukoplakia By Er:YAG Laser: Current Relevance To Dental Practice
Verica Pavlic1,2*, Smiljka Cicmil3, Mirjana Gojkov Vukelic4, Marwa Madi5, Milica Jeremic Knezevic6, Dragana Gabric7, Koji Mizutani8, Akira Aoki8 and Frank Schwarz9
1Department of Periodontology and Oral Medicine, Institute of Dentistry, Banja Luka, Bosnia and Herzegovina
2Department of Periodontology and Oral Medicine, Medical faculty University of Banja Luka, Bosnia and Herzegovina
3Department of Periodontology and Oral Medicine, Medical faculty Foca, University of East Sarajevo, Bosnia and Herzegovina
4Department of Periodontology and Oral Medicine, Dental faculty University of Sarajevo, Bosnia and Herzegovina
5Department of Preventive Dental Sciences, College of Dentistry, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
6Department of Prosthodontics, Medical faculty University of Novi Sad, Novi Sad, Serbia
7Department of Oral Surgery, School of Dental Medicine, University of Zagreb, Zagreb, Croatia
8Section of Periodontology, Department of Hard Tissue Engineering, Tokyo Medical and Dental University, Tokyo, Japan
9Department of Oral Surgery and Implantology, Carolinum, Goethe University, Frankfurt, Germany
- *Corresponding Author:
- Verica Pavlic
Department of Periodontology and Oral Medicine, Medical faculty University of Banja Luka, Republic of Srpska, Bosnia and Herzegovina,
Tel: +387 66/769-844
E-mail: verica.pavlic@med.unibl.org
Received Date: March 14, 2021; Accepted Date: August 18, 2021; Published Date: August 25, 2021
Citation: Pavlic V, Cicmil S, Vukelic MG, Madi M, Knezevic MJ, et al. (2021) Treatment of Oral Leukoplakia by Er:YAG Laser: Current Relevance to Dental Practice. Dent Health Curr Res 7:8. 158.
Copyright: © All articles published in Dental Health: Current Research are the property of SciTechnol, and is protected by copyright laws. “Copyright © 2021, SciTechnol, All Rights Reserved.
Abstract
Laser ablation/vaporization is considered to be a safe and effective alternative technique for treating oral leukoplakia (OL) lesions. Recently, more interest has been gained in using Er:YAG laser radiation for OL removal. The purpose of the current systemic review was to evaluate the effectiveness and safety of removing OL using Er:YAG laser. The main outcomes included were the improvement of wound healing and OL recurrence rate/malignant transformation following Er:YAG laser irradiation. The literature demonstrated that Er:YAG laser could be considered an efficient and reliable method for treating OL. Uncomplicated healing was observed, moreover, favourable healing with lower or no recurrence rate or malignant transformation was observed. However, the key factor preventing a final, solid conclusion for effective use of Er:YAG laser for OL removal was that the available literature are mainly comparative case reports. The heterogeneity of the used laser protocols (laser settings; different sizes, depths and localisations of the OL lesions) have hindered comparing the results and drawing a final conclusion. Therefore, further randomized control clinical trials with considerable power and longer follow-up periods are, however, recommended.
Keywords: oral leukoplakia, erbium laser, erbium laser therapy, dysplastic changes
Introduction
World Health Organization (WHO) in 2005 has defined oral leukoplakia (OL) term as “a white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer” [1]. OL cannot be scraped off or stripped off easily [1- 3]. The worldwide prevalence of OL is ranging from 0.5 to 3.4% [4]. The main aetiology of oral leukoplakia is still undetermined, however, many potential locally acting factors, have been considered, including: tobacco and alcohol use, chronic irritation, betel quid chewing habit, electro galvanic reactions, ultraviolet radiation, occlusal problems, candidiasis, syphilis and viral infection [2-5]. Almost all of OL patients were reported to be smokers, thus a close association was found between OL and excessive tobacco use [5]. OL is also frequently observed in patients undergoing immunosuppression treatment or with previous history of head and neck malignancy [5].
OL can be classified into two types: lesion at a single site and the other has multiple lesions [2]. Clinically, by macroscopic appearance, OL is classified depending on the texture, thickness, colour and regularity into homogenous (thin and thick) and non-homogeneous (erythroleukoplakia, verrucous, ulcerated) leukoplakia [2].
Homogenous plaques are mainly white, thin, and flat with consistent texture and corrugated surface [5]. Among nonhomogeneous OL, a very special entity is the proliferative verrucous OL [6-8], which is very specific and often a more aggressive form of OL, characterised by a tendency toward multifocality (field cancerization), resistance to treatment, high recurrence and increased malignant transformation rate [6-8].
Histologically leukoplakia includes epithelial hyperplasia, with/ without hyperkeratosis with minimum inflammation, and different epithelial dysplasia grades (abnormal growth of epithelium that can be only confirmed histopathologically). Dysplasia has been considered as the main indicator of malignant tendency, carrying a fivefold higher risk of malignant transformation than non-dysplastic oral leukoplakia [8].
Leukoplakia is usually asymptomatic, however, the appearance of white intraoral patch is the patients’ main concern [2]. It is considered a potentially malignant lesion, with elevated malignant transformation potential to squamous cell carcinoma [1-3,9]. The point prevalence is estimated to be 2.6%, with a reported rate of malignant transformation ranging from 0.13 to 34%, and a yearly malignant transformation rate of approximately 1% for all types of oral leukoplakia [1,10]. However, contemporary practice remains unable to predict lesion behaviour or quantify the risk of malignant transformation. Surely, malignant transformation is related to the clinical and histological classification (presence of epithelial dysplasia), the location of the lesion (lesions that are located on the borders of the tongue, floor of the mouth and soft palate) and factors associated with an increased risk of malignant transformation (increased age of patient, female gender, lesion size and depth, lesion duration, idiopathic lesions, surface changes of the lesion, presence of erythroplakia/speckled leukoplakia, presence of Candida albicans) [5]. Although no evidence exist that treatment of OL prevents its malignant transformation, treating OL is highly recommended at least to alleviate the patients concern [11].
To date, several different treatment approaches have been introduced, but since aetiopathology of OL cannot be established, treatment is usually symptomatic. The possible causative factors can be eliminated (e.g. smoothing sharp edges of the teeth or a restoration, smoking cessation, etc.) and a period up to four weeks seems to be a reasonable time to look for regression. If the OL lesion does not regress after elimination of causative factors, several non-surgical and surgical treatment options are available. Among non-surgical treatment options, topical or systemic pharmacologicals (e.g. carotenoids, retinoids, lycopene, vitamin A, C, K and E, bleomycin, non-steroidal anti-inflammatory drugs, and mouthwashes) and photodynamic therapy have been mentioned [12,13]. However, non-surgical treatments have their own limitations; for example retinoids, vitamin A and carotenoids might influence epithelial turnover, non-steroidal anti-inflammatory drugs might modulate specific prostaglandins possibly involved in carcinogenesis and chemotherapeutic agents can act on early neoplastic cells [14]. The rationale of surgical treatment is that it is indicated when the removal of OL altered tissue can prevent transformation of OL to oral cancer [14]. Surgical treatments include excision by conventional surgery (scalpel, electrocoagulation and cryosurgery) and the use of lasers [15]. Although conventional surgical treatments can provide an entire lesion for histopathological evaluation and can result in the resolution of leukoplakia in a certain number of patients, they have their own limitations that depends on the size and site of the lesions (unfavourable scarring and secondary functional alterations as surgical sequelae) [15]. Furthermore, even complete removal of the OL lesion by conventional surgery does not reduce the rate of either recurrence or malignant transformation at the same or another site [16,17]. The OL recurrence rates following scalpel excision were reported to vary from 10% to 34% [18].
Recently, different laser wavelengths have been recognised as an alternative advanced treatment modality in modern dentistry, namely due to the various intra- and postoperative benefits, such as extremely precise ablation and vaporisation, haemostasis (clearly visible working field), disinfection, decontamination, no need for sutures, minimal pain and swelling after surgery and, consequently, faster wound healing [19]. Apart from the documented benefits of lasers, patients seemed to prefer them over conventional surgical techniques. In the recent meta-analysis, the mean OL recurrence rate following lasers was reported to be 16.5% [18].
To date, various lasers have been used for OL removal, including CO2, semiconductor diode, Er:YAG, Er,Cr:YSGG, Nd:YAG and Tm:YAG lasers [10,19]. The use of Er:YAG lasers for OL removal has gained increasing importance in recent years, either for superficial ablation/vaporisation in cases of hyperkeratotic lesions, or complete ablation of the epithelium and adjacent tissues in dysplastic lesions [1-4]. Er:YAG lasers possess high absorption by water, minimising the thermal damage of the ablated tissue [19-22]. Since thermal damage at the edge of the incision is shallow, Er:YAG lasers cause fewer cytological artefacts at the borders of OL and are, therefore, the lasers of choice when it comes to oral excisional biopsies and histopathological evaluation [10,15].
Recently, some studies reported a lower recurrence rate and malignant transformation of OL lesions using different laser wavelengths [23-25]. Therefore, the aim of this study was to give a systematic overview of the effects of Er:YAG lasers on OL wound healing, recurrence rate and malignant transformation.
Materials and Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [26]. A systematic search of Medline/PubMed (National Library of Medicine, NCBI), Science Direct and Cochrane Library online databases was carried out from June 1st - July 30th 2020. When searching electronic databases, only English language studies published in peer reviewed reputed journal from 2000 to 2020 were obtained. Furthermore, the citations of all included articles were checked. In case of missing or insufficient data, corresponding authors were communicated. For Medline/PubMed (National Library of Medicine, NCBI) database search the following MeSH terms were used: ”oral leukoplakia”, “erbium yag laser”, “lasers, erbium doped yttrium aluminium garnet”, “erbium yag laser” AND “oral leukoplakia”, “laser, erbium yag” AND “oral leukoplakia” and “lasers, erbium doped yttrium aluminium garnet” AND “oral leukoplakia”. Other databases were searched using the following search words and terms: “Er:YAG laser”, “erbium yag laser”, “oral leukoplakia”, “erbium yag laser” AND “oral leukoplakia” and erbium doped yttrium aluminium garnet” AND “oral leukoplakia”.
Eligibility criteria
Article should have the following PICOS framework to be included: (1) Patients: papers reporting patients more 18 years old with histopathologically confirmed OL prior to any treatment; (2) Intervention: OL were excised or vaporized by Er:YAG laser; (3) Control: compared to other treatment or absence of control group; (4) Outcomes: primary outcome (OL wound healing following Er:YAG laser irradiation) and secondary outcomes (OL recurrence rate and malignant transformation following Er:YAG laser irradiation); (5) Study design: The retrospective/prospective observational studies, comparative studies and randomised controlled clinical studies were included. Studies excluded from this review were: reviews, editorials and abstracts, book chapters, letters to the editor, or conference abstracts; animal and experimental laboratory studies; duplicate studies; publications with not sufficient information regarding laser parameter settings, recurrence rates and/or malignant transformation; publications in language other than English.
The screening and study selection process according to the study inclusion/exclusion criteria were performed independently by two authors (V. P. and S. C.). The search words and terms were used to retrieve papers from databases. Duplicates were removed using software (Mendeley Desktop ©, Version 1.19.4, 2008–2019 Mendeley Ltd.). Articles were scanned upon title and abstract and those who did not fulfill the set criteria were excluded. Further, the selected articles were read in full (V. P. and S. C.) and those who did not fulfill the set criteria were excluded. Any disagreement regarding paper inclusion was settled by either discussion or by third author’s (M. GV.) decision (Figure 1). The collected data from the studies were: the first author’s name and year of publication, type of the study, Er:YAG laser parameters, number of OL patients treated with Er:YAG laser, control group information, follow-up period and findings related to wound healing (value of reduction in lesion area), recurrence rate (value of the presence of the new lesions) or malignant transformation following Er:YAG laser.
Risk of bias
After establishing the scores of quality assessment (Table 1), an overall estimation of risk of bias (low-met all of the criteria, moderate- met the criteria partly, and high-not met one or more criteria) was determined for each selected study (Table 2), according to the Cochrane Handbook for Systematic Reviews of Interventions. Two independent authors (V. P. and S. C.) carried out the quality assessment. Any disagreement regarding paper inclusion was settled by either discussion or by third author’s (M. GV.) decision.
| Category | Category description | Grading |
|---|---|---|
| A | Sample size calculation(number of the participants in order to achieve a statistically significant difference among compared groups) | 0꞊ no |
| 1꞊ possibly adequate | ||
| 2꞊ yes | ||
| B | Randomization and allocation concealment methods | 0꞊ no |
| 1꞊ possibly adequate | ||
| 2꞊ yes | ||
| C | Clear definition of inclusion/exclusion criteria | 0꞊ no |
| 1꞊ yes | ||
| D | Follow-up period completed | 0꞊ no |
| 1꞊ yes | ||
| E | Experimental and control group comparable at study baseline | 0꞊ no |
| 1꞊ unclear | ||
| 2꞊ yes | ||
| F | Presence of masking | 0꞊ no |
| 1꞊ unclear | ||
| 2꞊ yes | ||
| G | Appropriate statistical analysis | 0꞊ no |
| 1꞊ unclear | ||
| 2꞊ yes |
| Study(reference) | A(0-2) | B(0-2) | C(0-1) | D(0-1) | E(0-2) | F(0-2) | G(0-2) | Estimated risk of bias |
|---|---|---|---|---|---|---|---|---|
| Matulic N. et al.(10) | 2 | 2 | 1 | 1 | 2 | 0 | 2 | High |
| Monteiro L. et al.(27) | 2 | 2 | 1 | 1 | 2 | 0 | 2 | High |
| Arduino PG. et al.(28) | 2 | 2 | 1 | 1 | 2 | 0 | 2 | High |
| Gabric D. et al.(29) | 2 | 2 | 1 | 1 | 2 | 0 | 2 | High |
| Schwarz F. et al.(30) | 2 | 2 | 1 | 1 | 2 | 0 | 0 | Moderate |
Summary measures and synthesis of results
Wound healing of Er:YAG laser treated OL was our primary outcome. The secondary outcomes were OL recurrence rate and malignant transformation following Er:YAG laser irradiation. Metaanalysis could not be performed, due to the diversity of the included studies (variety of the Er:YAG parameters, study designs, findings and follow up periods).
Results
Study selection
Electronic databases search initially identified 10729 studies. Of these, 5342 duplicated articles were removed. After evaluating the titles and abstracts obtained, 5349 publications were excluded after authors’ agreement. The remaining 12 publications (related or possibly-related) were obtained in full-text format for more detailed analysis. According to required selection criteria 7 more studies were excluded, and finally this review comprised 5 studies (Figure 1).
Study characteristics
All selected studies were randomized controlled clinical studies [10, 27-30]. Selected studies compared the use of Er:YAG to Er,Cr:YSGG laser [10]; or Er:YAG to cold blade, Nd:YAG, CO2, QMR scalpel and scalpel [27]; or Er:YAG to scalpel [28] or Er:YAG laser to pharmaceutical (1% topical isotretinoin) [29]; or Er:YAG to CO2 laser [30]. 3 out of 5 studies performed careful randomisation of the patients according to the gender, size, depth and localisation of the OL lesion [27,28,30], one study based randomisation upon flipping a coin [10], and one study treated all patients whose re-measures revealed unsuccessful treatment with pharmaceuticals [29]. Regarding clinical examination, 4 studies reported precise data regarding the exact localization, measurement of the size (in millimetres) and shape of the OL lesion recorded [10,27-29] to access clinical change, and one study reported photography of the lesions for clinical evaluation [30].
Applying local anaesthesia was used in all studies using Er:YAG laser [10,27-30]. Four studies performed the recommended surgical protocol for ablation, having a 3 mm circumferential zone as a safety margin that also extends 3 mm below the clinically visible lesion [10,27,29,30]. Only one study did not perform the recommended surgical protocol for ablation [28].
Three studies implemented single laser irradiation [27,28,30], while 2 studies performed repeated laser irradiations according to the re-measurement of the OL lesion on the follow-up [10, 29]. The studies had different follow up protocols, but all of the selected studies recorded clinical response one week and six months following the irradiation [10,27,29,30]. Recurrence rate and malignant transformations were measured differently. Most of the studies reported OL recurrence rate and malignant transformations following Er:YAG laser only upon clinical observation (existence of residual lesions, incidence of recurrence mostly on the borders of the previous lesions and presence of the new lesions) [10,27-29]. One study reported assessment by positive response (including complete and partial response) and cytologic and DNA cytometric examinations [29]. Two studies reported assessment based on the visual analogue scale/VAS and Oral health impact profile/OHIP questionnaire [10, 29].
Risk of bias
Bias risk analysis of the contained 5 studies is summarized in Table 2. Four of the included studies have a low [10, 27-29], while one have a moderate risk of bias [30].
Discussion
Wound healing following Er:YAG laser irradiation
All authors agreed that Er:YAG laser ablation was precise, easy and fast using various delicate contact tips and specifically designed handpieces (Table 2). The Er:YAG laser is with specific wavelength that is highly absorbed by water. This dramatically decrease the thermal side effect on the treated tissues which accelerate wound healing as well as decreasing the post-operative discomfort, swelling, scarring and dysfunction [10,27-30]. Already minimal thermal alteration of the ablated tissue following Er:YAG laser irradiation was further minimalized by implementation of water irrigation during laser irradiation (Table 2). Er:YAG laser do not provide persistent heat to cause an immediate shrinkage of the blood vessels (especially vessels more than 0.5 mm in diameter), therefore it is not considered the laser of choice when perfect haemostasis is desired. However, all of the authors agreed that Er:YAG lasers demonstrated a spontaneous haemostasis achieved within several minutes postoperatively [10, 27- 30]. On the first week of follow up, the ablated surface demonstrated re-epithelialisation with a healthy appearance without swelling, infection or scarring. At week 4, the ablated site was completely re-epithelialized in the former defect area and was found to be the same as the normal untreated sites. This research group obtained and suggested similar results (Figures 2 and 3).
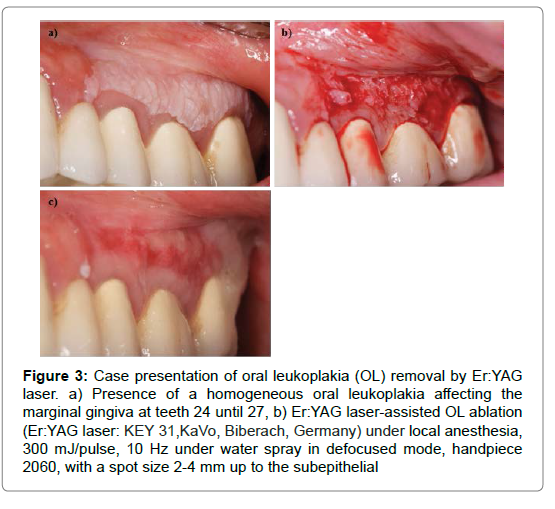
Figure 2: Case presentation of oral leukoplakia (OL) removal by Er:YAG laser. a) Presence of a homogeneous oral leukoplakia affecting the marginal gingiva at teeth 15 and 16. A cytologic diagnosis revealed the absence of any dysplasia, b)Er:YAG laser-assisted OL ablation (Er:YAG laser: KEY 31,KaVo, Biberach, Germany) under local anesthesia, 300 mJ/pulse, 10 Hz under water spray in defocused mode, handpiece 2060, with a spot size 2-4 mm. Tissue ablation was accomplished up to the subepithelial connective tissue zone, including a circumferential safety margin of about 3 mm. In the absence of any clinical signs of carbonization, a spontaneous hemostasis was achieved within 2 minutes, thus not necessitating any wound dressing, c) Situation at 4 weeks, showing a complete re-epithelialization of the former defect area, d) Situation at 12 weeks showing a complete remission of the former lesion. (Case details provided by Prof. Dr Frank Schwarz).
Figure 3: Case presentation of oral leukoplakia (OL) removal by Er:YAG laser. a) Presence of a homogeneous oral leukoplakia affecting the marginal gingiva at teeth 24 until 27, b) Er:YAG laser-assisted OL ablation (Er:YAG laser: KEY 31,KaVo, Biberach, Germany) under local anesthesia, 300 mJ/pulse, 10 Hz under water spray in defocused mode, handpiece 2060, with a spot size 2-4 mm up to the subepithelial
Er:YAG lasers can cause pain reduction by low tissue necrosis, since it is known that it have the least tissue penetration (1μm). Possible explanations for the decreased pain during Er:YAG laser irradiation could be the sealing of the ends of sensory nerves or protein coagulum formed on the wound surface that acts as a biological dressing [15]. Further, decreased pain, called “laser analgesia”, can be partly explained as a result of low-level laser therapy/LLLT [31]. Clinically observed analgesia was further confirmed by in vivo studies, wherein LLLT decreased the firing frequency of nociceptors [31].
As a result of this study, enhanced wound healing and less pain following Er:YAG laser irradiation when compared to scalpel [27, 28] and to CO2 lasers [30] was suggested. Er:YAG lasers demonstrated enhanced wound healing following ablation of OL lesions previously unsuccessfully treated with 1% topical isotretinoin [27]. However, when Matulic et al. [10] compared Er,Cr:YSGG to Er:YAG lasers, the results showed prolonged wound healing following Er:YAG laser irradiation. This finding was not in accordance with previous reports of lower thermal damage following Er:YAG when compared to Er,Cr:YSGG laser irradiation [22]. Matulic et al. suggested a possible explanation for this finding was most likely a result of the randomisation process by flipping a coin, where all the bigger and deeper OL lesions were in the Er:YAG laser experimental group [10].
Overall, Er:YAG laser was suggested as more pleasant, less painful treatment option with better aesthetic results [27-29]. Patients seemed to prefer it, especially when having larger lesions removed [29].
Recurrence rate and malignant transformation of OL lesions
OL recurrence rates vary, and this variety is strongly influenced by ethnic and smoking habits [32]. Since continuous smoking, alcohol consumption or chewing betel quid after surgical treatment leads to 19.8 or 9.7 times more recurrence, respectively, some of the authors advised the patients to quit using tobacco and alcohol before the beginning of treatment and during the follow-up [26,32]. In our study, some authors reported zero recurrence rate following Er:YAG laser after 1 year of follow up [10,29,30]. However, some authors reported recurrence rates of 15.2% [27], or even 40% [28]. Gabric et al. reported an even higher recurrence rate of 74.1% after the first Er:YAG laser application [29], but they worked with an Er:YAG laser with a digitally controlled handpiece, where the margins of the OL tissues are software-driven. They realised that when they removed OL lesions for the first time, ablation of the epithelial tissue was performed with little or no capillary bleeding from the connective tissue. Their study protocol consisted of repeated Er:YAG laser ablations if recurrence of the lesion was observed during follow up. They finally reported a zero recurrence rate following Er:YAG laser irradiation at six months and one-year follow up [29].
Gabric et al. reported that the recurrence rate was highly dependent on the localization of the OL lesion [29]. They realised that sublingual OL lesions were without recurrences upon Er:YAG laser ablation. This is probably due to the thinner mucosa and higher vascularisation of the sublingual region compared with other parts of oral cavity, due to the tongue acting as a mechanical barrier to the sublingual region and the protective role of saliva from the submandibular and sublingual glands [29]. Also, the authors suggested a higher OL recurrence rate in the buccal region, probably due to mechanical trauma and mastication [29]. Most probably in thicker mucosa, especially where the thickness of the OL lesion is not the same throughout whole lesion, there is a bigger chance that some OL fragments will remain non-ablated by the Er:YAG laser.
Arduino et al. explained the high recurrence rate (40%) by not following the standard surgical protocol of OL removal [28]. The standard surgical protocol includes a safety margin of clinically normal tissue of about 3 mm around each OL lesion and an extension in depth of 3 mm (when possible) below the clinically visible lesion, and this is recommended whenever possible [10,27,29,30]. The surgical protocol for ablation was mainly developed upon clinical studies that demonstrated that new OL patches mainly develop adjacent to the margins of the previously treated OL sites [33], suggesting that adjacent epithelia that consist of highly proliferating cells can be the origin of the recurrence, especially in the high-risk OL lesions [33-35]. In this context, by “field cancerization” of oral mucosal cancer we could possibly explain the presence of dysplastic cells adjacent to squamous cell carcinoma [30].
To date, there has been controversy over whether surgery can prevent the malignant transformation rate of OL, namely due to inconsistency in the data in the available literature [15]. Most of the authors agreed that malignant transformation of OL can be reduced by surgery, though it does not eliminate this risk completely [27]. Regarding malignant transformation of OL, the literature suggests that it is strongly associated with the presence of dysplasia in the biopsy (with a 31.4%–36.3% risk of malignancy for patients with any degree of dysplasia) [36,37]. Though malignant transformation is more likely to occur within dysplastic lesions, dysplasia is not mandatory. Also, studies suggest that malignant transformation does not exclusively arise from areas of previously diagnosed OL (the literature suggests that 41% of new cancers developed in sites distinct from prior OL sites). These findings indicate that OL is not considered a premalignant lesion only but rather a marker for an increased risk of cancer in the oral cavity [35-37]. Also, the risk of malignant development is considered to be approximately seven-fold higher for non-homogenous leukoplakia as compared to homogenous leukoplakia and a five-fold higher when the size of the lesion is bigger than 200 mm2. Moreover, recurrence of OL seems to be a prognostic indicator of oral malignant transformation [27]. Monteiro et al. reported malignant transformation in one patient (1.1%) after a period of 35 months [27]. To date, histological and biochemical studies suggested methods that can be used to identify which patients with leukoplakia will develop oral cancer, and which will not; however, a definitive evidence-based and clinically useful, commercially available predictor of malignant transformation for dysplastic and non-dysplastic leukoplakias is not available at the moment [14]. Therefore, availability and implementation of early tests/screening for differentiation of OL lesions should be mandatory [30].
Limitations
Although this systematic review included 6 studies, there are still several limitations to be underlined. A final conclusion could not be formulated concerning the optimal irradiation conditions for the Er:YAG lasers in treating OL due to the fact that the available studies were only comparative case reports. The studies offered different laser protocols (different power, distance and duration of laser irradiation), different sizes, depths and localizations of the OL, thus matching of the results and drawing final conclusion were even more difficult. That is the reason why further studies, especially randomised control clinical trials, with more sample size and longer follow-up periods are highly recommended to give a solid based guidance on the use of Er:YAG lasers for oral leukoplakia management (Table 3).
| Author and the year of the study | Type of the study | Er:YAG laser group information | Control group information | Nr. Of lesions treated/ Er:YAG vs control groups(s) | Follow-up time | Findings: 1. Wound healing 2.Recurrence rate/malignant transformation |
|---|---|---|---|---|---|---|
| Schwarz et al. 2005 | Randomized controlled clinical trial (Er:YAG vs CO2) | 300 mJ/pulse, 10 Hz, defocused mode, 2-4 mm spot size, handpiece 2060, water spray | CO2 laser: 4-6 W, 20-50 Hz, focused mode | 8 vs 8 | 24-96 weeks (at 12 weeks intervals postoperatively) | 1. Improved wound healing 2. No recurrence and no signs of malignancy or dysplasia during follow-up time |
| Monteiro et al. 2017 | Randomized controlled clinical trial (Er:YAG vs cold blade vs Nd YAG vs CO2 vs QMR scalpel) | 250mJ/pulse, 25 Hz, 1250 W/cm2, 50J/cm2, very short pulse | cold blade: Bard-Parker scalpel blade number 15 with a number 3 handle; Nd: YAG laser: 1064nm, 320-um fiber, 3.5 W and 70 Hz, CO2 laser: 10600nm, 2-mm spot, 5W (159.2 W/cm2, 159.2 J/cm2); QMR scalpel: used with the thin straight electrode (diameter: 0.15-mm) | 33 vs 17 vs 14 vs 15 vs 8 | 1-151 months (at 1, 4 weeks, every 6 months postoperatively) | 2. Recurrence rate of 15.2%. Recurrence rate is significantly lower for the lesions treated with Er: YAG laser when compared to traditional scalpel |
| Ard uino et al. 2018 | Randomized controlled clinical prospective studty (Er:YAG vs scalpel) | 150mJ/pulse, 10 Hz, 0.9 mm spot size, R O2 -C handpiece, water spray | scalpel: not performed the recommended surgical protocol for a blation | 59 vs 58 | 24- 108 months (every 6 months postoperatively) | 1. The wound healing without the significance difference among two proposed treatments 2. The same recurrence rate of 40% among two proposed treatments |
| Matulic et al. 2019 | Randomized controlled clinical trial (Er:YAG vs Er,Cr:YS GG) | 120 mJ/pulse, 20 Hz, quantum squared pulse mode, non-contact digitally controlled handpiece (circular, rectangular or hexagonal shape), 15 mm distance, water spray | Er, Cr:YSGG laser: power of 2.5 W, frequency 50 Hz, fluence 31.25 J/cm2, and air:water concentration ratio of 25%:60% | 27 vs 27 | up to 1 year (at 1, 2, 4 weeks, 6, 12 months postoperatively) | 1. Prolonged wound healing following Er, YAG laser irradiation 2. No recurrence during follow-up time |
| Gabric et al. 2020 | Randomized controlled clinical trial (Er:YAG laser vs 1% tropical isotretinoin) | 120 mJ/pulse, 20 Hz, quantum squared pulse mode, non-contact digitally controlled handpiece (circular, rectangular or hexagonal shape), 15 mm distance, water spray | 1% topical isotretinoin in orabase, mixed in the same amounts applied three times a day during the period of 1 year | 28 vs 28 | up to 1 year (at 1, 2, 4 weeks, 6, 12 months postoperatively) | 1. Improved wound healing 2. No recurrence during follow-up time |
Conclusion
The present review has showed that Er:YAG laser can be reliable and effective in treating OL lesions. Its application resulted in a more favourable wound healing without thermal side effects and/ or complications and, more importantly with lower recurrence rates and malignant transformation of OL. However, strong attention should be given to the necessity for continued clinical observation and monitoring of the patients on a regular basis, regardless of the outcome following Er:YAG laser intervention.
References
- Munde A, Karle R. (2016) Proliferative verrucous leukoplakia: An update. J Cancer Res Ther 12:469.
- Saibene AM, Rosso C, Castellarin P, Vultaggio F, Pipolo C, et al. (2019) Managing benign and malignant oral lesions with carbon dioxide laser: indications, techniques, and outcomes for outpatient surgery. Surg J (N Y) 5:69-75.
- Ribeiro AS, de Aguiar MC, do Carmo MA, de Abreu MH, Silva TA, et al. (2011) 660 AsGaAl laser to alleviate pain caused by cryosurgical treatment of oral leukoplakia: a preliminary study. Photomed Laser Surg 29:345-350.
- Kumar A, Cascarini L, McCaul JA, Kerawala CJ, Coombes D, et al. (2013) How should we manage oral leukoplakia?. Br J Oral Maxillofac Surg 51:377-383.
- Staines K, Rogers H. (2017) Oral leukoplakia and proliferative verrucous leukoplakia: a review for dental practitioners. Br Dent J 223:655-661.
- Kundoor VK, Patimeedi A, Roohi S, Maloth KN, Kesidi S, et al. (2015) Efficacy of diode laser for the management of potentially malignant disorders. J Lasers Med Sci 6:120.
- Kharadi UA, Onkar S, Birangane R, Chaudhari S, Kulkarni A, et al. (2016)Treatment of oral leukoplakia with diode laser: a pilot study on Indian subjects. Asian Pac J Cancer Prev 16:8383-8386.
- Giri D, Agarwal N, Sinha A, Srivastava S, Mishra A. (2016) Diode laser: In treatment of recurrent verrucous leukoplakia. Contemp Clin Dent 7:250.
- Thomson PJ, Goodson ML, Cocks K, Turner JE. (2017) Interventional laser surgery for oral potentially malignant disorders: a longitudinal patient cohort study. Int J Oral Maxillofac Surg 46:337-342.
- Matulić N, Bago I, Sušić M, Gjorgievska E, Kotarac Knežević A, et al. (2019) Comparison of Er: YAG and Er, Cr: YSGG laser in the treatment of oral leukoplakia lesions refractory to the local retinoid therapy. Photobiomodul Photomed Laser Surg 37:362-368.
- Nammour S, Zeinoun T, Namour A, Vanheusden A, Vescovi P. (2017) Evaluation of different laser-supported surgical protocols for the treatment of oral leukoplakia: a long-term follow-up. Photomed Laser Surg 35:629-638.
- Ribeiro AS, Salles PR, da Silva TA, Mesquita RA. (2010) A review of the nonsurgical treatment of oral leukoplakia. Int J Dent.
- Konopka K, Goslinski T. (2007) Photodynamic therapy in dentistry. J Dent Res 86:694-707.
- Lodi G, Franchini R, Warnakulasuriya S, Varoni EM, Sardella A, et al. (2016) Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev.
- Seoane J, González-Mosquera A, López-Niño J, García-Caballero L, Aliste C, et al. (2013) Er,Cr:YSGG laser therapy for oral leukoplakia minimizes thermal artifacts on surgical margins: a pilot study. Lasers Med Sci 28:1591-1597.
- Mogedas-Vegara A, Hueto-Madrid JA, Chimenos-Küstner E, Bescós-Atín C. (2016) Oral leukoplakia treatment with the carbon dioxide laser: a systematic review of the literature. J Craniomaxillofac Surg 44:331-336.
- Montebugnoli L, Frini F, Gissi DB, Gabusi A, Cervellati F, et al. (2012) Histological and immunohistochemical evaluation of new epithelium after removal of oral leukoplakia with Nd: YAG laser treatment. Lasers Med Sci 27:205-210.
- de Pauli Paglioni M, Migliorati CA, Faustino IS, Mariz BA, Roza AL, et al. (2020) Laser excision of oral leukoplakia: does it affect recurrence and malignant transformation? A systematic review and meta-analysis. Oral Oncol 109:104850.
- Mizutani K, Aoki A, Coluzzi D, Yukna R, Wang CY, et al. (2016) Lasers in minimally invasive periodontal and peri‐implant therapy. Periodontol 2000 71:185-212.
- Novakovic D, Rickert S, Blitzer A. (2011) Office-based laser treatment of oral premalignant lesions. Operative Tech Otoryno-Head Neck Surg 22:159-164.
- Sawabe M, Aoki A, Komaki M, Iwasaki K, Ogita M, et al. (2015) Gingival tissue healing following Er: YAG laser ablation compared to electrosurgery in rats. Lasers Med Sci 30:875-883.
- Kawamura R, Mizutani K, Lin T, Kakizaki S, Mimata A, et al. (2020) Ex vivo evaluation of gingival ablation with various laser systems and electroscalpel. Photobiomodul Photomed Laser Surg 38:364-373.
- Tewari M, Rai P, Singh GB, Kumar M, Shukla HS. (2007) Long term follow up results of Nd: YAG laser treatment of premalignant and malignant (Stage I) squamous cell carcinoma of the oral cavity. J surg oncol 95:281-285.
- Chandu A, Smith AC. (2005) The use of CO2 laser in the treatment of oral white patches: outcomes and factors affecting recurrence. Int J Oral and Maxillofacial Surg. 34:396-400.
- Jerjes W, Upile T, Hamdoon Z, Al-Khawalde M, Morcos M, et al. (2012) CO2 laser of oral dysplasia: clinicopathological features of recurrence and malignant transformation. Lasers Med Sci 27:169-179.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:1000097-1000106.
- Monteiro L, Barbieri C, Warnakulasuriya S, Martins M, Salazar F, et al. (2017) Type of surgical treatment and recurrence of oral leukoplakia: a retrospective clinical study. Med Oral Patol Oral Cir Bucal 22:520.
- Arduino PG, Cafaro A, Cabras M, Gambino A, Broccoletti R. (2018) Treatment outcome of oral leukoplakia with Er: YAG laser: a 5-year follow-up prospective comparative study. Photomed Laser Surg 36:631-633.
- Gabrić D, Brailo V, Ivek A, Krpan K, Matulić N, et al. (2019) Evaluation of innovative digitally controlled Er: YAG laser in the treatment of leukoplakia-trial research. Acta Clin Croat 58: 615-620.
- Schwarz F, Maraki D, Yalcinkaya S, Bieling K, Böcking A, et al. (2005) Cytologic and DNA‐cytometric follow up of oral leukoplakia after CO2 and Er: YAGlaser assisted ablation: a pilot study. Lasers Surg Med 37:29-36.
- Zeredo JL, Sasaki KM, Yozgatian JH, Okada Y, Toda K. (2005) Comparison of jaw-opening reflexes evoked by Er: YAG laser versus scalpel incisions in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:31-35.
- Ishii J, Fujita K, Munemoto S, Komori T. (2004) Management of oral leukoplakia by laser surgery: relation between recurrence and malignant transformation and clinicopathological features. J Clin Laser Med Surg 22:27-33.
- Meister J, Franzen R, Eyrich G, Bongartz J, Gutknecht N, et al. (2010) First clinical application of a liquid-core light guide connected to an Er: YAG laser for oral treatment of leukoplakia. Lasers Med Sci 25:669-673.
- Yang SW, Tsai CN, Lee YS, Chen TA. (2011) Treatment outcome of dysplastic oral leukoplakia with carbon dioxide laser-emphasis on the factors affecting recurrence. J Oral Maxillofac Surg 69:78-87.
- Zhang L, Poh CF, Lam WL, Epstein JB, Cheng X, et al. (2001) Impact of localized treatment in reducing risk of progression of low-grade oral dysplasia: molecular evidence of incomplete resection. Oral oncol 37:505-512.
- Zaffe D, Vitale MC, Martignone A, Scarpelli F, Botticelli AR. (2004) Morphological, histochemical, and immunocytochemical study of CO2 and Er: YAG laser effect on oral soft tissues. Photomed Laser Surg 22:185-189.
- Bewley AF, Farwell DG. (2017) Oral leukoplakia and oral cavity squamous cell carcinoma. Clin Dermatol 35:461-467.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi