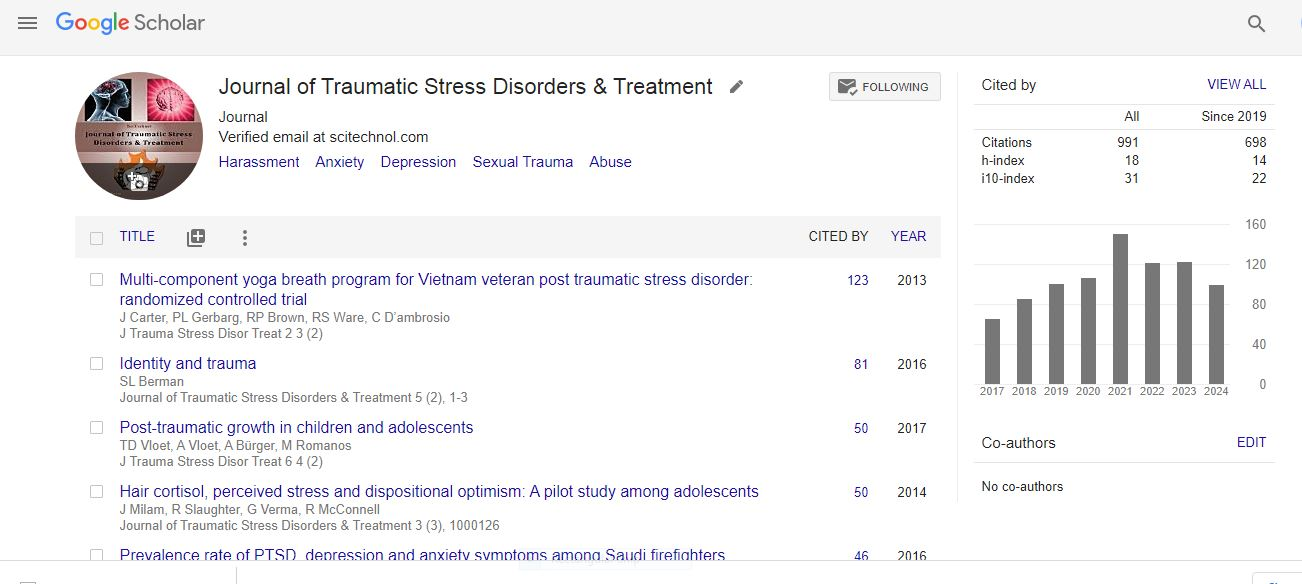
Briefreport, Jtsdt Vol: 13 Issue: 2
The Role of Neuroplasticity in Neurorehabilitation after Stroke.
Antonio Pinto*
Department of Cognitive Neuroscience, Harvard University, United States
*Corresponding Author: Antonio Pinto
Department of Cognitive Neuroscience, Harvard University, United States
E-mail: antoniop@harvard.edu
Received: 10-Apr-2024, Manuscript No. JTSDT-24-131953;
Editor
assigned: 11-Apr-2024, PreQC No. JTSDT-24-131953 (PQ);
Reviewed: 23-Apr-2024, QC No. JTSDT-24-131953;
Revised: 28-Apr-2024, Manuscript No. JTSDT-24-131953 (R);
Published: 30-Apr-2024, DOI:10.4172/2324-8947.100388
Citation: Pinto A (2024) The Role of Neuroplasticity in Neurorehabilitation after Stroke. J Trauma Stress Disor Treat 13(2): 388.
Copyright: © 2024 Pinto A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited
Introduction
Stroke is a leading cause of long-term disability worldwide, often resulting in motor, sensory, and cognitive impairments. While the initial damage caused by a stroke is often irreversible, the brain has a remarkable ability to reorganize and adapt in response to injury—a process known as neuroplasticity. Neurorehabilitation strategies leverage the principles of neuroplasticity to promote recovery and improve functional outcomes for stroke survivors. In this article, we explore the role of neuroplasticity in Neurorehabilitation after stroke, examining the underlying mechanisms, evidence-based interventions, and future directions in stroke rehabilitation [1].
Neuroplasticity refers to the brain's ability to reorganize its structure and function in response to changes in the environment, learning, and injury. Following a stroke, neuroplasticity plays a central role in the brain's recovery process. This dynamic process involves structural and functional changes at the cellular and network levels, including synaptic remodelling, axonal sprouting, and changes in cortical representation [2].
Synaptic Plasticity: Synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD), allows for the strengthening or weakening of synaptic connections between neurons. Following a stroke, synaptic plasticity contributes to the reorganization of neural circuits and the formation of new connections to compensate for lost function [3].
Cortical Reorganization: Stroke can lead to changes in cortical representation, with adjacent areas of the brain taking over functions previously performed by the damaged tissue—a phenomenon known as cortical reorganization or remapping. This adaptive process enables the brain to maintain or regain function despite injury [4].
Axonal Sprouting: Axonal sprouting involves the growth of new neural connections, allowing neurons to form alternative pathways to bypass areas of damage. Axonal sprouting plays a crucial role in functional recovery after stroke by facilitating the rewiring of neural circuits and the restoration of motor and sensory function [5].
Constraint-Induced Movement Therapy (CIMT): CIMT involves constraining the unaffected limb while engaging in intensive, task-specific training with the affected limb. By promoting repetitive and intensive practice of motor tasks, CIMT harnesses neuroplasticity to enhance motor function and facilitate recovery after stroke [6].
Task-Specific Training: Task-specific training focuses on practicing functional tasks relevant to the individual's goals and daily activities. By targeting specific motor skills and movements, task-specific training promotes neoplastic changes in the brain that support motor learning and recovery [7].
Virtual Reality Rehabilitation: Virtual reality (VR) rehabilitation utilizes immersive virtual environments to provide engaging and interactive rehabilitation experiences. VR-based interventions can enhance motor learning, sensory retraining, and cognitive rehabilitation through repetitive practice and feedback, capitalizing on neuroplasticity to drive recovery [8].
Non-Invasive Brain Stimulation: Non-invasive brain stimulation techniques, such as trans cranial magnetic stimulation (TMS) and trans cranial direct current stimulation (tDCS), modulate cortical excitability and promote neuroplasticity in stroke survivors. These techniques can be used to enhance the effects of rehabilitation interventions and facilitate recovery of motor function [9].
Personalized Rehabilitation Approaches: Advances in neuroimaging and biomarker research may enable the development of personalized rehabilitation approaches tailored to the individual's unique neuroanatomical and neurophysiological profile. Personalized interventions could optimize treatment outcomes by targeting specific neural circuits and mechanisms underlying recovery [10].
Conclusion
Neuroplasticity is a fundamental mechanism underlying recovery after stroke, offering hope for meaningful rehabilitation and improved quality of life for stroke survivors. By harnessing the brain's ability to adapt and reorganize, Neurorehabilitation interventions aim to facilitate recovery of motor, sensory, and cognitive function following stroke. As our understanding of neuroplasticity continues to evolve, so too will our approaches to stroke rehabilitation, with the ultimate goal of optimizing outcomes and maximizing the potential for recovery in individuals affected by stroke.
References
- Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol. 2012;69(2):161-7.
- Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol. 2013;26(6):609-16.
- Burnham ME, Esnault S, Roti Roti EC. Cholesterol selectively regulates IL-5 induced mitogen activated protein kinase signaling in human eosinophils. PLoS One. 2014;9(8):e103122.
- Cho H, Seo SW, Choi JY, et al. Predominant subcortical accumulation of 18F-flortaucipir binding in behavioral variant frontotemporal dementia. Neurobiol Aging. 2018;66:112-21.
- Cheng R, Smith SR, Kalpakjian CZ. Comorbidity has no impact on unplanned discharge or functional gains in persons with dysvascular amputation. J Rehabil Med. 2019;51(5).
- Khan F, Amatya B, Galea MP. Neurorehabilitation: applied neuroplasticity. J Neurol. 2017;264:603-15.
- Maier M, Ballester BR, Verschure PF. Principles of neurorehabilitation after stroke based on motor learning and brain plasticity mechanisms. Front Syst Neurosci. 2019;13:74.
- Albert SJ, Kesselring J. Neurorehabilitation of stroke. J Neurol. 2012;259(5):817-32.
- Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597-608.
- Alia C, Spalletti C, Lai S, et al. Neuroplastic changes following brain ischemia and their contribution to stroke recovery: novel approaches in neurorehabilitation. Front Cell Neurosci. 2017;11:76.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi