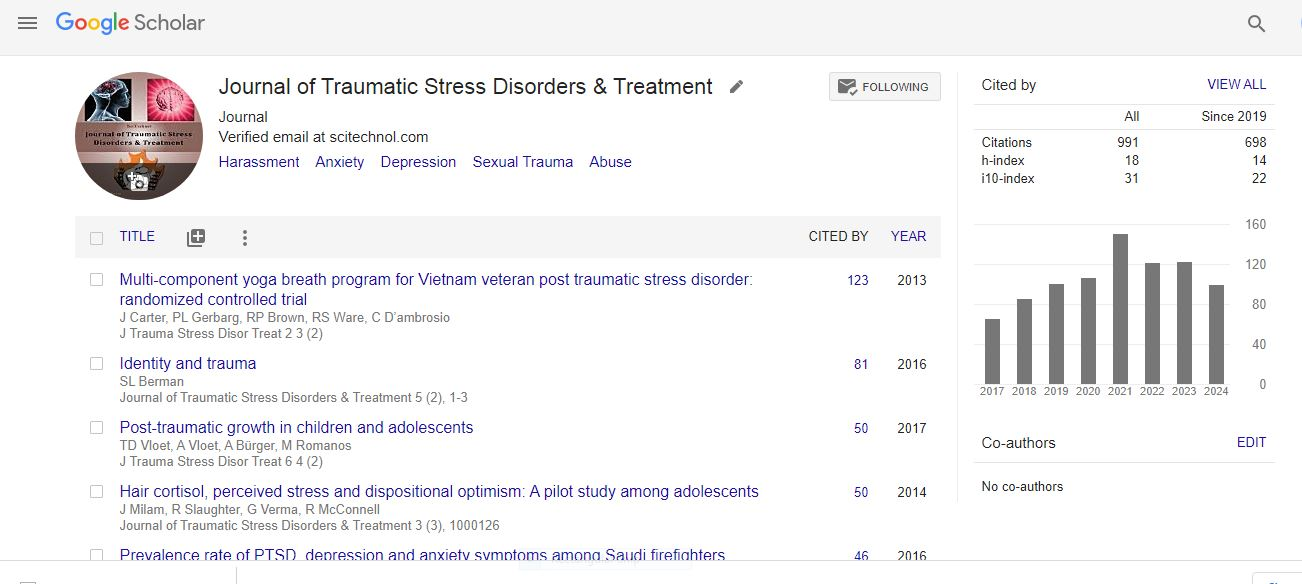
Editorial, Jtsdt Vol: 14 Issue: 1
The Role of Neuroinflammation in the Development of Nerve-Related Psychological Disorders
Oliver Davis*
Department of Social Psychology, Westbrook College of Social Sciences, United States
*Corresponding Author: Oliver Davis
Department of Social Psychology, Westbrook College of Social Sciences, United States
E-mail: oliver.davis@westbrook.edu
Received: 10-Feb-2025, Manuscript No. JTSDT-25-161131;
Editor assigned: 11-Feb-2025, PreQC No. JTSDT-25-161131 (PQ);
Reviewed: 23-Feb-2025, QC No. JTSDT-25-161131;
Revised: 25-Feb-2025, Manuscript No. JTSDT-25-161131 (R);
Published: 28-Feb-2025, DOI:10.4172/2324-8947.100440
Citation: Davis O (2025) The Role of Neuroinflammation in the Development of Nerve-Related Psychological Disorders. J Trauma Stress Disor Treat 14(1):440
Copyright: © 2025 Davis O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Introduction
Neuroinflammation, a response of the central nervous system (CNS) to injury or disease, has emerged as a key player in the development of various nerve-related psychological disorders. Once thought to be solely protective, chronic or uncontrolled neuroinflammation is now understood to have profound effects on the brain's structure and function, contributing to the pathogenesis of conditions such as depression, anxiety, schizophrenia, and neurodegenerative diseases like Alzheimer’s and Parkinson’s [1].
This article explores the mechanisms by which neuroinflammation affects brain health and contributes to the onset and progression of nerve-related psychological disorders. It also examines the potential for targeting neuroinflammatory pathways in the development of new treatments for these disorders. Neuroinflammation refers to the inflammatory response within the brain and spinal cord, primarily mediated by glial cells, particularly microglia and astrocytes. Microglia, the brain’s resident immune cells, become activated in response to various stimuli, including infections, trauma, or neurodegenerative processes [2].
Under normal circumstances, neuroinflammation is a protective mechanism that helps to clear pathogens, repair tissue, and restore CNS function. However, in cases of chronic or excessive activation, neuroinflammation can become harmful, leading to neuronal damage, disrupted synaptic connections, and altered neurotransmission. Growing evidence suggests that neuroinflammation plays a critical role in the development and maintenance of Major Depressive Disorder (MDD). Patients with depression often exhibit elevated levels of pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP), which are markers of systemic inflammation [3].
Increased neuroinflammation in depression is associated with alterations in key brain regions, including the prefrontal cortex, hippocampus, and amygdala, which are involved in mood regulation and cognitive processes. Chronic inflammation in these areas can lead to reduced neurogenesis, neuronal damage, and impaired synaptic plasticity, all of which contribute to the symptoms of depression. Neuroinflammation has also been implicated in anxiety disorders, such as Generalized Anxiety Disorder (GAD) and Post-Traumatic Stress Disorder (PTSD). Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, a key stress response system, is common in these disorders, and inflammation is known to modulate HPA axis function [4].
Chronic inflammation affects the amygdala, a brain region critical for fear processing and emotional regulation, which may lead to heightened anxiety and stress responses. Inflammatory processes can also impact the hippocampus, resulting in impaired memory consolidation, a hallmark of PTSD. Schizophrenia is a complex neuropsychiatric disorder characterized by symptoms such as hallucinations, delusions, cognitive impairments, and emotional disturbances. Neuroinflammation is thought to contribute to the pathophysiology of schizophrenia by disrupting synaptic transmission, altering neurodevelopmental processes, and promoting neuronal damage [5].
Post-mortem studies of individuals with schizophrenia have revealed increased microglial activation and elevated levels of inflammatory cytokines in the brain. These changes are particularly pronounced in the prefrontal cortex, a region involved in executive function, and the hippocampus, which plays a key role in memory and learning. Moreover, neuroinflammatory processes during early brain development are believed to contribute to the onset of schizophrenia in adulthood. Prenatal infections and maternal immune activation have been linked to an increased risk of schizophrenia, suggesting that neuroinflammation during critical developmental periods can have long-lasting effects on brain function [6].
Neuroinflammation is a well-established contributor to neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease, both of which have significant psychological components. Depression, anxiety, and cognitive decline are common in individuals with these conditions, and neuroinflammation is thought to play a role in these symptoms. In Alzheimer’s disease, microglial activation and chronic inflammation contribute to the accumulation of beta-amyloid plaques and tau tangles, hallmarks of the disease. This inflammatory response exacerbates neuronal damage and synaptic dysfunction, leading to cognitive decline and behavioral changes [7].
Similarly, in Parkinson’s disease, neuroinflammation contributes to the degeneration of dopaminergic neurons in the substantia nigra, a key brain region involved in movement and emotional regulation. The resulting loss of dopamine is associated with both motor symptoms and psychological disturbances, including depression and anxiety: Disruption of Neurotransmitter Systems: Neuroinflammation can alter the synthesis, release, and reuptake of neurotransmitters such as serotonin, dopamine, and glutamate. For example, inflammatory cytokines can reduce the availability of serotonin, a neurotransmitter involved in mood regulation, by increasing the activity of indoleamine 2,3-dioxygenase (IDO), an enzyme that degrades tryptophan, the precursor to serotonin [8].
Neuronal Damage and Synaptic Dysfunction: Chronic neuroinflammation can lead to the release of excitotoxic substances, such as glutamate, which cause neuronal damage and death. Inflammation can also disrupt synaptic plasticity, the brain’s ability to strengthen or weaken synapses in response to learning and experience, leading to cognitive deficits and mood disturbances. Blood-Brain Barrier (BBB) Dysfunction: Neuroinflammation can compromise the integrity of the blood-brain barrier, a protective barrier that regulates the movement of molecules between the bloodstream and the brain. BBB dysfunction allows peripheral immune cells and inflammatory mediators to enter the brain, further exacerbating neuroinflammation and contributing to psychological symptoms [9].
Neurogenesis Impairment: Inflammation can impair neurogenesis, the process by which new neurons are generated, particularly in the hippocampus. Reduced neurogenesis is associated with cognitive deficits and mood disorders, such as depression and anxiety. Anti-Inflammatory Medications: Nonsteroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents have shown potential in reducing depressive and anxiety symptoms in some patients. However, the long-term efficacy and safety of these treatments require further investigation [10].
Conclusion
Neuroinflammation plays a central role in the development of nerve-related psychological disorders, including depression, anxiety, schizophrenia, and neurodegenerative diseases. The chronic activation of microglia and astrocytes, coupled with the release of pro-inflammatory cytokines, can disrupt neurotransmitter systems, damage neurons, and impair synaptic function, all of which contribute to the onset and progression of psychological symptoms.
References
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immun. 2016;16(1):22-34.
- Dantzer R, O'connor JC, Freund GG. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46-56.
- Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neurosci. 2013;246:199-229.
- Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology?. Acta Neuropsych. 2018;30(1):1-6.
- Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17(8):497-511.
- Fernández Ó, Sörensen PS, Comi G, Vermersch P, Hartung HP. Managing multiple sclerosis in individuals aged 55 and above: a comprehensive review. Front Immunol. 2024;15:1379538.
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection. Lancet Neurol. 2009;8(4):382-97.
- Nobis A, Zalewski D, Waszkiewicz N. Peripheral markers of depression. J Clin Med 2020;9(12):3793.
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226-38.
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immun. 2011;11(9):625-32.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi