Research Article, Int J Glob Health Vol: 6 Issue: 4
The Global Lack of Surgical Trials and Strategies to Overcome Barriers: A Systematic Review and Narrative Meta-Synthesis
William S Bolton1*, Noel Aruparayil1, Bonnie Cundill2, D Julian A Scott1, Julia M Brown2, David Jayne1
1Departments of Surgery, Leeds Institute of Medical Research at St James’s, University of Leeds, Leeds, United Kingdom
2Department of Epidemiology and Population Health, Leeds Institute for Clinical Trials Research, Level 10 Worsley Building, Clarendon Way, University of Leeds, Leeds, United Kingdom
*Corresponding Author: William S Bolton,
Departments of Surgery, NIHR
Academic Clinical Fellow Room 7.19, Clinical Sciences Building, Leeds Institute of
Medical Research, St James’s University Hospital, LS9 7FT, United Kingdom
E-mail: w.s.bolton@leeds.ac.uk
Received date: 31 August, 2023, Manuscript No. IJGH-23-111827;
Editor assigned date: 04 September, 2023, PreQC No IJGH-23-111827 (PQ);
Reviewed date: 19 September, 2023, QC No. IJGH-23-111827;
Revised date: 27 September, 2023, Manuscript No. IJGH-23-111827 (R);
Published date: 05 October, 2023, DOI: 10.4172/Ijgh.1000188.
Citation: Bolton WS, Aruparayil N, Cundill B, D Julian A Scott DJA, Brown JM, et al. (2023) The Global Lack of Surgical Trials and Strategies to Overcome Barriers: A Systematic Review and Narrative Meta-Synthesis. Int J Glob Health 6:3.
Abstract
Background: Clinical trials are the accepted gold standard method to generate clinical effectiveness data. In global surgery, there is a significant lack of surgical trials in Low and Middle-Income Countries (LMICs) due to unique barriers in trial delivery. Understanding these barriers and exploring facilitators will increase the quantity and quality of LMIC surgical trials.
Materials and Methods: To determine the number of registered surgical trials globally, the World Health Organisation (WHO) International Clinical Trials Registry Platform (ICTRP) was searched by country and by all trials registered. To identify barriers and facilitators to surgical trial delivery in LMICs, a systematic search of MEDLINE (via OvidSP) and EMBASE (via OvidSP) was undertaken. A narrative meta-synthesis was conducted using inductively generated themes via thematic analysis.
Results: Only 3.4% of all registered clinical trials involved a surgical intervention. Globally, only 32% of all registered trials recruit patients from an LMIC. There were four main barrier themes: i) Lack of human resource capacity; ii) Lack of equipment, technology and resources; iii) Culture and contexts; iv) Methodology, design and implementation. There were three main facilitating strategies to overcome the barrier themes: i) Collaboration; ii) Flexible and efficient trial designs; iii) Funding and research culture.
Conclusions: The results of this study are widely relevant and provide facilitating strategies to address challenges in undertaking LMIC surgical trials. Given the significant barriers, it is important to explore flexible trial designs, with in-built trial training and qualitative methodologies.
Keywords: LMIC: Low and Middle-Income Countries; Surgery;
Trials
Introduction
Clinical trials, particularly Randomised Controlled Trials (RCTs), are the accepted gold standard to generate clinical effectiveness data. Surgical trials often involve complex interventions, presenting additional challenges for researchers including standardising the intervention across surgeons and settings, learning curve effects, and access to necessary technology [1-5]. Because of these challenges, there has historically been a lack of surgical trials globally with less than 1% of patients enrolled into surgical trials [6]. This phenomenon is ever more pronounced in Low And Middle-Income Countries (LMICs) [6,7]. Participation in clinical trials research enhances the overall quality of patient care and involving surgeons in clinical trials is one means of driving up the quality of services globally [8].
To ensure surgical innovation and technologies are implemented effectively, appropriate scientific evaluation is required. This process must be practical, feasible and affordable [9,10]. Once preliminary studies have been conducted to ensure the safety and feasibility of the intervention, a powered RCT is usually performed to evaluate clinical effectiveness. This data is often required to persuade policy makers and other surgeons to adopt an intervention [9]. A recent systematic review on the barriers to clinical trial delivery in LMICs did not include any papers on surgical trials [11].
The aim of this study was to investigate the number of surgical trials registered globally and to conduct a systematic review and narrative meta-synthesis of articles reporting on barriers or facilitators to LMIC surgical trial delivery. By understanding the barriers to LMIC surgical trial delivery and identifying facilitating strategies, this study aims to improve the quantity and quality of surgical trial research.
Materials and Methods
Study design
The study was conducted according to the principles of Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) and was registered prospectively on the PROSPERO database of systematic reviews (Registration number: CRD42019135349) [12].
Search strategy
Two independent investigators (WB, NA) performed a systematic search of MEDLINE (via OvidSP), EMBASE (via OvidSP), and all clinical trial registries listed on Primary Registries in the World Health Organisation (WHO) Registry Network via the WHO International Clinical Trials Registry Platform (ICTRP) (https://www.who.int/ictrp/ network/primary/en/). The ICTRP was used to access all primary registries meeting the WHO International Standards for Clinical Trial Registries. The search strategy for the Platform employed the ‘intervention’ menu of the advanced search function using the topic ‘surgery’ and system self-generated synonyms. The database provided the recruiting countries, and countries were categorised manually into LMICs or High-Income Countries (HICs) according to the World Bank Development Assistance Committee (DAC) classification. All trials registered as open to recruitment, in follow-up or completed were included.
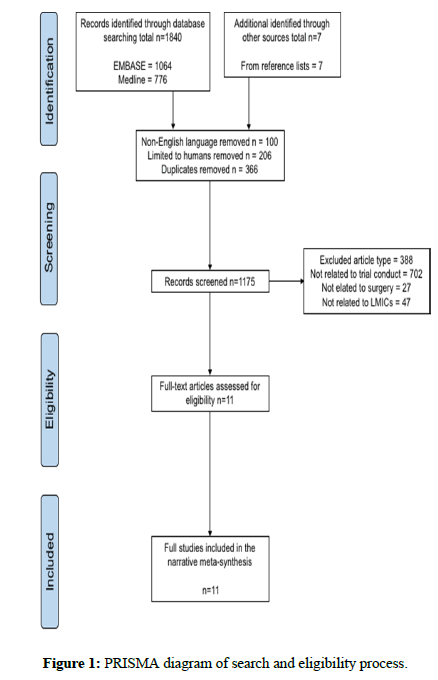
For the MEDLINE and EMBASE databases, all studies published up until May 2019 inclusive were considered for eligibility. All searches were conducted on 25th June 2019. This represents a Pre- COVID 19 pandemic global status. The effects of the COVID-19 pandemic are not considered. Identified article’s titles and abstracts were screened for relevance against the eligibility criteria below (Figure 1). Eligible manuscripts were fully inspected. Reference lists from eligible studies and published systematic reviews were inspected to further identify relevant studies. The search strategy for these databases is described in Appendix A. In brief, search terms included LMIC countries (as classified by the World Bank DAC list), articles relating to the delivery of clinical trials in any surgical speciality and reporting on barriers and/or facilitators to trial delivery. Non-English articles were excluded to prevent potential misinterpretation of the information.
Manuscript eligibility criteria
Figure 1 illustrates the selection process. The eligibility criteria for manuscripts were:
Inclusion Criteria: The criteria are as follows
1. Manuscript type: systematic reviews (+/- meta-analysis), randomised controlled trials, cohort and case control studies, case series, case reports, study protocols.
2. Participants: Humans in LMICs.
3. Procedure: Any surgical technology or operation defined here broadly as any technologies, medical devices, procedures aimed at improving surgical care.
4. Outcome: Reports on barriers, challenges or facilitating factors of trial delivery.
Exclusion Criteria: The criteria are as follows
1. Manuscript: All editorials, news, comments, letters, technical notes, and conference abstracts.
2. Language: All non-English language articles.
3. Participants: Studies not involving humans, or humans in HICs only.
Data extraction and analysis
To identify barriers and facilitators, a meta-synthesis approach guided by Grennhalgh et al., and Thomas et al., was used to inductively generate analytical themes from the findings of the included studies, as described and used in previous systematic reviews [11,13-18]. All text under the ‘‘results or findings’’ or ‘‘conclusion or discussion’’ section of each article was extracted and entered verbatim in vivo. This was coded and analysed by WSB using the Framework method for thematic analysis [19]. If the article was a review, then all text within the main body of the article was included. Once the coding process was completed, the meta-synthesis was conducted by organising the data using in vivo. Patterns within the data were observed, themes were identified and refined using an iterative process, and used to draw interpretations before the evidence was then synthesised to provide a narrative relevant to the research questions. This process followed the European Social Research Council Guidance on the Conduct of Narrative Synthesis in Systematic Reviews [20]. To improve reliability of the evidence synthesised, identified themes were discussed within the research group, examined and changes made where necessary. This process was repeated until consensus was reached regarding the sufficiency and appropriateness of the themes and subthemes developed. Given the qualitative methodology and synthesis of a heterogenous literature on the topic, formal methods for assessing risk of bias were not undertaken [21-30].
Results
WHO International Clinical Trials Registry Platform (ICTRP) findings.
A total of 488,120 (136,327 listed as recruitment ongoing) trials were listed on the WHO ICTRP. Of these, 16,510 (4,823 listed as recruitment ongoing) listed ‘surgery’ (including synonyms) as an intervention. This represents only 3.4% of all registered trials globally. A total of 154,613 (49,931 listed as recruitment ongoing) of all trials registered globally were listed as recruiting from at least one LMIC, representing 31.7% of all registered trials. Within LMICs, 7,077 (2,140 listed as recruitment ongoing) trials listed ‘surgery’ (including synonyms) as an intervention, representing 4.6% of the trials with recruitment from LMICs (Table 1).
| First author and date | LMIC country/countries | Study design | Participant population | Primary aims |
|---|---|---|---|---|
| Ibrahim et al., 2015 [25] | Chile, Ethiopia, Kenya, Nigeria, Ukraine | Qualitative | Surgeons n=18 | Develop a framework for the monitoring international surgical initiatives in LMICs |
| Vischer et al., 2017 [28] | Kenya, Ghana, Burkina Faso, Senegal. | Qualitative | Surgeons, clinical researcher staff n=60 | Determine internal factors slowing down clinical trials in Sub-Saharan Africa |
| Rendon et al., 2017 [22] | Brazil | Qualitative | Surgeons n=13 | Identify barriers and facilitators experienced in collaborative prospective research in orthopaedic surgery |
| Aveling et al., 2016 [23] | Ethiopia | Qualitative | Surgical staff, clinical research staff n=66 | Barriers to implementation of interventions to improve surgical services |
| Fallah et al., 2017 [30] | HIC members working with various LMIC collaborators | Qualitative | Surgeons, anaesthetists, physicians, residents, nurses, academics, and administrators n=68 | Framework for improving international surgical teaching collaborations |
| Conradie et al., 2018 [21] | 27 African countries | Mixed methods | Surgeons, clinical researcher staff n=134 | Barriers to clinical research in Africa |
| Grover et al., 2017 [24] | na | Review | na | Identify challenges to clinical trials in LMICs |
| Moraes et al., 2013 [29] | na | Review | na | Identify challenges to multicentre trials in orthopaedic and trauma surgery |
| Clement et al., 2018 [26] | na | Review | na | Identify the use of qualitative methods in trials globally |
| Søreide et al., 2013 [6] | na | Review | na | Strategies to improve surgical research through international collaboration |
| Skrzynno et al., 2018 [27] | na | Review | na | Challenges in surgical consent in trials |
Table 1: Summary of included studies, study design, country and primary aims.
Barriers to surgical trials in LMICs
The thematic presentation of barriers meta-synthesised from the literature are summarised in Table 2, highlighting which references were drawn upon to generate each theme and subtheme. There were four main barrier themes, each having multiple sub themes:
1. Lack of human resource capacity
2. Lack of equipment, technology and resources
3. Culture and contexts
4. Methodology, design and implementation. To describe the core of each theme, the following narrative draws on examples from the included studies.
| No | Thematic barriers | Subtheme | References |
|---|---|---|---|
| 1 | Lack of human resource capacity | Lack of surgical and allied healthcare workforce | [6,21–25,28,30] |
| Lack of general clinical research delivery workforce | |||
| Lack of trial methodology and delivery workforce | |||
| Lack of knowledge and skills | |||
| Lack of time | |||
| Lack of incentive or motivation | |||
| Under developed research culture | |||
| 2 | Lack of equipment, technology and resources | Lack of surgical technology for trial and wider care delivery | [21–24,28] |
| Lack of data collection and information technology | |||
| Lack of research and service delivery infrastructure | |||
| Lack of financial resources | |||
| 3 | Culture and contexts | Cultural sensitives and a lack of patient/community education | [6,21–28] |
| Varying ethics and other regulatory affairs | |||
| Lack of trial adaptation to differing populations and systems | |||
| Surgeon and researcher attitude to research, randomisation and influence of surgeon preference | |||
| 4 | Methodology, design and implementation | Lack of planning and context understanding | [6,21,22,24–28] |
| Lack of LMIC consultation in design | |||
| Lack of trial design and delivery knowledge and skills | |||
| Challenging outcome and data collection | |||
| Standardising interventions and trial delivery procedures | |||
| Lack of qualitative process evaluation |
Lack of human resource capacity: The lack of human resource capacity was a significant barrier in seven out of the eleven studies. In their mixed methods study Conraide et al., highlighted a critical lack of surgical and allied healthcare workforce to conduct the necessary study interventions [21]. These are essential to deliver the components of any trial, but the execution of a complex surgical intervention is even more dependent on human resource skillset. In their review article, Søreide et al., highlighted that the lack of clinical research delivery skills and motivation among the surgical workforce adds to the human resource gap [6]. A qualitative paper by Rendon et al., exploring barriers to collaborative orthopaedic trial research in LMICs, highlighted that while improving the skillset of personnel is important, researchers often have a limited bandwidth and find it difficult to balance research with clinical delivery [22]. Finally, they often experience a personal sense of fatigue from constantly fighting barriers.
Lack of equipment, technology and resources: Five studies found a lack of equipment, technology or resources, such as financial resources, was a key barrier. In their qualitative process evaluation of a surgical quality improvement project, Aveling et al., highlighted that it is essential to audit the amount of functioning equipment from both within and outside the research study [23]. This ensures safe and efficient running of the research, but also builds and leaves clinical capacity after the study has finished. In their study involving 27 Africa countries, Conradie et al., found that information technology to collect data and share information was among their most frequently reported barrier [21]. Although the internet may have penetrated larger urban centres, rural hospitals (where a large proportion of surgical care is delivered) have little access to the information technologies that are often essential for research. Financial resource is also essential to research delivery. In their review article, Grover et al., discussed how funding for clinical trials does not always prioritise the conditions or areas of greatest need in LMICs [24]. Further, even if clinical trials do demonstrate efficacy in LMICs, researchers are often unable to provide a plan for sustainable implementation of these interventions. Grover et al., argue this poses a major challenge to global surgical funding panels and ethics committees [24].
Culture and contexts: The most frequently reported barrier was adapting to the wide range of cultural and contextual factors that are intrinsic to international global surgical research. In an international qualitative study of surgeons from HICs and LMICs, Ibrahim et al., demonstrated that understanding cultural sensitivities and locoregional contexts was essential when navigating varying ethical and regulatory affairs, as well as exploring surgeon and researcher attitudes to trials [25]. A systematic review by Clement et al., showed the usefulness of process evaluations and other qualitative methodologies during trials to inform the understanding of culture and context as they examined a total of 615,311 registered trials [26]. The authors highlighted that trials of surgical interventions used qualitative methods the least, and in general, use in LMICs was infrequent. In particular, De Skrzynno et al., highlighted that culturally appropriate patient and public engagement is vital to ensure good conduct during surgical trials [27].
Methodology, design, and implementation: A total of eight studies discussed important barriers concerning the theme of methodological design and trial implementation. Vischer et al., conducted a qualitative study of internal factors responsible for slowing down trials in Sub-Saharan Africa [28]. They identified two broad themes: ‘planning’ and ‘site organisation’. Planning referred to the work done during the design phases of studies. Importantly, there seems to be a chronic lack of LMIC consultation during the trial methodology design stages. This can translate into poorer quality data collection that reduces the overall impact of the trial. These themes were represented in several included articles [25,27]. Ongoing site organisation can be monitored and improved with in-built qualitative process evaluations, a theme which was frequently reported the literature [26].
Facilitating strategies to surgical trials in LMICs
The thematic presentation of facilitators meta-synthesised from the literature are summarised in Table 3, highlighting which references were drawn upon to generate each theme and subtheme. There were three main facilitator themes, each having multiple sub themes: i) Collaboration; ii) Flexible and efficient trial designs; iii) Funding and research culture. Facilitators are presented here as strategies to overcome barriers. Often a facilitating factor can be identified as the inverse or counterpoint to a barrier, but in the narrative here, the core of each facilitating theme is discussed to cover distinctive and perhaps cross-cutting ideas to overcome the identified barriers.
| No | Thematic facilitators | Subtheme | References |
|---|---|---|---|
| 1 | Collaboration | International HIC-LMIC collaboration | [6,21,22,24,25,29,30] |
| Loco-regional collaboration | |||
| Interdisciplinary collaboration and team working | |||
| Multi-centre collaboration to increase sample sizes and capacity building | |||
| Surgical trial leadership | |||
| 2 | Flexible and efficient trial design | External pilot phases | [6,21–24,28,29] |
| LMIC lead planning and trial design | |||
| Evaluation of implementation and process | |||
| Cost-effective and efficient design | |||
| Use of technology in design and delivery | |||
| 3 | Funding and research culture | Financial resources and capacity building | [6,21–29] |
| Encouraging a research culture, recognising academics | |||
| Professional development |
Table 3: Meta-synthesised thematic and sub thematic presentation of facilitators to delivering surgical trials in LMICs.
Collaboration: Collaboration was discussed as an essential facilitator in several papers. In particular, Søreide et al., stressed that international collaboration is mutually beneficial, and Ynoe de Moraes et al., highlighted the importance of considering loco-regional collaboration within countries [6,29]. This is principally important to reduce the urban-rural disparity. Importantly, the interdisciplinarity (between/within healthcare workforce members and collaboration with key partners such as industry and governments) of the collaboration was discussed as a facilitating factor that improved trial delivery [22,30]. These collaborations foster surgical trial leadership and multiple centres increases capacity to gain larger sample sizes when necessary and improve generalisability of trial findings. Overall, collaboration is needed to address the key barrier in the lack of human resource capacity, where surgeons and researchers within and between countries establish training and knowledge transfer collaborations.
Flexible and efficient trial designs: Most of the included literature emphasised that local contexts should be considered at every stage of the trial. Particular additional features discussed as facilitating strategies included conducting external pilots and front-loading qualitative evaluations during the design and planning phases [6,28]. These can inform the design and prevent avoidable pit falls and failures, as well as pulling in key members of the team in the design process locally. Using in-built qualitative methodology can allow researchers to evaluate the trial implementation and delivery in realtime, thus adapting to changing situations where needed and boosting efficiency [22,26,28]. Overall, it is essential to keep trials affordable and efficient in their design. The use of digital technologies to collect, store and share data, as well as to deliver training and trial implementation, are further facilitating strategies to improve the efficiency of trial conduct [6,21].
Funding and research culture: Almost all included literature highlighted that adequate funding, and a robust research culture are essential components for increasing surgical trials capacity globally. Recognising and developing clear career pathways and offering professional development opportunities for clinical academics is key, especially in LMICs [6,25,29,30]. This will encourage new research leadership and ensure academic effort is rewarded, including financially.
Creating mutually interesting and beneficial research priorities across countries will develop a deeper, more productive surgical trials research culture whilst ensuring collaborative large-scale funding and impact can be achieved.
Discussion
The results demonstrate a global lack of surgical trials, driven by a range of challenges. The delivery of standardised interventions and procedures, whilst ensuring they are context and culture specific, presents significant barriers for surgical researchers in LMICs. This is compounded by the frequent lack of healthcare resource and research infrastructure. In contrast, flexible trial design, employing additional methods from qualitative research, and collaboration and training opportunities all seem to facilitate surgical trials in LMICs.
The lack of surgical trials in LMICs is particularly concerning given the contribution that surgical care makes to tackling the global disease burden [31,32]. This review provides an insight into the (Pre- COVID 19 pandemic) challenges faced by those attempting to deliver this much needed research. Even in the most difficult circumstances however, there are strategies to overcome these barriers which should be a target for future use. A key strength of this review is the ability to synthesise and display these strategies in one place. The use of a qualitative meta-synthesis design permitted the exploration of a broad research question and interrogation of heterogenous evidence from a range of contexts and study designs. This rich synthesis of the evidence and resulting narrative does not solely focus on the challenges but highlights widely useful facilitating strategies for both policymakers and surgical researchers alike.
A weakness of the review is the incomplete representation of the research pathway. Whilst clinical trials are often required to persuade policy makers to adopt an intervention, many other types of research are needed to answer other questions pertaining to the impact of interventions in the short and long term. An examination of non-trial surgical research in LMICs may identify further gaps and facilitating strategies along the pathway. Another weakness is that only papers written in English were included the review owing to the lack of translation facilities available to the research team. Some countries are not represented in this review, and as barriers may be context specific, this important data could be missing. Further, of the papers included, appraisal of study quality was challenging with such a variety of study designs included. The authors felt that given the lack of literature on the topic, an inclusive approach would increase the diversity of the data. Finally, the qualitative methods used in this review are inherently subjective, affected by the authors’ particular knowledge paradigms and experiences. However, the value of the resulting synthesis lies in presenting the exploration of meanings in a clinically useful narrative, and explicitly acknowledging the subjectivity and bias this creates.
This study raises important considerations for both the design and delivery of surgical trials in LMICs. Standardising interventions within surgical RCTs is essential to derive high quality, meaningful data [33]. In LMIC surgical trials, ensuring standardisation across multiple surgeons, hospitals and countries is equally important but often more challenging. To address this, the current study highlights the need to ensure a flexible and efficient trial design, and to take differing cultures and contexts into consideration. Another facilitating strategy we identified within the literature is in-building qualitative methodologies during the design and delivery phases of surgical trials. Despite the infrequent use of qualitative methodologies, surgical trials can benefit from qualitative methodology to explore surgical behaviour, recruitment issues and factors affecting equipoise [26]. For example, Donovan et al., embedded a surgical trial within qualitative research and demonstrated the positive impact on recruitment and trial delivery [34]. Employing qualitative methodology may be especially important for surgical trials recruiting in LMICs, where there may be a greater need to identify challenges, explore cultural contexts and the acceptability of interventions. By front-loading qualitative exploration during the trial design phase, researchers may be able to identify and tackle specific human resource and training needs, thereby addressing a significant barrier to research upfront.
Conclusion
It seems clear that surgical trial research in LMICs has been neglected. Human resource gaps and global trial funding remain unmet challenges and international collaboration from all members of the surgical trial community is required to overcome these. Cultivating surgical trial leadership from within LMICs will be a critical step in strengthening the international surgical research culture. Addressing the lack of qualitative methodology in surgical trials will hopefully provide a cross-cutting facilitating strategy that can target multiple elements of trial design and delivery. The findings are relevant to all members of the global surgical trial community, providing facilitating strategies to address the challenges in undertaking surgical trials in LMICs. This will in turn help drive up the overall standard of surgical care globally.
Funding
This research was funded by the National Institute for Health Research (NIHR) (16/137/44) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care.
Dissemination Declaration
The results will be disseminated on social media platforms and reports back to funders.
Contributions
WSB and DGJ conceptualised the study. WSB and NKA performed systematic searches, study assessments, and data extraction. All authors fed into the interpretation of the generated themes. WSB prepared the first draft of the manuscript, which was subsequently edited by all authors. DGJ is the study guarantor.
Declarations
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years.
References
- Mcculloch P, Taylor I, Sasako M, Lovett B, Griffin D (2002) Randomised trials in surgery : problems and possible solutions. BMJ 324 (7351):1448-51
- Gattellari M, Ward J, Solomon M, Randomized (2001) Controlled Trials in Surgery. Dis colon rectum 44: 1413-20
- Neugebauer AM, Rath A, Antoine S, Eikermann M, Seidel D, et al. (2017) Specific barriers to the conduct of randomised clinical trials on medical devices. Trials 1: 427
- Djurisic S, Rath A, Gaber S, Garattini S, Bertele V, et al. (2017) Barriers to the conduct of randomised clinical trials within all disease areas. Trials 18: 360
- Duley L, Antman K, Arena J, Avezum A, Blumenthal M (2008) Specific barriers to the conduct of randomized trials. Clin Trials 1: 40–8.
- Søreide K, Alderson D, Bergenfelz A, Beynon J, Connor S, et al. (2013) Strategies to improve clinical research in surgery through international collaboration. Lancet 382: 1140-51
- Meara JG, Leather AJM, Hagander L, Alkire BC, Alonso N, et al. (2015) Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 386: 569-624
- Majumdar S, Roe M, Peterson E, Chen A, Gibler W, et al. (2008) Better outcomes for patients treated at hospitals that participate in clinical trials. Arch Intern Med 168: 657-62
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, et al. (2009) No surgical innovation without evaluation: the IDEAL recommendations. Lancet 374: 1105-12
- Bolton WS, Aruparayil N, Quyn A, Scott J, Wood A, et al. (2019) Disseminating technology in global surgery. Br J Surg 106: 34-43
- Alemayehu C, Mitchell G, Nikles J (2018) Barriers for conducting clinical trials in developing countries-a systematic review. Int J Equity Health 1: 37
- Moher D, Liberati A, Tetzlaff J, Altman D, Group P (2009) Moher Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 151: 264-9
- Thomas J, Harden A (2008) Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 8: 45
- Joseph PD, Caldwell PHY, Tong A, Hanson CS, Craig JC (2016) Stakeholder Views of Clinical Trials in Low-and Middle-Income Countries: A Systematic Review. Pediatrics 137: e20152800
- Forrester J, Powell B, Forrester J, Fast C, Weiser T (2018) Surgical Instrument Reprocessing in Resource-Constrained Countries: A Scoping Review of Existing Methods, Policies, and Barriers. Surg Infect (Larchmt) 19: 593-602
- Thorne S, Jensen L, Kearney M, Noblit G, Sandelowski M (2004) Qualitative metasynthesis: reflections on methodological orientation and ideological agenda. Qual Heal Res 14: 1342-65
- Franzen SRP, Chandler C, Lang T (2017) Health research capacity development in low and middle income countries : reality or rhetoric ? A systematic meta-narrative review of the qualitative literature. BMJ Open 7:e012332.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, et al. (2005) Storylines of research in diffusion of innovation : a meta-narrative approach to systematic review. Soc Sci Med. 61: 417-30
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S (2013) Using the framework method for the analysis of qualitative data in multi-disciplinary health research-Supplementary material: Illustrative example of the use of the Framework Method. BMC Med Res Methodol 13: 1-11
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L,et al. (2006) Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version 1(1):b92.
- Conradie A, Duys R, Forget P, Biccard B (2018) Barriers to clinical research in Africa: a quantitative and qualitative survey of clinical researchers in 27 African countries. Br J Anaesth 121(4):813-821.
[Crossref] [Google scholar] [Pubmed]
- Rendon JS, Swinton M, Bernthal N, Boffano M, Damron T, et al. (2017) Barriers and facilitators experienced in collaborative prospective research in orthopaedic oncology: A qualitative study. Bone Joint Res 6(5):307-14.
[Crossref] [Google scholar] [Pubmed]
- Aveling EL, Zegeye DT, Silverman M (2016) Obstacles to implementation of an intervention to improve surgical services in an Ethiopian hospital: a qualitative study of an international health partnership project. BMC Health Serv Res 16(1):1-2.
[Crossref] [Google scholar] [Pubmed]
- Grover S, Xu M, Jhingran A, Mahantshetty U, Chuang L, et al. Clinical trials in low and middle-income countries-successes and challenges. Gynecologic oncology reports. 2017;19:5-9.
[Crossref] [Google scholar] [Pubmed]
- Ibrahim GM, Cadotte DW, Bernstein M (2015) A framework for the monitoring and evaluation of international surgical initiatives in low-and middle-income countries. PLoS One.;10(3):e0120368.
[Crossref] [Google scholar] [Pubmed]
- Clement C, Edwards SL, Rapport F, Russell IT, Hutchings HA (2018) Exploring qualitative methods reported in registered trials and their yields (EQUITY): systematic review. Trials. 19(1):1-8.
[Crossref] [Google scholar] [Pubmed]
- Dunin De Skrzynno SC, Di Maggio F (2018) Surgical consent in sub-Saharan Africa: a modern challenge for the humanitarian surgeon. Trop Doct 48(3):217-20.
[Crossref] [Google scholar] [Pubmed]
- Vischer N, Pfeiffer C, Limacher M, Burri C (2017) "You can save time if…"—A qualitative study on internal factors slowing down clinical trials in Sub-Saharan Africa. Plos one 12(3):e0173796.
[Crossref] [Google scholar] [Pubmed]
- Moraes VY, Belloti JC, Faloppa F, Bhandari M (2013) Collaborative multicenter trials in Latin America: challenges and opportunities in orthopedic and trauma surgery. Sao Paulo Med J 131:187-192.
[Crossref] [Google scholar] [Pubmed]
- Fallah PN, Bernstein M (2017) Unifying a fragmented effort: a qualitative framework for improving international surgical teaching collaborations. Global Health 13:1-3.
[Crossref] [Google scholar] [Pubmed]
- Shrime MG, Bickler SW, Alkire BC, Mock C (2015) Global burden of surgical disease: an estimation from the provider perspective. The Lancet Global Health 3:S8-S9.
[Crossref] [Google scholar] [Pubmed]
- Isaakidis P, Swingler GH, Pienaar E, Volmink J, Ioannidis JP (2002) Relation between burden of disease and randomised evidence in sub-Saharan Africa: survey of research. BMJ 324(7339):702.
[Crossref] [Google scholar] [Pubmed]
- Blencowe NS, Mills N, Cook JA, Donovan JL, Rogers CA, et al. (2016) Standardizing and monitoring the delivery of surgical interventions in randomized clinical trials. Journal of British Surgery 103(10):1377-84.
[Crossref] [Google scholar] [Pubmed]
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, et al. (2002) Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Bmj 325(7367):766.
[Crossref] [Google scholar] [Pubmed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi