Research Article, J Nanomater Mol Nanotechnol Vol: 13 Issue: 3
Synthesis and Characterization of ZnO Nanoparticles by Co-Precipitation Method
Faizal Mirza1*, Vivekanand Mishra2 and Divyabahen Hadiyal3
1Department of Physics, Saurashtra University, Rajkot, 360005, India
2Department of Science, Alliance University, Bangalore, 562106, India
3Department of Science, B.V. Shah (Vadivihar) Science College, C.U. Shah University, Gujarat, 363030, India
- *Corresponding Author:
- Faizal Mirza
Department of Physics,
Saurashtra University,
Rajkot,
360005,
India,
Tel: 91-9157841470;
E-mail: fzl5381@gmail.com
Received date: 13 April, 2023, Manuscript No. JNMN-23-95565;
Editor assigned date: 17 April, 2023, PreQC No. JNMN-23-95565 (PQ);
Reviewed date: 01 May, 2023, QC No. JNMN-23-95565;
Revised date: 13 June, 2024, Manuscript No. JNMN-23-95565 (R);
Published date: 20 June, 2024, DOI: 10.4172/2324-8777.1000409
Citation: Mirza F, Mishra V, Hadiyal D (2024) Synthesis and Characterization of ZnO Nanoparticles by Co-Precipitation Method. J Nanomater Mol Nanotechnol 13:3.
Abstract
Zinc oxide powders have been synthesized by a co-precipitation method using zinc acetate dehydrate and methanol as the reactants. A number of process parameters such as reaction temperature, solution basicity or pH and heating time are the main factors affecting the morphology and physical properties of the ZnO nanostructures The synthesized particles were characterized to study their microstructural properties by scanning electron microscopy and the presence of the functional group the Fourier Transform-Infrared spectroscopy (FT-IR) has been carried out and analysed.
Keywords: Nanoparticles; Zinc oxide; Spectroscopy; Coprecipitation method
Introduction
Today, Nanotechnology (NT) is operating in various fields of science via its operation for materials and devices using different techniques at nanometer scale nano particles are a part of nanomaterials that are defined as a single particle 1 nm-100 nm in diameter. From last few years, nanoparticles have been a common material for the development of new cutting edge application in communication, energy storage, sensing, data storage, optics, transmission, environmental protection, cosmetics, biology and medicine due to their important optical, electrical and magnetic properties. In perticuler the unique properties and utility nanoparticles also arise from a variety attributes including the similar size of nanoparticles and biomolecules such as proteins and polynucleicacids [1]. Additionally, nanoparticles can be fashioned with a wide range of metal and semiconductor core material that impart useful properties such as fluorescence and magnetic behavior [2]. Moreover, unlike their bulk counterpart nanoparticles have reduced size associated with high surface/volume ratios that increase as the nanoparticles size decreases. As the particle size decrease to some extent a large number of constituting atoms can be found around surface of the particle which makes the particles highly reactive with prominent physical properties. Nanoparticle of particular material show unique material properties, hence manipulation and control of the material properties mechanistic means is needed. In addition, synthesis of nanoparticle having uniform shape and size easy synthetic routes is the main issue in nanoparticle growth. For the past decade scientists have been involved in the development of new synthetic routes enabling the precise control of the morphology and size of the nanoparticles. In addition, nanoparticle synthesis can be possible via liquid (chemical method), solid and gaseous media [3].
Nanostructure have received much attention because of their novel properties, which differ from those of bulk materials [4,5]. Control of dimension and morphology of material has aroused the interest of researchers in the design of functional device due to the optical and electronic properties of nanometer and micrometer sized material which determine their application, can be adapted by varying their size and shape [6]. Zinc Oxide (ZnO), a versatile semiconductor material has been attracting attention because of the commercial demand for optoelectronic devices operating at the blue and violet regions [7]. ZnO is quartzite type semiconductor with band gap energy of 3.37 eV and it has very large excitation binding energy (60 meV) at room temperature [8]. Recently special attention has been devoted to the morphology as ZnO can from different nanostructure [9-11]. Thermal stability irradiation resistance and flexibility to form different nanostructure are the advantages that expedite its potential wide application in photo detectors, surface acoustic wave device, ultraviolet nano-laser, varistors, solar cell, gas sensor, biosensor, ceramics, field emission, and nano-generator [12-21].
Materials and Methods
Experimental work
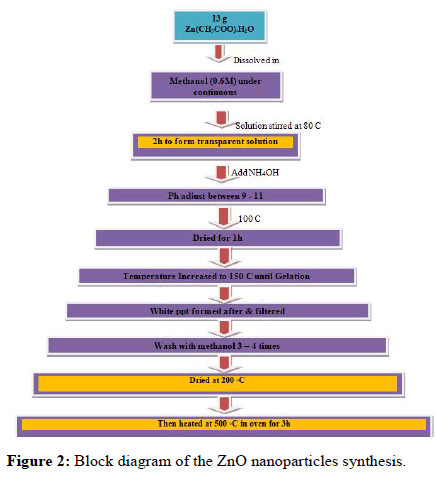
ZnO nanoparticles were synthesized using chemical precipitation method. In a typical synthesis 13 g of zinc acetate dehydrate Zn(CHOO)2.2H2O was dissolved in 0.6 M methanol solution under the continuous stirring. The mixed solution was stirred with a magnetic stirrer at 80℃ for 2 hour to form a transparent solution. Then the pH was adjusted with ammonia between 9 and 11. After adjusting the pH by using the pH meter. Then the solution was dried at 100℃ for 1 hour and then the temperature was increased up to 150℃ until the precipitate occurred. The white precipitate formed after the evaporation was filtered washed with methanol 3-4 times. Then the dried at room temperature. Then after the power sample are grind using the pastle and mortar to get homogenious particles. After drying the powder sample was heated in an oven at 500℃ temperature. Achieved by the oven after 3 hours. Then the temperature kept constant at 500℃ for 3 hrs. After heating for 3 hrs the further was cooled to room temperature. Then after the powder sample are grind using pestle and mortar to get homogenious particles.
Figure 1 shows the experimental figures of the ZnO nanoparticles synthesized by the co-precipitation method.
Basic diagram prepared nanoparticles
The block diagram shows the overall process done to produce the Zinc Oxide (ZnO) nanoparticles by co-precipitation method precursor materials are zinc acetate dissolved in 0.6 M methanol. We already discussed the overall process earlier. Then the obtained sample materials were characterized by the SEM and FTIR spectroscopy (Figure 2).
Results and Discussion
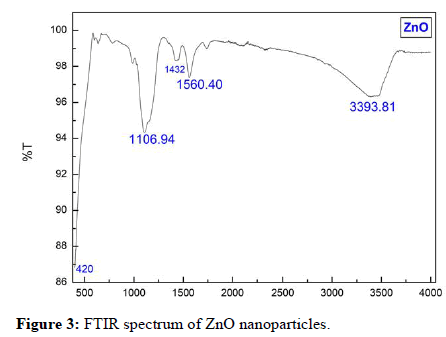
Figure 3 shows the FTIR spectra of the ZnO nanoparticle sample. For the IR spectra, a series of absorption peaks from 400 cm-1 to 4000 cm-1 can be found. Metal oxides generally give absorption bands in fingerprint region i.e. below 1000 cm-1 arising from interatomic vibrations. The peak at 420 cm-1 is the characteristic absorption of Zn- O bond. To be more specific, a broad band at 3393 cm-1 is assigned to the O-H stretching mode of hydroxyl group. Peaks between 1680 cm-1 and 3000 cm-1 are due to C-H stretching vibration. The peaks observed at 1560 cm-1 and 1432 cm-1 are due to the asymmetrical and symmetrical stretching of the zinc carboxylate, respectively. Together this suggests that these FTIR identified impurities mainly exist near ZnO surfaces. This result is a good agreement with other works.
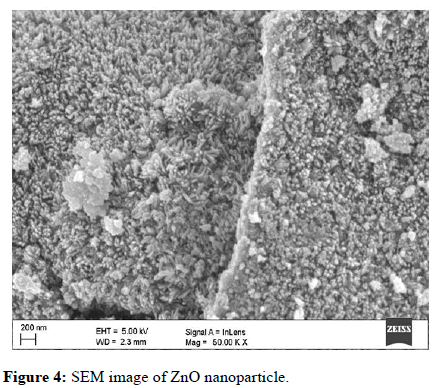
The morphology of the prepared ZnO powder was investigated by Scanning Electron Microscopy (SEM). As shown in Figure 4, the average size of ZnO particles was estimated to be around 40 nm-50 nm. The SEM photograph shows that the powder ZnO NPs are spherical in nature and are agglomerates of nano-crystallitesand homogeneous. A similar result for SEM analysis was reported by Mirza, et al.
Conclusion
Nano powder zinc oxide with less than 50 nm average gain size was synthesized by a co-precipitation method and the powder shapes were almost uniform. Films have been characterized using optical and structural measurement. For the FTIR spectra of series of absorption peaks from 4022 cm-1 to 4000 cm-1 can be found. FTIR identified impurities mainly exist near ZnO surface. The morphology of the prepared ZnO powder was investigated by SEM. The average size of ZnO particle was estimated to be around 40 nm-50 nm.
References
- Zheng M, Davidson F, Huang X (2003) Ethylene glycol monolayer protected nanoparticles for eliminating nonspecific binding with biological molecules. J Am Chem Soc 125:7790-7791.
[Crossref] [Google Scholar] [PubMed]
- Ferrari M (2005) Cancer nanotechnology: Opportunities and challenges. Nature reviews Cancer 5:161-171.
- Vaseem M, Umar A, Hahn YB (2010) ZnO nanoparticles: Growth, properties, and applications. Metal Oxide Nanostr App 5:10-20.
- Baron R, Campbell FW, Streeter I, Xiao L, Compton RG (2008) Facile method for the construction of random nanoparticle arrays on a carbon support for the development of well-defined catalytic surfaces. Int J Electrochem Sci 3:556.
- Moghaddam AB, Kazemzad M, Nabid MR, Dabaghi HH (2008) Improved voltammograms of hydrocaffeic acid on the single walled carbon nanotube/graphite film surfaces. Int J Electrochem Sci 3:291.
- Milliron DJ, Hughes SM, Cui Y, Manna L, Li J, et al. (2004) Colloidal nanocrystal hetero structures with linear and branched topology. Nature 430:190-195.
[Crossref] [Google Scholar] [PubMed]
- Look DC (2001) Recent advances in ZnO materials and devices. Mater Sci Eng B 80:383-387.
- Lieber CM (1998) One dimensional nanostructures: Chemistry, physics and applications. Solid State Commun 107:607-616.
- Zhang Y, Suenaga K, Colliex C, Iijima S (1998) Coaxial nanocable: Silicon carbide and silicon oxide sheathed with boron nitride and carbon. Science 281:973-975.
[Crossref] [Google Scholar] [PubMed]
- Vayssieres L, Keis K, Hagfeldt A, Lindquist SE (2001) Three dimensional array of highly oriented crystalline ZnO microtubes. Chem Mater 13:4395-4398.
- Pan ZW, Dai ZR, Wang ZL (2001) Nanobelts of semiconducting oxides. Science 291:1947-1949.
[Crossref] [Google Scholar] [PubMed]
- Rodriguez JA, Jirsak T, Dvorak J, Sambasivan S, Fischer D (2000) Reaction of NO2 with Zn and ZnO: Photoemission, XANES, and density functional studies on the formation of NO3. J Phys Chem B 104:319-328.
- Shin WC, Wu MS (1994) Growth of ZnO films on GaAs substrates with a SiO2 buffer layer by RF planar magnetron sputtering for surface acoustic wave applications. J Cryst Growth 137:319-325.
- Huang MH, Mao S, Feick H, Yan H, Wu Y, et al. (2001) Room temperature ultraviolet nanowire nanolasers. Science 292:1897-1899.
[Crossref] [Google Scholar] [PubMed]
- Cooray NF, Kushiya K, Fujimaki A, Okumura D, Sato M, et al. (1999) Optimization of Al doped ZnO window layers for large area Cu (InGa) Se2 based modules by RF/DC/DC multiple magnetron sputtering. Jpn J Appl Phys 38:6213.
- Paneva R, Gotchev D (1999) Non-linear vibration behavior of thin multilayer diaphragms. Sens Actuat A: Phys 72:79-87.
[Crossref] [Google Scholar] [PubMed]
- Topoglidis E, Cass AE, O'Regan B, Durrant JR (2001) Immobilisation and bio electrochemistry of proteins on nanoporous TiO2 and ZnO films. J Electroanal Chem 517:20-27.
- Gao L, Li Q, Luan W, Kawaoka H, Sekino T, et al. (2002) Preparation and electric properties of dense nanocrystalline zinc oxide ceramics. J Am Ceram Soc 85:1016-1018.
- Xu CX, Sun XW (2003) Field emission from zinc oxide nanopins. Appl Phys Lett 83:3806-3808.
- Gao PX, Ding Y, Mai W, Hughes WL, Lao C, et al. (2005) Conversion of zinc oxide nanobelts into superlattice structured nanohelices. Science 309:1700-1704.
[Crossref] [Google Scholar] [PubMed]
- Mirza F, Makwana H (2021) Synthesis and characterization of Magnesium Oxide (MgO) nanoparticles by co-precipitation method. Int J Innov Res Technol 8:436-441.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi