Research Article, Int J Cardiovasc Res Vol: 13 Issue: 3
Study the Effect of Type II Diabetes Alone and Diabetes Associated with Hypertension on Left Atrial Volume as a Sensitive Predictor of Left Atrial Dysfunction: A Case-Control Study
Nancy Ibraheem Mohammed Abdo, Mahmoud Mohammed Abdou Youssof, Ayman Ahmed Abd EL-Aziz and Ahmed Hassan Hosny Eladawy*
1Department of Cardiology, Mansoura University, Mansoura, Egypt
*Corresponding Author: Ahmed Hassan Hosny Eladawy,
Deaprtment of Cardiology,
Mansoura University, Mansoura, Egypt
E-mail: dr_ahmed_hosny@msn.com
Received date: 14 February, 2024, Manuscript No. ICRJ-24-127545;
Editor Assigned date: 16 February, 2024, PreQC No. ICRJ-24-127545 (PQ);
Reviewed date: 01 March, 2024, QC No. ICRJ-24-127545;
Revised date: 08 March, 2024, Manuscript No. ICRJ-24-127545 (R);
Published date: 15 March, 2024, DOI: 10.4172/2324-8602.1000538.
Citation: Eladawy AHH et al. (2024) Study the Effect of Type II Diabetes Alone and Diabetes Associated with Hypertension on Left Atrial Volume as a Sensitive
Predictor of Left Atrial Dysfunction: A Case-Control Study. Int J Cardiol Res 13:1.
Abstract
Introduction: It has been shown that the volume of the left chamber is a delicate mark of the level of diastolic brokenness. The objective of this study was supposed to evaluate left atrial volume in patients with Type 2 Diabetes Mellitus (T2DM) and level of diastolic brokenness in patients with type 2 DM in presence or nonappearance of crucial hypertension and relationship of this limits with section and clinical revelations.
Results: Twenty-two diabetic patients were taken into account and divided into two groups: Patient gathering: were broken down into two groups: Subgroup I: contained 30 patients with straightforward sort 2 Diabetes Mellitus (DM) without foundational blood vessel hypertension. II Subgroup: involved 30 patients with straightforward kind 2 DM with fundamental blood vessel hypertension. Systolic left ventricular function was normal in all of the study’s participants. Control subjects: comprised 60 subjects who were matched according to age (48 years ± 8 years), had normal clinical examination and Electrocardiogram (ECG), and were all free of cardiovascular symptoms. Just the pulse fundamentally expanded in the diabetes mellitus bunch among the segment information of our patients and control gatherings. Left Atrial Volume (LAV) and Left Atrial Volume record both increased significantly in the diabetic group in comparison to the control group. Despite the fact that LA breadth was not significantly different between the two gatherings. Because these results indicate enlargement of the Left Atrium (LA) in diabetic patients, LAV and LAVI perform better than the conventional M-mode LA dimension in assessing the size of the LA. Diabetic patients had significantly lower E wave peak velocity, A wave peak velocity, E/A ratio, and Tissue Doppler Imaging (TDI) E’ and A’ when compared to the control group.
Conclusion: The biplane area length method is easier to use to calculate left atrial volume than the antero-posterior diameter measurement. Left atrial volume record expanded in asymptomatic sort 2 diabetes mellitus, and especially reflects LV diastolic brokenness in type 2 diabetes mellitus.
Left atrial volume amplification is related with LA brokenness in type 2 diabetes mellitus as assessed by trans-mitral Doppler and tissue Doppler imaging. The presence of hypertension was the most free indicator of the expanded Left Atrial Volume Index (LAVI) in type II DM, with expansion in the gamble.
Keywords: Left atrial; Volume; Type II diabetes mellitus
Abbreviations
2DE: 2-Dimensional Echocardiography; 2-D: 2 Dimensions; 3D: 3 Dimensions; 3DE: 3-Dimensional Echocardiography; A wave Transmitral flow velocity during atrial contraction; A2C: Apical 2 chamber; A4C: Apical 4 chamber; ACC: American College of Cardiology; ADA: American Diabetes Association; AF: Atrial Fibrillation; AHA: American Heart Association; AL: Biplane Area Length; A Max: Maximum A wave velocity; AVTI: A wave Velocity Time Integral; ANOVA: Analysis of Variance; ASE: American Society of Echocardiography; AUC: Area Under the Curve; BMI: Body Mass Index; BP: Blood Pressure; BSA: Body Surface Area; CAD Coronary artery disease; CBC: Complete blood picture; CHD: Coronary Heart Disease; CHF: Congestive Heart Failure; CKD: Chronic Kidney Disease; cm/s: Centimeter/Second; CMR: Cardiac Magnetic Reasonance; CVD: Cardio Vascular Disease; DBP Diastolic blood pressure; DCM: Diabetic Cardio Myopathy; DD: Diastolic Dysfunction; DDT: Diastolic Deceleration Time;D.M: Diabetes Mellitus; DT: Deceleration Time; DTI: Doppler Tissue Imaging; E wave: Transmitral flow velocity during early ventricular filling; E’: Tissue Doppler velocity at mitral annulus during early ventricular filling; ECG: Electrocardiogram; EF: Ejection Fraction; EF: Ejection Fraction; E Max: Maximum E wave velocity; E Dec: E wave Deceleration time III; ESC: European Society of Cardiology; FS: Fractional Shortening; GDM: Gestational Diabetes Mellitus; HbA1C: Glycosylated Hemoglobin; HDL: High Density Lipoprotein; HF: Heart Failure; HR: Heart Rate; HRV: Heart Rate Variability; HTN: Hypertension; IFG: Impaired Fasting Glucose; IGT: Impaired Glucose Tolerance; IHD: Ischemic Heart Disease; ISH: Isolated Systolic Hypertension; IVRT: Isovolumetric Relaxation Time; IVS: Interventricular Septum; JNC: The Joint National Committee; L: Area Length; LA: Left Atrium; LAA: Left Atrial Appendage; LAD: Left Atrial Diameter; LADI: Left Atrial Diameter Index; LAP: Left Atrial Pressure; LAV: Left Atrial Volume; LAVI: Left Atrial Volume Index; LVIDd: Left Ventricular Diastolic diameter; LDL: Low Density Lipoprotein; LV: Left Ventricle; LVH: Left Ventricular Hypertrophy; LVIDd: Left Ventricular Internal Dimensions in Diastole; LVIDs: Left Ventricular Internal Dimensions in Systole; LVM: Left Ventricular Mass; LVMI: Left Ventricular Mass Index; M: Meter; MACE: Major Adverse Cardiovascular Events; MBP: Mean Blood Pressure; MI: Myocardial Infarction; mmhg: Millimeter Of Mercury; PVD: Peripheral Vascular Disease; PVS: Pulmonary Veins; PWT: Posterior Wall Thickness; ROC: Receiver Operating Characteristic; RT3DE: Real Time Three Dimensional Echocardiography; RV: Right Ventricle; SBP: Systolic Blood Pressure; SD: Standard Deviation; SIMP: Biplane Modified Simpson Method; TDI: Tissue Doppler Imaging; T2DM: Type 2 Diabetes Mellitus; WHO: World Health Organization.
Introduction
Type 2 Diabetes Mellitus (T2DM) and Hypertension (HTN) tend to become more common as people get older. Explores show that cardiovascular confusions are the significant reasons for mortality and handicap in patients with T2DM [1]. Cardiovascular difficulties of diabetes are the most serious danger to patients as a result of their metabolic and microvascular results prompting decrease of heart capability [2]. Diabetes is frequently connected with blood vessel hypertension, which is thusly connected with horrible cardiovascular results and disabled diastolic capability. As a result, diabetes and hypertension frequently have comorbidities and conditions in common, such as obesity and Left Ventricular (LV) hypertrophy, both of which have the potential to affect LV structure and mechanics [3]. The essential elements of left chamber, incorporate; storing blood in a reservoir during LV systole; a conductor for traveling blood from pneumonic veins to LV during right on time and center diastole, and a functioning contractile chamber to increment LV filling at late diastole [4]. Patients with moderate to severe hypertension experience left atrial enlargement, which causes Left Ventricular (LV) hypertrophy and diastolic dysfunction. Left atrial volume is a sensitive indicator of the severity of diastolic dysfunction, as has been demonstrated [5]. It has additionally been found that, diastolic brokenness is an early indication of diabetic heart muscle illness going before the systolic harm and can be available even without any modifications of (LV) systolic capability [6].
Materials and Methods
TThe present study was carried out as a case-control comparative study, aimed to evaluate left atrial volume in patients with type II diabetes mellitus in presence and absence of systemic arterial hypertension. Comprised 60 healthy subjects and 60 patients with type 2 DM with a mean age of 48 years ± 8 years. They were collected from the (outpatient clinic-Departments of cardiology and endocrinology-Mansoura specialized hospital – Mansoura university) in the period between March, 2012 and December 2014. Diagnosis of diabetes mellitus was based on American Diabetes Association definitions in patients that have been receiving treatment in the form of insulin or oral hypoglycemic drugs.
The patients were further divided into two subgroups
Subgroup I: Includes 30 patients with uncomplicated type 2DM without systemic arterial hypertension.
Subgroup II: Includes 30 patients with uncomplicated type 2 DM with systemic arterial hypertension.
Control subjects were matched for age (48 year ± 8 years) they were all non-diabetic, non-hypertensive and free from any cardiovascular symptoms and had normal clinical examination and ECG. The protocol was approved by our ethical committee, and an informed consent was taken from the patients and the control.
Inclusion criteria: All subjects have uncomplicated type 2 DM with and without systemic “arterial hypertension with normal left ventricular systolic function.
Exclusion criteria: Patients with valvular heart disease, heart failure (due to impaired systolic function), and congenital heart disease, coronary artery disease (excluding those with a history of angina, chest pain, abnormal Tread Mill Tests (TMT), non-sinus rhythm, and conduction defect are all included. Past heart medical procedure, Poor transthoracic echocardiographic window, high level renal or liver sickness, history of stroke or fringe vascular infection and danger.
All patients were undergoing
Complete history taking: That includes, Onset, course and duration of type 2 DM, History of dyspnea, angina, palpitation, Risk factors (dietary habits/obesity, smoking, amount of exercise, personal and family history of C.V disease, DM, hypertension, hyperlipidemia). History of drug intake (antihypertensive medication, oral hypoglycemic drugs, insulin therapy if present).
General examination and cardiac examination: General examination was carried out with special emphasis on blood pressure measurement (Two measurements should be made at least 5 minutes apart and if there’s a discrepancy of more than 5 mmHg, a third reading was taken, the reading should be averaged. Pulse pressure is calculated as the difference between systolic and diastolic Bp). Mean Blood Pressure (MBP) is calculated as diastolic BP plus one third of pulse pressure. The diagnosis of hypertension was based on a systolic blood pressure more than 140 mm hg and a diastolic blood pressure more than 90 mm hg in resting supine position or current use of antihypertensive drugs.
Anthropometric assessment including: Height in meter, weight in Kilograms (KG)-Body Mass Index evaluation (BMI), Body Surface Area (BSA)
Laboratory: Kidney function tests, Liver function tests, Lipid profile and Urine analysis. Resting standard 12-lead surface ECG will be carried out, to detect Left Ventricular Hypertrophy (LVH), and chamber enlargement, to detect Left Atrium (LA) enlargement. And to exclude patients with Ischemic Heart Diseases (IHD), conduction abnormality and non-sinus rhythm.
Echocardiographic examination: Complete routine two dimensional, M-mode and doppler transthoracic echocardiography was done to all patients and control group using GE (Vivid 3 pro) and GE (S5) Norway, using 3.5 MHZ multi-frequency transducer.
Pulsed wave doppler: In the apical four chamber view, using a 1-2 mm sample volume, PW doppler cursor is placed between the atrial leaflet tips during diastole. From the mitral inflow profile, the E and A-wave velocity, E-Deceleration Time (DT), and E/A velocity ratio will be measured. The E/A ratio is the ratio of the Early (E) to late (A) ventricular filling velocities.
Tissue doppler: Peak systolic (S’) and early (E’) and late (A’) diastolic velocities of the lateral mitral annulus were measured by pulsed wave tissue doppler imaging from the apical four-chamber view to acquire mitral annular velocities. The sample volume was positioned at or 1 cm within the lateral insertion site of the mitral leaflets and adjusted as necessary (usually 5-10 mm) to cover the longitudinal excursion of the mitral annulus in both systole and diastole (Dokainish et al.,)
Statistical analysis
Information were arranged, coded then investigated utilizing the PC program SPSS (Measurable bundle for sociology) rendition 17.0 to get Illustrative information. The following descriptive statistics were calculated: Mean ± Standard Deviation (SD) and Recurrence (Number-percent).
Student’s t-test: Used to compare the mean of two groups of numerical (parametric) data in the statistical comparison between the groups, this test was used to determine whether or not there was a significant difference. Additionally, post-hoc Tukey and ANOVA (Analysis of Variance) are utilized to compare more than two groups of numerical (parametric) data. Different parameters were correlated using the Pearson correlation coefficient® test.
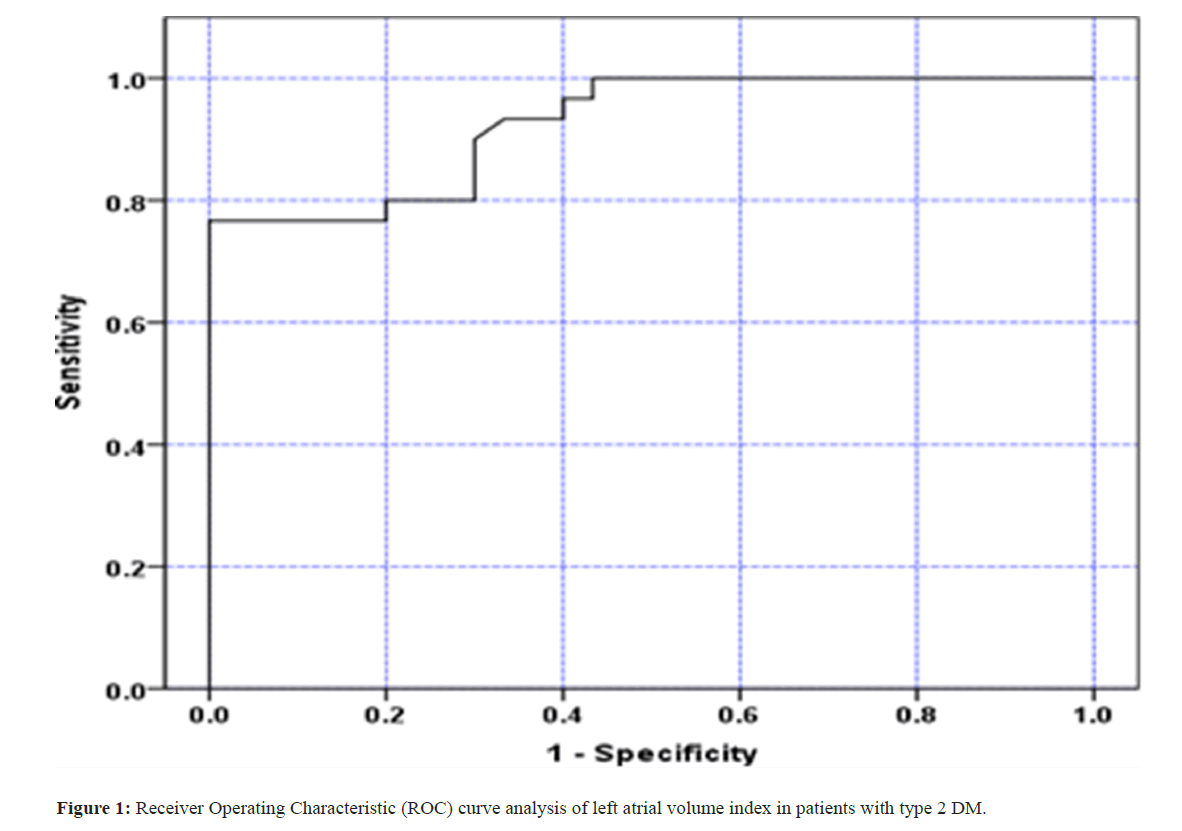
In patients with type 2 diabetes mellitus, the Receiver Operating Characteristic (ROC) curve was used to determine the LAVI cutoff value. A significance level of 0.05 was deemed statistically significant. In addition, all analyses regarded a P value of less than 0.0001 as highly significant.
Results
This research includes 120 subjects including 60 subjects as a control group and 60 patients with type 2 DM, 30 of them are hypertensive as a test group. There is statistically significant difference between patient and control groups regarding heart rate with no statistically significant differences regarding demographic parameters including; age, gender, BSA, and BMI (Table 1).
| Gender | Groups | P | |||
|---|---|---|---|---|---|
| Control n=60 | Patients n=60 | ||||
| Mean ± SD | Mean ± SD | ||||
| Male | Female | Male | Female | ||
| No=9 (15%) | No=51 (85%) | No=11 (18.3%) | No=49 (81.7%) | 0.9 | |
| Age (year) | 47.3 ± 5.1 | 47.6 ± 4.0 | 0.8 | ||
| BMI (kg/m2) | 29.23 ± 7.57 | 31.08 ± 3.22 | 0.4 | ||
| BSA (m2) | 1.965 ± .244 | 2.021 ± .074 | 0.09 | ||
| HR (Beat/min) | 74.6 ± 5.9 | 81.5 ± 14.7 | 0.0001 | ||
Table 1: Comparative analysis of demographic data between control and patients.
Table 2 shows LAV and LAVI were significantly higher in the patient group than in the control group (61.4 ± 16.7 vs. 38.7 ± 5.4; p<0.0001) and (30.43 ± 8.36 vs. 19.15 ± 2.68; p<0.0001) respectively, while There was no statistically significant difference in LAD between both groups (Table 2).
| Groups | |||
|---|---|---|---|
| Control | Patients | P | |
| Mean ± SD | Mean ± SD |
|
|
| LAD (cm) | 3.67 ± .39 | 3.80 ± .44 | 0.1 |
| LAV (ml) | 38.7 ± 5.4 | 61.4 ± 16.7 | <0.0001 |
| LAVI (ml/m2) | 19.15 ± 2.68 | 30.43 ± 8.36 | <0.0001 |
Table 2: Comparative analysis of echocardiographic data of the studied groups (patient and control groups) regarding LAD, LAV, and LAVI.
There was statistically significant differences between test and control groups regarding (IVSd, LVIDd, LVPWD), mitral peak E and A velocity early (E’) diastolic late (A’) diastolic velocities (Table 3). Patients with type 2 DM had significantly higher IVSd (1.05 ± 0.23 vs. 0.83 ± .06; p<0.0001), IVDd (4.84 ± .35 vs. 4.50 ± 0.53; p<0.0001), PWD (0.99 ± 0.19 vs. 0.8 ± 0.08; p<0.0001) , while they had lower mitral peak E velocity (0.70 ± 0.15 vs. 0.92 ± .09; p< 0.0001) , mitral peak A velocity (0.81 ± 0.17 vs. 0.97 ± .07; p<0.0001) , early(E’) diastolic velocity (.09 ± 0.03 vs. 0.11 ± .01; p<0.0001), and late (A’) diastolic velocities of the lateral mitral annulus (0.11 ± 0.03 vs. 0.13 ± .02; p<0.0001). There were no statistically significant differences between test and control groups regarding other echocardiographic parameters (Table 3).
| Groups | |||
|---|---|---|---|
| Control | Patients | P | |
| Mean ± SD | Mean ± SD |
|
|
| IVSd (cm) | .83 ± .06 | 1.05 ± 0.23 | <0.0001 |
| LVIDd (cm) | 4.50 ± 0.53 | 4.84 ± 0.35 | <0.0001 |
| Lvpwd (cm) | .80 ± .08 | .99 ± 0.19 | <0.0001 |
| EF% | 64.5 ± 4.6 | 64.2 ± 5.8 | 0.8 |
| FS% | 36.0 ± 5.3 | 35.2 ± 4.4 | 0.4 |
| E (m/s) | .92 ± .09 | .70 ± 0.15 | <0.0001 |
| A (m/s) | .97 ± .07 | .81 ± 0.17 | <0.0001 |
| E/A | .93 ± 0.9 | .87 ± .22 | 0.048 |
| DT(m/s) | 235.8 ± 24.5 | 235.0 ± 49.4 | 0.9 |
| S' (m/s) | .32 ± 1.54 | .09 ± 0.02 | 0.25 |
| E' (m/s) | .11 ± .01 | .09 ± 0.03 | <0.0001 |
| A' (m/s) | .13 ± .02 | .11 ± 0.03 | <0.0001 |
| E/E' | 8.72 ± 1.38 | 8.42 ± 2.76 | 0.5 |
| LV MASS (g) | 154.1 ± 12.6 | 163.3 ± 29.5 | 0.03 |
| LVMI (g/m2) | 76.39 ± 6.86 | 81.12 ± 14.91 | 0.03 |
Table 3: Comparative analysis of echocardiographic data of the studied groups (patient and control groups).
As regrade the segment and clinical information between diabetic patients just and diabetic patients with hypertension, there was no measurably huge contrasts between understanding gatherings (Table 4).
| Groups | P | ||
|---|---|---|---|
| DM only | DM+HTN | ||
| Mean ± SD | Mean ± SD | ||
| Age (year) | 47.0 ± 4.4 | 48.1 ± 3.5 | 0.26 |
| BMI (kg/m2) | 30.12 ± 3.34 | 32.04 ± 2.83 | 0.02 |
| HR (B/min) | 83.1 ± 12.5 | 79.9 ± 16.6 | 0.4 |
| Duration of diabetes (y) | 6.1 ± 2.1 | 6.0 ± 2.0 | 0.8 |
Table 4: Demographic data of diabetic only and diabetic hypertensive groups.
We additionally saw that LAV and LAVI were fundamentally higher in the patient gathering than in the diabetic hypertensive gathering (74.3 ± 11.5 versus. 48.6 ± 9.7 vs. p<0.0001) and (36.82 ± 5.85 versus 24.04 ± 4.85; p<0.0001) separately, while There was no measurably massive contrast in fellow between the two gatherings (Table 5).
| Groups | |||
|---|---|---|---|
| DM only patients | DM+HTN Patients | P | |
| Mean ± SD | Mean ± SD | ||
| LAD (cm) | 3.79 ± .41 | 3.81 ± .46 | 0.9 |
| LAV (ml) | 48.6 ± 9.7 | 74.3 ± 11.5 | <0.0001 |
| LAVI (ml/m2) | 24.04 ± 4.85 | 36.82 ± 5.85 | <0.0001 |
Table 5: Comparative analysis of echocardiographic data of the diabetic only and diabetic hypertensive groups regarding (LAD, LAV, LAVI).
Compared to the diabetic only group, patients in the diabetic hypertensive group had significantly higher LV MASS (187.6 20.4 vs. 139.0 11.5). LVMI was also higher (93.17.10.55 vs. 69.07.35; p 0.0001) and other echocardiographic parameters did not show any statistically significant differences between the two groups (Table 6, p 0.0001).
| Groups | P | ||
|---|---|---|---|
| DM only patients | DM+HTN patients | ||
| Mean ± SD | Mean ± SD | ||
| IVSd (cm) | 1.08 ± .24 | 1.02 ± .21 | 0.3 |
| LVIDd (cm) | 1.02 ± .21 | 4.45 ± .51 | 0.5 |
| Lvpwd (cm) | 1.0 ± .19 | .98 ± .18 | 0.6 |
| EF% | 63.9 ± 5.8 | 64.5 ± 5.9 | 0.7 |
| FS% | 35.2 ± 4.2 | 35.3 ± 4.5 | 0.9 |
| E (m/s) | .69 ± .13 | .70 ± .16 | 0.9 |
| A (m/s) | .84 ± .17 | .77 ± .17 | 0.1 |
| E/A | .85 ± .21 | .88 ± .24 | 0.6 |
| DT (m/s) | 226.4 ± 17.4 | 243.5 ± 67.1 | 0.2 |
| S' (m/s) | .09 ± .02 | .09 ± .02 | 0.3 |
| E' (m/s) | .09 ± .03 | .09 ± .03 | 0.8 |
| A' (m/s) | .11 ± .03 | .10 ± .03 | 0.6 |
| E/E' | 7.95 ± 2.83 | 8.90 ± 2.66 | 0.2 |
| LV MASS (g) | 139.0 ± 11.5 | 187.6 ± 20.4 | <0.0001 |
| LVMI (g/m2) | 69.07 ± 6.35 | 93.17 ± 10.55 | <0.0001 |
Table 6: Comparative analysis of echocardiographic data of the diabetic only and diabetic hypertensive groups.
As respect the Connection of left atrial volume file in patients with type 2 DM with various clinical and echocardiographic information, the Left Atrial Volume List (LAVI) had a critical positive relationship with left ventricular mass (r=0.88, p.000), with left ventricular mass list (r=.86, p.000) in the patients with type 2 DM. Table 7 shows that there was no significant correlation with any other demographic or echocardiographic data (Table 7).
| Parameter | LAVI in the patient group (n=60) | |
|---|---|---|
| R | P | |
| Age (years) | 0.066 | 0.616 |
| BMI (kg/m2) | 0.236 | 0.07 |
| Duration of DM (y) | 0.037 | 0.776 |
| IVSd (cm) | -.170- | 0.194 |
| LVIDd (cm) | -.058- | 0.66 |
| Lvpwd (cm) | -.069- | 0.602 |
| EF% | 0.034 | 0.794 |
| FS% | 0.044 | 0.737 |
| HR (beat/min.) | 0.004 | 0.978 |
| E (m/s) | 0.027 | 0.836 |
| A (m/s) | -.208- | 0.11 |
| E/A | 0.108 | 0.412 |
| DT (m/s) | 0.173 | 0.186 |
| S' (m/s) | -.033- | 0.8 |
| E' (m/s) | 0.054 | 0.683 |
| A' (m/s) | -.096- | 0.465 |
| E/E' | 0.08 | 0.543 |
| LAD (cm) | 0.142 | 0.279 |
| LV MASS (g) | 0.88 | 0 |
| LVMI (g/m2) | 0.86 | 0 |
Table 7: Correlation of left atrial volume index in patients with type 2 DM with different clinical and echocardiographic data.
There is a critical backwards connection between the Left Atrial Volume file (LAVI) in the DM bunch with age (r=-.47, p .09), with huge positive connection with E/A proportion (r=.388, p=.034), and E’speed. No huge connection to other segment and echocardiographic information was accounted for (Table 8).
| Parameter | LAVI in DM only patient no=30 | |
|---|---|---|
| R | P | |
| Age (years) | -0.47 | 0.009 |
| BMI (kg/m2) | -0.194 | 0.305 |
| Duration of DM (y) | 0.14 | 0.46 |
| IVSd (cm) | -0.053 | 0.78 |
| LVIDd (cm) | -0.007 | 0.972 |
| Lvpwd (cm) | 0.069 | 0.718 |
| EF% | -0.129 | 0.498 |
| FS% | 0.003 | 0.985 |
| HR (beat/min.) | 0.024 | 0.898 |
| E (m/s) | 0.276 | 0.14 |
| A (m/s) | -0.131 | 0.49 |
| E/A | 0.388 | 0.034 |
| DT (m/s) | 0.145 | 0.445 |
| S' (m/s) | 0.141 | 0.456 |
| E' (m/s) | 0.377 | 0.04 |
| A' (m/s) | 0.076 | 0.691 |
| E/E' | -0.234 | 0.214 |
| LAD (cm) | 0.177 | 0.35 |
| LV MASS (g) | 0.264 | 0.158 |
| LVMI (g/m2) | 0.129 | 0.497 |
Table 8: Correlation of left atrial volume index in type 2 DM only group with different clinical and echocardiographic data.
In the diabetic hypertensive group, there was a significant positive correlation (r=.9, p 0.0001) between the Left Atrial Volume Index (LAVI) and LV mass and Left Ventricular Mass Index (LVMI). With no huge connection to other segment and echocardiographic information was accounted for (Table 9).
| Parameter | LAVI in DM+HTN patient group no=30 | |
|---|---|---|
| R | P | |
| Age | 0.329 | 0.076 |
| BMI | 0.201 | 0.287 |
| Duration of DM | 0.063 | 0.74 |
| IVSd | -.135- | 0.477 |
| LVIDd | 0.059 | 0.758 |
| Lvpwd | -.107- | 0.574 |
| EF% | 0.102 | 0.591 |
| FS% | 0.087 | 0.647 |
| HR | 0.214 | 0.257 |
| E | -.152- | 0.422 |
| A | -.028- | 0.884 |
| E/A | -.147- | 0.438 |
| DT (m/s) | 0.051 | 0.789 |
| S' | 0.083 | 0.662 |
| E' | -.095- | 0.617 |
| A' | -.180- | 0.341 |
| E/E' | 0.043 | 0.82 |
| LAD | 0.211 | 0.264 |
| LV MASS | 0.9 | <0.0001 |
| LVMI | 0.9 | <0.0001 |
Table 9: Correlation of left atrial volume index in type 2 DM, hypertensive group with different clinical and echocardiographic data.
Beneficiary Working Trademark (ROC) bend: Our study demonstrated that the Left Atrial Volume Index (LAVI) with a cutoff point of 29.52 ml/m2 had an Area Under the Curve (AUC) of (.925), sensitivity of (80%), specificity of (80%), appositive predictive value of (80%), and negative predictive value of (80%) in patients with type 2 diabetes. (Figure 1) (Table 10).
| LAVI | Area under the curve | Cutoff value | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|
| 92.5% (86.2%-98.8%) | 29.52 | 80% | 80% | 80% | 80% | 80% |
Table 10: ROC curve analysis of LAVI in patients with type 2 DM.
Discussion
Patients with type 2 Diabetes (DM2) have an extended bet of making cardiovascular infection, achieving gigantic heart distressingness and mortality [7]. In DM2 morphological changes suggestive of coronary disease could appear before aftereffects arise and the transcendence of subclinical Left Ventricular (LV) brokenness is extended among these patients. A couple of parts could underlie LV brokenness in DM2, including atherosclerosis, microinfarctions, mitochondrial brokenness, lipotoxicity and assortment of state of the art glycation end-product, provoking myocyte hypertrophy, perivascular fibrosis and extended measures of cross section collagen [8]. All proposed parts provoking LV brokenness in DM2 consolidate principal characteristics that can in like manner influence the capacity of the left chamber.
Though M-mode LA angle is easy to get, its authenticity has actually been tried. An estimation of volume rather than region or a straight aspect better reflects LA size because the left chamber is a topsy-turvy hole. Additionally, estimation of anteroposterior aspect is likely to disregard changes in LA size, and LA dilatation is unlikely to be evenly distributed across all planes [9].
Among the section data in our patients and control social events, simply the beat was on a very basic level brought up in diabetes mellitus pack. Hidden heart autonomic neuropathy may be the cause of the general increase in pulse rate in diabetics. It is possible to experience incidental pulse increases of up to 130 bpm and resting pulses of 90 to 100 bpm. Anomalies in Heart Rate Variability (HRV) are early revelations of cardiovascular autonomic neuropathy, resting tachycardia and a fair heartbeat are brand name late disclosures in diabetic patients with vagal deterrent. Cardiovascular autonomic neuropathy may be associated with irregularities in LV systolic and especially diastolic capability in diabetic patients without any evidence of heart disease, according to a review by [10].
Our chose patients were of more youthful age with mean of 47.6 ± 4 years as in, to stay away from the likely effect of the expanded age on the LA size and LAV. It has been recommended that rising age is related with a rising LA size. Nonetheless, LA extension isn’t viewed as a feature of the ordinary maturing process [11]. The outcomes showed an exceptionally critical expansion in Left Atrial Volume (LAV), and Left Atrial Volume file (LAVI) in the diabetic gathering contrasted with the benchmark group, while there was no tremendous distinction in LA width between the two gatherings. These discoveries show LA amplification in diabetic patients, and raise the predominance of LAV and LAVI in evaluation of LA size over the regular M-mode LA aspect. Our outcomes were like showed moderate-to-extreme LA development (LA volume record 32 ml/m2) in 33% of their review populace containing 305 subjects with DM [12,13].
Similar outcomes were seen by Chillo et al., and Kadappu et al., they assess Left Atrial (LA) volume and capability as evaluated by endlessly strain rate got from 2D spot following in 73 patients with DM in examination with age-and orientation matched typical controls [6,14]. Different strategies for LA volume estimation have been depicted, the biplane or single plane region length technique, the biplane or single plane Simpson’s plate strategy, the prolate-ellipsoid technique, and the circular model [15,16].
In our review, most extreme LAV (not long before mitral valve opening) was estimated utilizing the biplane region length technique. This is as per the proposals of the American culture of echocardiography for measurement of LA volume utilizing biplane 2D echocardiography utilizing the region length strategy which can precisely and reproducibly be performed utilizing 2D echocardiography. Likewise, heart attractive reverberation (CMR) precisely gauges of LA volume utilizing comparable cine pictures from vertical and even log hub view. In our review we ordered the LAV to the body surface region as the left atrial size increments with expanding body size and ought to be listed to body size to permit significant correlations. Estimation of anteroposterior LA straight aspect by M-mode echocardiography is basic and advantageous however not dependably precise which was affirmed in this review, considering that the LA is an uneven 3D construction and may not expand similarly along its three tomahawks, yet there is not a really obvious reason for why, in certain patients, the front back width is saved [17]. When contrasted and attractive reverberation, 3D echocardiography or figured tomography, LA2D volumes have been reliably demonstrated to be more exact than direct aspect to analyze LA expansion [18].
In our review diabetic patients had critical lessening in E wave top speed, A wave top speed, E/A proportion, and TDI Em, and Am contrasted with the benchmark group as in Table 8, giving a proof of left ventricular diastolic brokenness in type II diabetes mellitus without plain cardiovascular sickness with safeguarded LV systolic capability reflected by the absence of distinction in LVEF, FS, and TDI top Sm. This diminishing in LV consistence in type 2 DM patients might be brought about by microvascular sickness, adjusted myocardial digestion, and underlying changes in the myocardium with expanded fibrosis [19]. Different examinations shows that the essential practical irregularity in a diabetic heart is the impedance of LV diastolic capability (DD) mirroring the diminished LV filling and that even the systolic useful modification is the consequence of decreased LV filling. In addition, the predominance of LVDD in patients with DM is altogether more noteworthy than that in everyone and is accounted for somewhere in the range of 43% and 75% [20].
Whether the subclinical diastolic anomalies found in our diabetic patients progress to clinically obvious coronary illness isn’t clear. A 3-year follow-up concentrate by Pritchett et al., of a gathering of asymptomatic diabetics with slow filling and wall diminishing verifiable on echocardiography showed 31% creating cardiovascular breakdown and 19% that passed on [21]. Consequently, unusual diastolic capability reminiscent of diminished LV consistence bringing about a ‘Solid’ myocardium gives off an impression of being the lobby sign of the particular kind of diabetic heart muscle infection. The presence or expansion of hypertension or coronary vein sickness will unquestionably put another weight on the allaround ‘rebellious’ myocardium in well-established diabetics despite everything microvascular entanglements. In our patients with diastolic dysfunction, the Left Atrial Volume Index (LAVI) significantly increased, making it more useful as a measure of LV diastolic function. Expanded LA volume typically reflects elevated ventricular filling pressures in subjects without essential atrial pathology or inborn heart or mitral valve disease. The LA is exposed to the LV’s tensions in ventricular diastole. LA pressure ascends because of expanded LV firmness or resistance to keep up with satisfactory LV filling, and the expanded atrial wall strain causes chamber dilatation and atrial myocardial stretch [22,23]. Hence, the seriousness of diastolic brokenness is related with an expansion in LA volume.
The chronicity of exposure to abnormal filling pressures may be expressed by the structural changes in the LA, which can provide predictive information in addition to diastolic function grade. In addition, the indexed LA volume has the highest discriminative value when comparing the trans mitral filling pattern to a pseudo-normal pattern [24]. In addition, Poulsen et al., reasoned that LA volume will reflect “ongoing” LA pressure over-burden in type II diabetics [12].
As shown in Table 8, there was no significant difference between diabetics and controls in left ventricular systolic function as measured by LVEF and TDI peak S wave. Our outcomes were contrasted and different investigations. Dikshit et al., they also found that 66% of diabetic patients had an E/A ratio that was significantly abnormal compared to the normal controls [1,25]. Our review shows critical expansion in interventricular septal, back wall thickness, LV mass, and LV mass record in diabetic gathering contrasted with the benchmark group (Table 8). Dikshit et al., reported the same thing, who suggest that insulin resistance and its associated hyperinsulinemia may be exacerbated by metabolic and/or hormonal factors in the development of greater ventricular mass [25].
However, the rise in LVMI and wall thickness is explained by the fact that 50% of patients in our study had associated hypertension. Transmitral peak A wave and mitral annular peak Am were significantly lower in diabetic patients than in controls in our study. Their abatement in our patients mirrors some level of LA contractile brokenness particularly in the setting of higher grades of diastolic brokenness. Atrial fibrosis, which impairs LA function, is known to be caused by diabetes mellitus. Trans mitral pinnacle A wave speed, its time speed essential gives experiences into LA mechanical capability and turns out to be progressively vital to the conservation of cardiovascular execution within the sight of diminished LV consistence and is missing in Atrial Fibrillation (AF) [26]. Different examinations have involved trans mitral pinnacle A wave as a file of LA capability evaluation. This assessment, in any case, is impacted by age, stacking conditions, and pulse [27]. Tissue Doppler peak Am, on the other hand, is relatively load-independent. Numerous studies have demonstrated a strong correlation between atrial function and the mitral annulus Am [28]. In patients with varying degrees of LV diastolic dysfunction, it correlates very well with LA ejection fraction, LA ejection force, and LA kinetic energy [29].
Be that as it may, TDI A′ evaluation gives data on worldwide LA capability as it were. It doesn’t permit point by point provincial LA useful evaluation. Tissue Doppler velocities may be affected by the translational and tethering effects of nearby myocardium, as well as their angle dependence, just like those of other Doppler techniques [30]. When we compared the diabetic hypertensive group to those with associated hypertension, the diabetic hypertensive group only had significant increases in the LAV, LAVI, LV mass, and LV mass index. Systolic and diastolic capability boundaries were unaffected (Table 5). As indicated by the expansion of the LV mass file in diabetic hypertensive patients, the additional impact of tension overburden and underlying changes in the heart (LVH) contributed to the expansion of LAVI. Hypertension, a typical heart risk consider diabetics who likewise have diabetes. Atrial hypertrophy and fibrosis are both exacerbated by the raised LV end-diastolic strain, similar to a debilitation of development limit because of expanded solidness [31].
In the Solid Heart study and the HyperGen study, evident ramifications of a particular diabetes impact on LV unwinding of the myocardium were tracked down in hypertensive sort 2 diabetes patients [32-34]. The effects of diabetes and hypertension on LV function were the subject of these studies. This is likely to hinder glycemic control, cause microangiopathy or interstitial collection of elastin and collagen, which also increases LV mass and strength in diabetics [35]. Every diabetic patient has a significant positive correlation between their LAVI, LV mass, and LV mass file. The LAVI and LV mass rise in tandem with the severity of LV diastolic dysfunction. Chen et al. who reasoned that LAVI is influenced by the volume and mass of the left ventricle on their own [36].
In diabetic patients without hypertension, there was a significant positive correlation between LAVI, E/A ratio, and TDI Em. [30,31]. This demonstrates the correlation between the various parameters of the assessment of diastolic dysfunction, as trans mitral inflow provides information about instantaneous pressures in the LV and LA and LAV/LAVI is a less load-dependent measure of chronicity and severity of diastolic dysfunction. LAV, according to Tsang and others, was a symptom of diastolic dysfunction [22]. LAV enlargement was found to be a feature that was consistent with the diagnosis of moderate diastolic dysfunction [37]. LAVI with an endpoint of (29.52 ml/m2) produced a region under the bend (AUC) of 0.925 in the current review, with a responsiveness of 80% and specificity of 80% for the detection of left atrial broadening in type II diabetes. Chillo et al., observed results that were comparable to who discovered that diabetic patients considered a LA volume indexed to body surface area (LAVI) of 29 ml/m2 to be excessive [6]. Poulsen and others found that LAVI 32 ml/m2 was an independent and incremental predictor of cardiovascular morbidity and mortality due to CVD in T2DM patients without a history of cardiovascular disease [12].
Both the biplane Simpson’s method and the biplane area-length method measured the left atrial volume, which was 20.6 ml/m2. Our ordinary gathering’s mean listed LA volume was 19.1 ml/m2, which is lower than the past figure. This may be because our patients are younger. In a similar vein, the size of the left atrium has been shown to be a predictor for adverse cardiovascular events as well as major outcomes. Atrial fibrillation, cerebrovascular events, severe myocardial infarction, congestive heart failure, and all-cause mortality have all been linked to LA size’s prognostic value in predicting these outcomes. In addition, in type II DM, hypertension was the most accurate predictor of an expanded LAVI, increasing the risk by 14.6 times, according to our research. Changes in LA size and capability are also linked to hypertension, a common cardiovascular risk factor that occurs concurrently [7]. Primary changes in the heart (LVH) and/ or diastolic dysfunction could be the cause of the LA expansion, but atrial fibrosis in diabetic patients is more common.
Conclusion
That is the very thing that this study contemplated, the left atrial volume can be really obtained by biplane locale length technique, and its manager to the antero-back expansiveness assessment, while, the left atrial volume record extended in asymptomatic sort 2 diabetes mellitus, and particularly reflects LV diastolic brokenness in type 2 diabetes mellitus. In the current review, LAVI with an endpoint of (29.52 ml/m2) yielded a region under the bend (AUC) of 0.925, with a responsiveness of 80% and specificity of 80% for the location of left atrial growth in type 2 diabetes mellitus, as assessed by trans-mitral Doppler and tissue Doppler imaging. The most accurate predictor of an expanded LAVI in type II diabetes was hypertension, which increased the risk by 14.6 times.
References
- Moura AL, Freire CM, Barbosa M, Machado LJ, Nogueira AI (2007) Left ventricle diastolic dysfunction in diabetes: An update. Arq Bras Endocrinol Metabol 51(2):168-175.
- Leung DY, Boyd A, Ng AA, Chi C, Thomas L (2008) Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J 156(6):1056-1064.
- Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A et al. (2001) Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) Study. Circulation 103(1): 102–107.
- Yuda S, Muranaka A, Tsuchihashi K, Hashimoto A, Nakata T, et al. (2009) Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography 26(3):262-271.
- Barnes ME, Tsang TS, Gersh BJ, Bailey KR, Seward JB (2002) Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 90(12):1284-1289.
- Chillo P , E. Rieck A , Wakatare J , Lutale J, Gerdts E (2013) Left atrial volume index as a marker of left ventricular diastolic dysfunction in asymptomatic Tanzanian diabetic patients. Blood Pressure 22(2):86-93.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in non-diabetic subjects with and without prior myocardial infarction. N Engl J Med 339(4):229–234.
- Devereux RB, Roman MJ, Paranicas, M O’Grady MJ, Lee ET, et al. (2000) Impact of diabetes on cardiac structure and function: The strong heart study. Circulation 101(19):2271–2276.
- Lang RM, Bierig M, Devereux RB (2006) Recommendations for chamber quantification. Eur J Echocardiogr. 7: 79 – 108.
- Hayat SA, Patel B, Khattar RS, Malik RA (2004) Diabetic cardiomyopathy: Mechanisms, diagnosis and treatment. Clin Sci (Lond) 107(6): 539–557.
- Pearlman JD, Triulzi MO, King ME Abascal VM, Newell J (1990) Left atrial dimensions in growth and development: Normal limits for two-dimensional echocardiography. J Am Coll Cardiol 16(5): 1168-1174.
- Poulsen MK, Dahl JS, Henriksen JE, Hey TM, Høilund-Carlsen PF et al. (2013) Left atrial volume index: Relation to long- termclinical outcome in type 2 diabetes. J Am Coll Cardiol 62(25): 2416‑2421.
- Poulsen MK, Henriksen JE, Dahl J, Johansen A, Gerke O et al. (2010) Left ventricular diastolic function in type 2 diabetes mellitus: Prevalence and association with myocardial and vascular disease. Circ Cardiovasc Imaging 3(1):24–31.
- Kadappu KK, Boyd A, Eshoo S, Haluska B, Yeo AE et al. (2012) Changes in left atrial volume in diabetes mellitus: More than diastolic dysfunction? Eur Heart J Cardiovasc Imaging 13(12):1016‑1023.
[Crossref][GoogleScholar] [PubMed]
- Schabelman S, Schiller N, Anschuetz R, Silverman N, Glantz S (1978) Comparison of four two-dimensional echocardiographic views for measuring left atrial size (abstr). Am J Cardiol 41(2): 391.
- Kircher B, Abbott JA, Pau S, Gould RG, Himelman RB et al. (1991) Left atrial volume determination by biplane two-dimensional echocardiography: Validation by cine computed tomography. Am Heart J 121(3):864 –871.
- Sahn DJ, DeMaria A, Kisslo J, Weyman A (1978) Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of chocardiographic measurements. Circulation 58(6): 1072–1083.
- Viera MJ, Teixeira R, Gonçalves L, Gersh BJ (2014) Left atrial mechanics: Echocardiographic assessment and clinical implications. J Am Soc Echocardiogr 27(5): 463–478.
- Watts GF, Marwick TH (2003) Ventricular dysfunction in early diabetic heart disease: Detection, mechanisms and significance. Clin Sci 105(5): 537–540.
- Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE (2004) Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 93(7): 870– 875.
- Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL et al. (2005) Diastolic dysfunction and left atrial volume: A population-based study. J Am Coll Cardiol 45(1): 87–92.
- Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC et al. (2001) Left atrial volume: Important risk marker of incident atrial fibrillation in 1,655 older men and women. Mayo Clin Proc 76:467–475.
- Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB (2002) Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 90(12):1284–1289.
- Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA (2007) How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20): 2539-2550.
- Dikshit1 N, Wadia P, Shukla D (2013) Diastolic dysfunction in diabetes mellitus. Nat J Med Res 3(3):249-252.
- Rosca M, Lancellotti P, Popescu BA, Piérard LA (2011) Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart 97(23):1982–1989.
- Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA et al. (2003) Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study). Am J Cardiol 91(9):1079‑1083.
[Crossref][GoogleScholar] [PubMed]
- Rodrigues AC, Scannavacca MI, Caldas MA, Hotta VT, Pisani C et al. (2009) Left atrial function after ablation for paroxysmal atrial fibrillation. Am J Cardiol 103(3):395–398.
- Khankirawatana B, Khankirawatana S, Peterson B, Mahrous H, Porter TR (2004) Peak atrial systolic mitral annular velocity by Doppler tissue reliably predicts left atrial systolic function. J Am Soc Echocardiogr 17(4):353– 360.
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK et al. (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler catheterization study. Circulation 102(15):1788–1794.
- Bukowska A, Lendeckel U, Hirte D, Wolke C, Striggow F et al. (2006) Activation of the calcineurin signaling pathway induces atrial hypertrophy during atrial fibrillation. Cell Mol Life Sci 63: 333–342.
- Ti Tsai C, Hwang J, Shih Y, Chiang FT, Lai LP et al. (2008) Evolution of Left Atrial Systolic and Diastolic Functions in Different Stages of hypertension: Distinct effects of blood pressure control. Cardiol J 109(3):180-187.
- Bella JN, Devereux RB, Roman MJ, Palmieri V, Liu JE et al. (2001) Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in american Indians (the Strong Heart Study). Am J Cardiol 87(11): 1260– 1265.
- Liu JE, Palmieri V, Roman MJ, Bella JN, Fabsitz R et al. (2001) The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: The Strong Heart Study. J Am Coll Cardiol 37(7): 1943–1949.
- Van Hoeven KH, Factor SM (1990): A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 82(3): 848–855.
- Yuan Chen, Hirotomo Sato, Nobuhide Watanabe, Adachi T, Kodani N et al. (2012) Factors influencing left atrial volume in treated hypertension . J Cardiol 60(2):133–138.
- Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK et al. (2006) Left atrial size: Physiologic determinants and clinical applications. J Am Coll Cardiol 47(12):2357–2363.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi