Review Article, J Regen Med Vol: 12 Issue: 6
Stem Cells: An Approach for the Treatment of Various Diseases
Vivek Sharma, Sapna Thakur and Sneh Sharma*
Department of Biotechnology, College of Horticulture and Forestry Neri, Hamirpur, India
- *Corresponding Author:
- Sneh Sharma
Department of Biotechnology,
College of Horticulture and Forestry Neri,
Hamirpur, India;
Tel: 7986839619;
E-mail: snehasharma_ss@yahoo.co.in
Received date: 15 May, 2023, Manuscript No. JRGM-23-98863;
Editor assigned date: 18 May, 2023, PreQC No. JRGM-23-98863 (PQ);
Reviewed date: 01 June, 2023, QC No. JRGM-23-98863;
Revised date: 14 July, 2023, Manuscript No. JRGM-23-98863 (R);
Published date: 21 July, 2023, DOI: 10.4172/2325-9620.1000275
Citation: Sharma V, Thakur S, Sharma S (2023) Stem Cells: An Approach for the Treatment of Various Diseases. J Regen Med 12:6.
Abstract
Stem cells are described as the basic components of tissues and organs. These are cells of all multicellular creatures. Stem cells are also known as universal cell. Stem cells have the capacity to develop into a variety of adult cells. Stem cells can be collected from numerous tissues, including bone marrow, amniotic cell adipose tissue, umbilical cord, and placental tissue. The major properties of stem cells that differentiate it from other cells include totipotency and self-renewal. Due to their capacity to develop into the distinct cell types needed for the repair of damaged tissues makes them the ideal choice when tissue and organ transplantation is necessary. Very early embryonic stem cells exhibit to totipotency, but adult stem cells also exhibit multipotency and differential plasticity, which can be used to provide new therapeutic alternatives in the future. Stem cell based treatments have shown significant success in treating genetic blood illnesses, neurodegenerative illnesses like Parkinson’s and Alzheimer’s in human body. They play a vital role in the maintenance, development of our muscles, skin, bones, and other organs. To replace neurons lost during some disease conditions, stem cell give promise in the fight against neurodegeneration. Stem cells are utilized for the treatment of cancer, heart damage, brain damage, blindness and currently have potential contribution in COVID-19 also. Here, we have talked about the role of mesenchymal stem cells therapy in the fight against Corona virus. They have great potential in the regeneration and repairing of damaged tissues, but there is still much to understand about stem cell biology and safety before achieving its full therapeutic potential. Stem cells have immense role in clinical as well as in medical field for the treatment of various diseases. Hence, the current review is focused on the sources, transplantation, applications, role of stem cells in COVID-19, stem cell banking and ethical issues as well as risks related to stem cell research and to make it easier for the reader to comprehend the following chapters.
Keywords: Stem cells; Embryonic stem cells; Stem cell banking; Stem cell therapy; Diseases
Introduction
Stem cells are undifferentiated cells and often referred to as unspecialized cells of human body that give rise to another type of cells. It is also known as blank cells and are mainly present in the embryonic, fetal, and adult stages of human life [1]. These cells are essential mediators in the development of newborns as well as in restorative processes following injury or disease [2]. The main properties of stem cells which distinguish it from another types of cells are that they stand unspecialized means they are not originated into cells to perform a specific function, stem cells have the major property of self-renewal that is it can undergo division and develop copies of themselves, the another property of stem cells corresponds to its differentiation means that these cells divide and originate such cell that can become other specific sort of cell, organs or tissue [3]. Now a days, it is possible to cultivate stem cells artificially and differentiate them into specialized cell types with traits like those of cells from other tissues, like muscles or nerves [4]. Autologous embryonic stem cells and embryonic cell lines are generated through therapeutic cloning have been suggested as interesting prospects for upcoming treatments. So, the present study is focused on the benefits of stem cells which can be used to cure human diseases [5]. Theoder Heinrich Boveri and Valentin Haecker introduced stem cells terminology in 1888. In the human body, stem cells have the capacity to differentiate into a wide variety of cell types that perform a variety of activities. Histologist Franz Ernst Christian Neumann and Alexander Alexandrowitsch Maximov conducted investigation on bone marrow in 1902 which showed that how common progenitor cells give rise to adult blood cells, a process known as hematopoiesis. Based on their proliferating and differentiating characteristics Maximov derived the idea of polyblast from this research, and Ernst Haeckel later termed these cells stem cells. In 1969 the first bone marrow transplantation is conducted by Dr. E. Donnall Thomas in the US. After that the first murine embryonic stem cells were established by Evans and Kaufman and G.R. Martin in 1981. The stromal cells later were renamed as “mesenchymal stem cells” by A.I. Caplan. The induced pluripotent stem cells were first discovered by S. Yamanaska and K Takahashi in 2007. In 2022 the reported data of 10 years post-administration of the OPC1 product in spinal cord injury’s treatment [6]. Stem cell research is one of the most exciting areas of modern biology. Stem cells research has shown the understanding of how organisms grow from a single cell and how healthy cells replace damaged ones in adult creatures.
It has been recently reported that mesenchymal stem cells can effectively reduce lung injury, improve lung function, and reduce the severe inflammatory response caused by SARS-CoV-2 in patients. Therefore, there is an urgent need in the field of stem cells for the treatment of COVID-19. Stem cells are a newer method of research from contemporary times in biology field, but also needs further research for its validation.
Classification
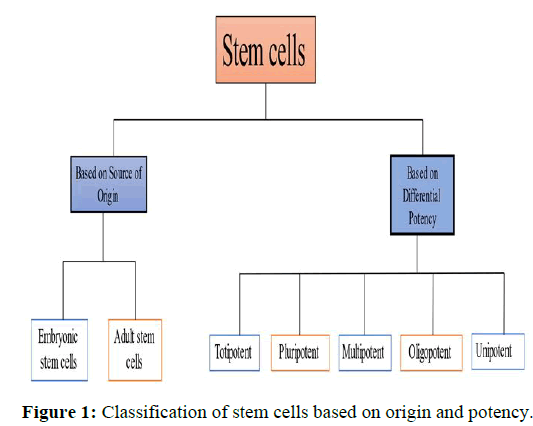
Stem cells have been broadly classified based on source of origin and based on their potency (Figure 1).
Based on its source of origin stem cells have been classified into embryonic and adult stem cell. But according to its differential potency stem cells have been broadly classified into totipotent, pluripotent, multipotent, oligo potent and unimportant stem cells.
Literature Review
Based on source of origin
Embryonic stem cells in 1981, these cells were initially isolated from mice and were later discovered inside the human embryo in 1998. Embryonic stem cells are also known by the name pluripotent stem cells as they have ability to develop all type of cell in human body, such cell can build a whole organism. Embryonic stem cells originated from the blastocyst, a very young stage of development that consists of a mainly hollow ball of between 150 and 200 cells that are hardly visible to the human eye. These stem cells are likely to be employed in a clinical context as they can produce a variety of cells, but their usage is constrained by several ethical issues [7].
In vitro generation technique of embryonic stem cells
Cell culture is a procedure which is utilized in the lab condition to grow human embryonic stem cells. The first embryonic stem cells were isolated by removing the inner mass of cell into a culture dish having culture medium of nutrient or broth. These culture dishes have been placed under suitable humidity and temperature so that the cell can divide or proliferate all over the surface of the culture dish containing culture medium [8].
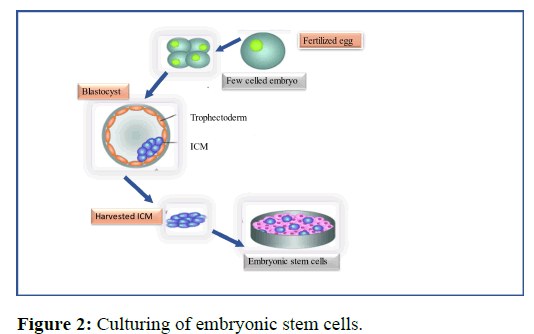
The colony of human embryonic stem cells are extracted either from dividing zygote or from the adult tissue. In the presence of extracellular matrices like matrigel and fibronectin, HESC can be sustained in feeder free conditions. While basic fibroblast growth factor supplementation and medium conditioning by feeder fibroblast cells were initially employed in such feeder free settings to keep hESC in an undifferentiated state. Commercially available feeder-free culture systems that only use human sourced recombinant proteins have been created for the cultivation of hESC maintenance have been devised, more research is required to identify the variables influencing the stability of hESC lines (Figure 2) [9].
Embryonic stem cells are produced from early stage embryos. The production of embryonic stem cells requires a fertilized embryo. After 4-5 days from fertilization, the blastocyst with ICM (Inner cell mass) are formed. ESCs from ICM are successfully transmitted on a dish.
Adult stem cells a type of stem cells which have been identified in various tissues throughout the body including the skeletal, blood vascular and brain, skin, the liver, and muscles which are obtained only after embryonic development. Among them bone marrow is rich source of adult stem cells. Adult stem cells are also named somatic cells as they play a vital role in replacement and healing and growth of cells that are lost every day. They can regenerate in the body, producing exact duplicates of them and become specialized to produce the cell types of the origin tissues. There is no ethical concern related to the use of adult stem cells and these are widely used in clinical and research purpose. In this group Mesenchymal Stem Cells (MSCs), Neural Stem Cells (NSCs), and Hematopoietic Stem Cells (HSCs) are often utilized in the treatment of cancer.
Mesenchymal Stem Cell (MSCs), are derived from various tissues and organs, plays important roles on repairing tissues and regeneration. They can rapidly proliferate and give rise to specialized cell types in vitro, like adipocytes, chondrocytes and osteocytes.
Neural Stem Cells (NSCs), originally found in the central nervous system, can generate and self-renew new neurons and glial cells. To treat both primary and metastatic breast, lung, and, prostate cancers in murine models.
Hematopoietic stem cells, are present in bone marrow, can produce all mature blood cells in the human body. The only stem cell treatment, which is FDA approved to treat multiple myeloma, leukaemia, and some types of blood system abnormalities is the infusion of HSCs obtained from cord blood.
Based on potency
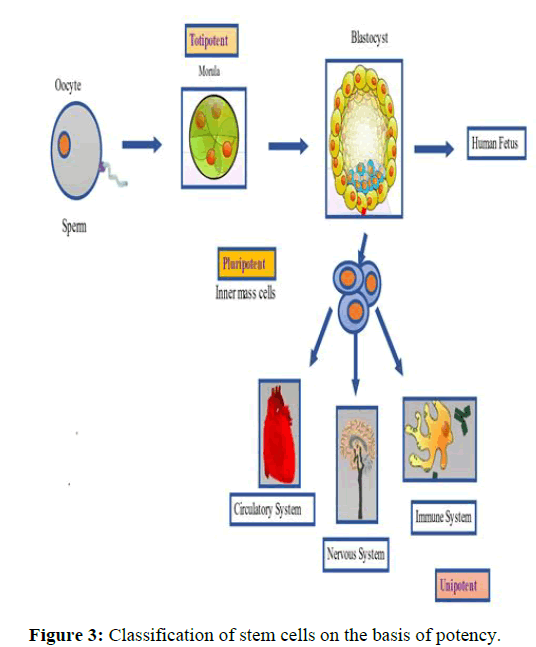
Totipotent stem cells are the type of stem cells which have the capacity to divide and give rise to all types of cells in an organism. Zygote is an example of a totipotent stem cell which is created when an egg and a sperm fertilized. Totipotency, which enables cells to create both extra embryonic and embryonic structures, has the highest differentiation potential. These stem cells have the potential to either develop into one of the three germ layers or a placenta in the future. The inner cell mass of the blastocyst develops pluripotency after around 4 days (Figure 3).
Inner cell mass within the blastocyst are the source of pluripotent embryonic stem cells. All body tissues can develop from these stem cells, with the exception of the placenta. Only the morula’s cells are totipotent, with the ability to develop into every type of tissue and a placenta.
Pluripotent stem cells are those cells which can’t form extraembryonic structure but are able to form cells of all germ layers. Examples may include embryonic stem cells and induced pluripotent stem cells [8].
Multipotent stem cells have less differentiation spectrum than pluripotent stem cells, but these cells can specialize in different types of cells of specific lineages. Hematopoietic stem cell is an example of multipotent stem cell because various types of blood cells is produced by hematopoietic stem cell. HSC are turned into oligopotent stem cells after the process of differentiation.
Oligopotent stem cells are those types of stem cells which can generate only a few types of cells after differentiation, an example of such type of cells are myeloid stem cells or lymphoid.
Unipotent stem cells have the capacity to originate one type of cell, but what sets them apart from non-stem cells is their ability for selfrenewal. A germ line stem cell, which produces sperm, and an epidermal stem cell which produces only skin are examples of unipotent stem cells (Figure 4) [9].
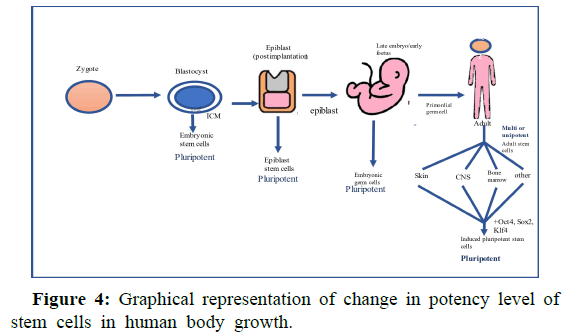
The range of potential cells in human organism includes unipotent cells of the skin, Central Nervous System (CNS), and bone marrow as well as pluripotent cells of the blastocyst.
Sources of stem cells
• Bone marrow.
• Placental tissues.
• Umbilical cord.
• Adipose tissue.
Bone marrow: Bone marrow is mainly found between the centers of the bones. Historically, bone marrow has been considered as the predominant harvesting site for the collection of the stem cells and is the denser source of stem cells compare to another because one can get 18 times more cell yield than other peripheral blood progenitor cell harvesting technique. But the procedure of harvesting stem cell from bone marrow is quite painful. The cells which is present within the bone marrow is known as Hematopoietic Stem Cells (HSCs) that provide a supply of the various types of blood cells which are eventually needed for the proper immunological and physiological functions [10].
Placental tissues: The type of cells present within the placental tissues are epithelial cells as well as stem cells which has the incredible ability to differentiate into different types of tissue including the pancreatic, hepatogenic, myogenic, pulmonary, cardiac, osteogenic, neurological, and endothelial. The special ability of placental cells is to differentiate into all these different tissues is due to lineages that come from various regions of the placenta, such as the hematopoietic cells that come from the chorion, yolk, and allantois [11].
Umbilical cord: The umbilical cord driven stem cells can be collected from different sites such as umbilical cord perivascular cells, umbilical cord blood, umbilical cord lining, umbilical cord endothelial cells, chorion, and amnion. Since 1998, the umbilical cord has been considered as a great source of hematopoietic stem cells and can be collected with lowest risk to the donor. Stem cells derived from umbilical cord are more frequently available as compared to other sources such as bone marrow driven stem cells because about 100 million people or even more rate of birth per year basis provides opportunity to get stem cells from the umbilical cord blood.
Stem cells derived from adipose tissues: These cells have been extracted from human fat, typically by a procedure named liposuction. The cell population which is derived from the adipose tissues mainly resembles the bone marrow derived mesenchymal stem cells in several respects. Adipose derives stem cells from humans that have been shown to differentiate in the lab into muscle, cartilage, fat, bone, and neurons. This makes them a potential source for use in the clinic in the future [11].
Stem cell therapy
Any disease or medical condition treated using stem cells is referred to as stem cell-based therapy [12]. Stem cell therapy is also known as regenerative medicine. The first bone marrow transplant took place in 1956, stem cells first played a part in contemporary regenerative medicine in the 1950's. This innovation clarified the future treatments may be made possible with further clinical procedure development and improvement, which paved the path for the stem cell therapies that are currently available. Stem cell therapies are increasingly recommended for a variety of clinical disorders outside of their core indications to cure genetic blood illnesses and have achieved notable success where other therapies have failed. Stem cells help to cure various diseases like cancer, retinal, dental, heart and injuries related to spinal cord. Induced pluripotent stem cells (compared to traditional sources, cells derived from somatic cells that have undergone programming factors to return to a pluripotent state can be harvested less invasively), a fast developing source of stem cells, are now applied in clinical settings [2].
Similarly embryonic stem cells have been utilized in the treatment of deafness. Li et al., stated that inner ear stem cells have the ability of differentiating into hair cell like cells stem cell treatment propose enormous potential in the field of organ transplantation and regeneration of tissue [13]. Both autologous, also known as “self to self” therapy, which uses the patient’s own cells, and allogenic sources, which use cells from a healthy donor, are acceptable sources of stem cells-based therapies. The current investigation performs a complete study concerning the use of stem cells in the medical field.
Hematopoietic stem cell transplantation
Hematopoietic stem cells are significant because they are by far the most extensively defined tissue specific stem cells after all, they have been experimentally investigated for more than 50 years. These stem cells have the potential to be used in regenerative medicine and seem to offer an appropriate paradigm model system to investigate tissue specific stem cells. The most common stem cell treatment now is multipotent hematopoietic stem cell transplantation. Target cells are often derived from the peripheral blood, umbilical cord, or bone marrow. The process might be autologous (using the patient’s own cells), syngeneic (using cells of identical twin) or allogenic (stem cell used from the donor). The production of all functional hematopoietic lineages in blood, such as erythrocytes, platelets and leukocytes, is carried out by HSCs. The transplantation of HSCs treats conditions like anemia and leukemia which is caused by inappropriate activities of the hematopoietic system. There are few limitations while we take conventional source of HSC under consideration. First off, there are only a certain number of transplantable cells available, and an effective method of obtaining them has not yet been developed. Finding a suitable antigen matched donor for transplantation is another issue, and conventional HSC transplants suffer from reduced effectiveness due to virus contamination or other immunoreactions. The highest immunological compatibility and highest likelihood of success come from using a patient’s own unspecialized somatic cells as stem cells [14].
Stem cell-based treatment: An overview of current clinical applications
Neurodegenerative disease: The origin of neural cells from stem cells in vitro paved the way for the current stem cell based trials aiming neurodegenerative diseases. These treatments focus on totally curing such problems rather than only slowing the progression of irreversible neurodegenerative diseases like Parkinson’s, Amyotrophic Lateral Sclerosis (ALS), Alzheimer’s and multiple sclerosis [15].
Parkinson’s Disease (PD): It is a neurological movement disorder predominantly brought on by the nigrostriatal dopaminergic system being damaged and degenerating. Several significant pathways have emerged as targets for prospective therapeutics because of improved understanding of the etiology and pathogenesis of Parkinson’s disease. With new genetic insights, there is possibility to use neuroprotective preventive treatments for people at risk of developing PD, thereby delaying the onset and progression of the disease. Strategy like conventional therapeutics for treating the symptomatic stage of PD remain in use. Researchers are looking into stem cells as potential replacement for sick neurons or aged tissues in addition to efforts to prevent and control symptoms of PD.
Amyotrophic Lateral Sclerosis (ALS): It is defined as motor neuron disease that progresses over time and causes destruction of upper and lower motor neurons at the spinal and bulbar levels. Stem cell therapy offers a novel method for treating diseases that were previously incurable. The federal drug administration (United State) has only approved riluzole and edaravone as treatments for ALS, and they only slow the diseases course and extend life by 3-6 months.
Alzheimer’s Disease (AD): Memory loss and cognitive impairment are hallmarks of Alzheimer’s disease, a progressive neurological illness. Due to the significant loss of neurons in the brains of AD patients, nearly all advanced clinical trials targeting certain pathways associated to the disease have failed to date. But with the development of stem cell technology and the differentiation of these cells into various types of neurons and glial cells of the central nervous system, stem cells now have improved features of self-renewal, proliferation, and recombination. Success of stem cell therapy has been proved in animal models with AD, stem cell treatment for AD has shown promise in recent preclinical research [16].
Multiple sclerosis: A progressive condition, multiple sclerosis is marked by increasing disability. More than 2 million people are said to be affected by multiple sclerosis globally, and it is thought to be the most prevalent non-traumatic cause of impairment among young adults (>50 years) adults in Europe [17]. Multiple sclerosis can be treated with the help of MSC therapy via the process of neuroprotection, neuroregeneration and immunomodulation. Due to an imbalance between the harmful immune response and the ineffective mechanism, damage to the central nervous system of MS patients may be lessened with the help of these qualities. The patient with multiple sclerosis having MSC injection improved clinical parameters and stopped the course of the disease without causing any negative side effects [18].
Chronic graft versus host disease: HSC or tissue transplantation have also been used to treat Chronic Graft Versus Host Disease (GVHD). Many body organs as well as the peripheral or central nervous systems may show symptoms of this extremely dangerous illness. With mixed results, at least ten clinical trials using MSC have been reported. However, many of them demonstrate a sizable degree of positive response. Patient recruitment in phase 3 trials for both newly diagnosed acute GVHD and steroid refractory acute GVHD has been completed by one business, Orisis [3].
Cardiovascular diseases: Around 17 million people die from cardiovascular disease each year, making it the leading cause of mortality worldwide. Research from the America heart association predicts that by 2030, there would be over 23 million cardiovascular deaths worldwide. In the United States, heart disease, stroke and other cardiovascular disorders claimed about 787,000 lives. Both embryonic and non-embryonic stem cell (adult stem cells) are the most crucial tool in regenerative medicine because these cells perhaps differentiating into cardiomyocytes. Therefore, it would be helpful to learn whether the differentiated cells can restore and improve heart function in a safe and efficient manner [19].
Stem cell based therapies for treatment of diabetes
In type I diabetes mellitus, pancreatic beta cells are destructed, because of disorders in the immune system and mesenchymal stem cell play important role in treating such disease but in type II diabetes the deficiency is due to the failure of the beta cell to generally produce insulin. In both cases the cells which is affected are beta cells. The type of stem cells that are used to replace the beta cell are pluripotent stem cells. In recent development researchers can persuade embryonic stem cells in laboratory to turn in to the beta cell.
Stem cell based treatment for spinal cord injury
Researchers and medical professionals working on spinal cord injuries are about to implement promising new experimental discoveries into patient therapy. The Christopher Reeve Paralysis Foundation (CRPF) funds research to treat or cure paralysis brought on by spinal cord injuries or other CNS illnesses. Advances in stem cell research have led to the development of several transplantation techniques that promote axonal regrowth and partial functional recovery in spinal cord injury [20,21].
Stem cell therapy for cancer
Brain cancer is hard to cure by using conventional methods of treatment. Human neural stem cells can be transplanted into the brain of rodents that have intracranial tumors [22]. After few days, the cells start moving into the area which is affected by cancer and cytosine demines (a type of enzyme that converts a non-toxic drug into a chemotherapeutic agent) are produced. The injected material reduced tumor bulk by 81% [23].
Vision impairment and blindness
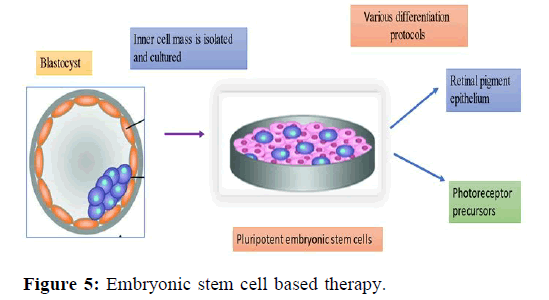
Corneal stem cells are transplanted into the defected eyes for vision restorement. Some individuals find it offensive that the team’s usage of sheets of retinal cells that were taken from aborted fetuses. The stem cells stimulate fresh healing and eventually restore once these sheets are implanted over the injured cornea (Figure 5).
The inner cell mass is isolated from the blastocyst and cultured. The pluripotent embryonic stem cells are then differentiated into retinal pigment epithelium, photoreceptor precursors or other cell types using various methods.
Stem cell treatment for Sjogren’s Syndrome (SS)
Sjogren’s syndrome is a type of systemic autoimmune disease that is marked by dry eyes and mouth. A therapeutic method for SS involving the infusion of MSCs in 24 patients was presented by Xu et al. The technique was based in the immunologic regulatory action of MSCs. After infusing mesenchymal stem cell in the patient, it begins to move towards the inflammatory location in a stromal cell derived factor-1 dependent manner. According to clinical experiment findings, the autoimmunity in SS patients decreased before salivary gland secretion was recovered.
Stem cells in dentistry
The isolation of stem cells from human teeth allowed researchers to observe the cells capacity to reconstruct dental tissues. Dental tissues such as the apical papilla, dental follicle, periodontal ligament have all been documented to successfully produce mesenchymal stem cells [24]. Dental stem cells have shown higher immunomodulation capacities, either through cell to cell communication or a paracrine effect. Stem cells from human exfoliated deciduous teeth are utilized in the formation of mineralized tissue, which are further used for the enhancement of the orofacial bone regeneration.
Stem cell treatment for COVID-19
The pathogenic bacteria known as Coronaviruses are known to cause colds as well as more serious illnesses including SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome). The global pandemic of the 2019 Coronavirus illness (COVID-19) has reached an emergency stage. The strain of Coronaviruses, that has never been discovered in human beings before is known as SARS-CoV-2. There is yet no specific, efficient medication for the COVID-19. But mesenchymal stem cell transplantation has been proven in numerous prior trials to be a viable treatment technique for COVID-19 infected patients. Mesenchymal stem cells are basically multipotent stromal cells which can heal injured epithelial cells and inhibit apoptosis. Several clinical studies have demonstrated that MSC therapy is a potentially viable treatment option for COVID-19 patients, particularly for those with acute respiratory distress syndrome, without the risk of toxicities or significant side effects. The effectiveness of this therapy will need to be confirmed in future research [25].
Mechanism of stem cell therapy for COVID-19
The most effective and potential cell based therapy for COVID-19 is mesenchymal stem cell therapy because they have significant immunoregulatory capabilities and can control both the adaptive immune system as well as the innate immune system. To control overactive immune responses and CSS, MSCs can release a variety of soluble substances by paracrine secretion, including nitric oxide, prostaglandin E2, in doleamine 2,3-dioxygenase, IL-10 and TGF-β. To control the balance and strength of the immune response, MSCs can also interact directly with a variety of immune cells, including lymphocytic T cells, B cells neutrophils, macrophages, and NK cells [26,27]. Additionally, studies have shown that while stem cells can be independent of interferon and can continuously active many antiviral genes, adult cells only produce interferon when the virus invades, which activates hundreds of genes that resist viral infection and draws in immune cells to resist the viral infection.
Mesenchymal stem cells derived from human umbilical cord
The first case for treating COVID- 19 patient with the help of hUCMSCs was reported by Alturi et al., who described the treatment of 65-years old COVID-19 patient from China using human umbilical cord mesenchymal stem cells, the patient received three doses of 50 million allogenic umbilical cord MSC spaced three days apart. Like this, Zhang et al., described the case of a 54 years old man who received hUC-MSC intravenously. Both patients received the same conventional treatment at the same time because they were in critical condition with severe pneumonia, shortness of breath, low oxygen saturation, respiratory failure and multi-organ failure that required mechanical ventilation and the frequent standard treatment were given to them.
In addition, Peng et al., described the case of a 66 years old woman who developed a fever, cough, and sore throat after interacting with a COVID-19 patient. On the 10th day Dr. came to know that she had SARS-CoV-2. Together with the UC-MSCs, convalescent plasma was administered to the patient, and certain clinical, laboratory and radiological data were tracked. After administering UC-MSC, no infusion or allergic reactions were noted. She tested negative after a few days, made full recovery, and was released from the hospital [28].
Bone marrow derived mesenchymal stem cells
Leng, et al., pioneering’s investigation on stem cell therapy for COVID-19 found that administering MSCs intravenously to seven COVID-19 patients improved their functional results and aided in rehabilitation. The Beijing you a hospital in China enrolled seven COVID-19 patients between January 23, 2020 and February 16, 2020 (1 critically severe, 4 severe and 2 common type). 1 × 106 MSCs per kilogram of body weight were infused intravenously into each patient. During two hours of transplantation, no immediate adverse or allergic infusion related symptoms were noticed. The patient initially came with a high fever, shortness of breath, weakness, and hypoxia. Yet 2-4 days following the transplant, all symptoms vanished, and each patient’s lung function had greatly improved.
Allogeneic adipose derived MSCs
Adipose derived MSCs were systemically delivered in a single center, double blind, randomized research by Zheng et al., to evaluate the feasibility and safety of doing so in ARDS patients. At least 18 years of age was required for the recruitment of 12 patients, and a diagnosis of hypoxia was obtained after 48 hours with a PAO2/FiO2 ratio of less than 200. Six patients were assigned to the MSC group and six to the placebo group. Patients in both groups received a single infusion of 1 × 106 cells/kg of cells or saline over the course of 60 minutes, with a follow-up period of 28 days. Except for 2 patients (1 had diarrhea and the other got a rash on the chest that went away in a week), there were no adverse drug reactions noted.
Ethical issues related to stem cell treatment: Moral status
Stem cells are distinctive in many respects, although they have several possible clinical advantages, as revealed by controlled clinical trials, there are also unanticipated risks associated with their use. However, several issues are brought up due to the biological characteristics of these cells and the results of their handling ex vivo. A significant portion of these are unique to their collection, processing, storage, and usage for clinical applications. It must be acknowledged that the donor has the exclusive right to learn about any information pertaining to his or her health and safety. It is essential to create efficient cell preservation techniques free of DMSO. As it is associated with negative side effects upon infusion as well as cell specific epigenetic effects, DMSO is not approved for use in human infusions. Even for cell types that have been successfully stored in the past, the necessity for developing alternative techniques of cell preservation will increase as the clinical usage of stem cells increases [29]. Embryonic stem cell research is ethically controversial because of the method utilized to generate novel embryonic stem cell lines involve the death of human embryos. This is the major disadvantage to the origin of new stem cell based treatments. According to some who think that life begins at conception, the blastocyst is a human life, and it is immoral and inappropriate to kill it [30]. First, the most crucial concern is completely understanding the process by which stem cells act initially in animal models [31,32]. It has been proposed that one of the most difficult ethical concerns now confronting the field of stem cell based therapy is the growing number of clinics offering unproven stem cell based treatments. So, it is the moral responsibility of researchers to make sure that success in clinical translation does not come at the expense of ethical issues [33-35]. To fully understand the potential of both adult and embryonic stem cells in the fascinating new sector, additional research is required.
Stem cell banking
A stem cell bank, also referred to as life bank, provides facilities where stem cells are prepared, collected, stored, provided, and used for research on a big scale. In 2004, the British government launched the first global stem cell bank [36-38]. The establishment of cord blood banks led to the beginning of the newborn stem cell banking more than 25 years ago. In the early 1990's, the successful demonstration that cord blood can reconstitute a patient’s blood and immune system, together with the confirmation that cord blood can be cryopreserved for later use, led to the formation of cord blood banks, and thus the newborn stem cell banking industry. About 800,000 cord blood units are thought to be cryopreserved in public banks, while over 5 million more are kept in private cord blood banks. During the last three decades, more than 40,000 hematopoietic stem cell transplants with the help of cord blood have been achieved [39]. The umbilical cord is meant to be the rich source of mesenchymal stem cells. These stem cells can be stored successfully with the help of cryopreservation technique and are used in various treatments to prevent the patient with life threatening diseases in the future. Stem cells of Human Exfoliated Deciduous (SHED) teeth has the capacity to originate into different types of tissues than other stem cells. For the isolation, collection, and storage of SHED the techniques are noninvasive and simple [40]. Yet, there is still much to be learned, such as how to prevent immune rejection and regulate the ideal in vivo settings for stem cell development, proliferation, and differentiation. The modernization and organized management of stem cell banks have expanded the range of applications for stem cells and given people many advantages (Table 1).
| Location | Facilitating organization | Number of banks |
|---|---|---|
| Gurugram | Cryobank international, Life cell international, Unistem biosciences, Totipotent RX cell therapy. | 4 |
| Karnatka and Gujrat | Narayana Hrudayalaya, Cryo save India, Stem cyte India, Best well care management services. | 4 |
| Maharashtra | Reliance life sciences, Ree laboratories, Regenerative medical services. | 3 |
| Tamil Nadu, Andhra Pradesh, West Bengal | Life cell international, Path care labs, Cord life sciences. | 3 |
Table 1: Cord blood banks in India.
Stem cell therapy market
Since bone marrow transplantation was made possible in 1968, stem cells have been applied in medicine. Cancer and hereditary blood abnormalities are both treated with bone marrow transplantation. Peripheral and umbilical cord blood are now more frequently used as sources of hematopoietic stem cells than bone marrow. Annually 60,000 procedures are carried out, with about 35,000 utilizing autologous HSCs and roughly 25,000 utilizing allogeneic HSCs.
Risks of stem cell therapy
Many factors influence the long term outcome of stem cell based treatments, including improvements in the survival, engraftment, regeneration, and proliferation of transplanted cells. The survival and therapeutic efficacy of stem cell therapy are critically dependent on the epigenetic and genomic integrity of cell lines that have undergone in vitro manipulation before transplantation. However, the major concern following by post transplantation is that stem cells have a high ability for replication, immunological rejection of donor cells by the host immune system [41]. According to recent research, the majority of donor cell death takes place in the hours to days following transplantation, which reduces the effectiveness and therapeutic potential of stem cell based treatment. Stem cell transplantation may also lead to the huge risk for example, after bone marrow transplant (an operation to replace the old bone marrow with healthy bone marrow stem cell, also known as hematopoietic stem cell transplant, or simply a stem cell transplant which helps to treat specific cancers and other bone marrow related illnesses) there is high risk of complications such as infections, anemia, infertility, and organ damage (Table 2).
| Year | Corporation | Stem cell type | Clinical application | Name of bank |
|---|---|---|---|---|
| 2012.01 | Cuepistem/Anterogen | Mesenchymal stem cells from autologous fat. | Crohn’s disease complicating and fistula. | Food and drug administration in South Korea. |
| 2012.01 | Cartistem/Medi-post | Mesenchymal stem cells derived from cord blood. | Degenerative arthritis and cartilage damage in the knee joint. | Food and drug administration in South Korea. |
| 2011.11 | Multistem/Athersys Inc, USA | Allogenic bone marrow stem cell. | Type I mucopolysaccharidosis. | American food and drug administration. |
| 2011.11 | Hemacord/New York blood center | Cord blood hematopoietic progenitor cells. | Acquired hematopoietic disease or hereditary. | Biological license of American Food and Drug Administration (FDA). |
| 2011.07 | Hearticellgram-AMI/FCB-Pharmicell | Autologous bone marrow mesenchymal stem cells. | Acute myocardial infarction. | Food and drug administration in South Korea. |
| 2010.1 | MPC/Mesoblast | Autologous mesenchymal precursor cells. | Bone reconstruction. | Production permit of Therapeutic Goods Administration (TGA) in Australia. |
| 2009.12 | Prochymal/Osiris Therapeutics, Inc., USA | Mesenchymal stem cells from human allogenic bone marrow. | Graft Versus Host Disease (GVHD) and Crohn’s diseases. | American food and drug administration. |
Table 2: Internationally approved stem cell drugs.
Conclusion
Recent developments in stem cell technology present a new path for patients with illnesses and problems that have not yet been treated. Clinical experiments using stem cells have changed the course of the developing field in recent years. The opportunity to create cutting edge stem cell based medicines in this quickly expanding field is highly alluring. Stem cell research has potential to result in a variety of interesting discoveries, such as illness treatments and cures. The proliferation and differentiation of stem cells are influenced by a variety of physiological and mechanical stimuli. With stem cells, controversy is a major problem that probably won’t go away. There must be agreement between people in favor of and against stem cell research. Before stem cell treatment may be successfully employed to cure diseases, more research must be done.
Acknowledgments
The authors sincerely acknowledge the department of biotechnology in college of horticulture and forestry Neri, Hamirpur for providing the necessary instructions related to this study.
Conflict of Interest
It has been stated by the authors that they have no conflict of interest regarding the study.
Funding
None.
References
- Jagiri AG, Gotte P, Singireddy S, Kadarla RK (2019) Stem cell therapy-An overview. Asian J Pharma Res Dev 7:92-102.
- Poliwoda S, Noor N, Downs E, Schaaf A, Cantwell A, et al. (2022) Stem cells: A comprehensive review of origins and emerging clinical roles in medical practice. Orthop Rev 14:01-09.
[Crossref] [Google Scholar] [PubMed]
- Devkate GV, Tupe A, Bhujbal A (2016) Stem cell: A review. Int J Pharmacy Pharm Res 8:1.
- Agius CM, Blundell R (2007) What are stem cells? Review paper. Int J Mol Med 3:45-50.
- Fan BS, Liu Y, Zhang JY, Chen YR, Yang M, et al. (2021) Principles for establishment of stem cell bank and its application on management of sports injuries. Stem Cell Res Ther 12:307.
[Crossref] [Google Scholar] [PubMed]
- Hoang DM, Pham PT, Bach TQ, Ngo AT, Nguyen Q T, et al. (2022) Stem cell based therapy for human diseases. Signal Transduct Target Ther 7:271.
[Crossref] [Google Scholar] [PubMed]
- Bezie M, Mesfin Y, Biyazen H (2016) Stem cell biology and its role in regenerative medicine: A concept shaping the future of medicine. J Regen Med 5:1.
- Bonthagarala B (2016) Stem cell: Past, present and future-a review article. Int J Exp Pharmacol 3:11-20.
- Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z (2019) Stem cells: Past, present and future. Stem Cell Res Ther 10:68.
[Crossref] [Google Scholar] [PubMed]
- Baltimore MB, Derseh HB, Akalu Y (2017) Stem cell biology and its role in regenerative medicine: A concept shaping. Regen Med 5:1-8.
- Avasthi S, Srivastava RN, Singh A, Srivastava M (2008) Stem cell: Past, present and future-a review article. Internet J Medic Update 3:22-30.
- Bansal R, Jain A (2015) Current review on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med 6:29-34.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Belmonte JCI (2016) Stem cells: A renaissance in human biology research. Lead Edge 16:72-85.
[Crossref] [Google Scholar] [PubMed]
- Klaver-Flores S, Zittersteijn HA, Cante-Barrett K, Lankester A, Hoeben RC, et al. (2022) Genomic engineering in human hematopoietic stem cells: Hype or hope. Front Genome Edit 2:1-9.
[Crossref] [Google Scholar] [PubMed]
- Aly RM (2020) Current state of stem cell-based therapies: An overview. Stem Cell Investig 7:8.
[Crossref] [Google Scholar] [PubMed]
- Liu XY, Yang LP, Zhao L (2020) Stem cell therapy for alzheimer’s disease. World J Stem Cells 12:787-802.
[Crossref] [Google Scholar] [PubMed]
- Mahajan PV, Kumar AA, Subramanian S (2016) Role of autologous stem cell therapy in relapsing multiple sclerosis. J Dent Med Sci 15:27-30.
- Causcut FX, Hutton GJ (2019) Stem cell based therapies for multiple sclerosis: Current perspectives. Biomedicines 7:1-26.
[Crossref] [Google Scholar] [PubMed]
- Terashvili Maia, Bosnjak ZJ (2019) Stem cell therapies in cardiovascular disease. J Cardio Vas Anesthes 33:209-222.
[Crossref] [Google Scholar] [PubMed]
- Dalkara D, Goureau O, Marazova K, Sahel JA (2015) Let there be light: Gene and cell therapy for blindness. Hum Gene Ther 27:34-47.
[Crossref] [Google Scholar] [PubMed]
- Sengupta S, Kizhakedathil MPJ, Sankar D (2018) Stem cells-regulation and applications: A brief review. Res J Pharm Technol 10:35-40.
- Sagar J, Chaib B, Sales K, Winslet M, Seifalian A (2017) Role of stem cell in cancer therapy and cancer stem cells: A review. Cancer Cell Int 7:01-11.
[Crossref] [Google Scholar] [PubMed]
- Chu DT, Nguyen TT, Tien NLB, Tran DK (2020) Recent progress of stem cell therapy in cancer treatment: Molecular mechanisms and potential applications. PMC. 9:1-13.
[Crossref] [Google Scholar] [PubMed]
- Bhat M, Shetty P, Shetty S, Khan FA, Rahman S, et al (2019) Stem cells and their application in dentistry: A review. J Pharm Bioallied Sci 11:82-84.
[Crossref]
- Zheng ZX (2021) Stem cell therapy: A promising treatment for COVID-19. World J Clin Cases 9:48-55.
[Google Scholar] [Crossref] [PubMed]
- Li S, Zhu H, Zhao M, Liu W, Wang L, et al. (2022) When stem cells meet COVID-19: Recent advances, challenges and future perspectives. Stem Cell Res Ther 13:9.
[Crossref] [Google Scholar] [PubMed]
- Arabpour E, Khosdel S, Tabatabaie N, Akhgarzad A, Zangiabadian M, et al. (2021) Stem cells therapy for COVID-19: A systematic review and meta-analysis. Front Med 8:73590.
[Crossref] [Google Scholar] [PubMed]
- Mahendiratta S, Bansal S, Sarma P, Kumar H, Choudhary G, et al. (2021) Stem cell therapy in COVID-19: Pooled evidence from SARS-CoV-2, SARS-CoV, MERS-CoV and ARDS: A systematic review. Biomed Pharmaco Ther 111300.
[Crossref] [Google Scholar] [PubMed]
- Hanna J, Hubel A (2009) Preservation of stem cells. Organ 5:34-37.
[Crossref] [Google Scholar] [PubMed]
- King NMP, Perrin J (2014) Ethical issues in stem cell research and therapy. Stem Cell Res Ther 5:85.
[Crossref] [Google Scholar] [PubMed]
- Verma V, Tabassum N, Yadav CB, Kumar M, Singh AK, et al. (2016) Cord blood banking: An Indian perspective. Cell Mol Biol 62:65-68.
- Leventhal A, Chen G, Nergo A, Boehm M (2012) The benefits and risks of stem cell technology. Oral Dis 18:17-22.
[Crossref] [Google Scholar] [PubMed]
- Golchin A, Seyedjafari, Ardeshirylajimi (2020) Mesenchymal stem cell therapy for COVID-19: Present or future. Stem cells Rev Rep 16: 427-433.
[Crossref] [Google Scholar] [PubMed]
- Jose N (2017) Stem cell therapy. Int J Immunol Nursing. 32-44.
- Ebrahimi A, Ahmadi H, Ghasrodashti ZP, Tanide N, Shahriarirad R, et al. (2021) Therapeutic effects of stem cells in different body systems, a novel method that is yet to gain trust. Transl Clin Res 6:672-700.
[Crossref] [Google Scholar] [PubMed]
- Kwak KA, Lee SP, Yang JY, Park YS (2018) Current perspectives regarding stem cell based therapy for Alzheimer’s disease. Stem Cell Int 1-14.
[Crossref] [Google Scholar] [PubMed]
- Liu D, Zheng W, Pan S, Liu Z (2020) Concise review: Current trends on applications of stem cells in diabetic nephropathy. Cell Death Differ 11: 01-14.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Cheung HH (2020) Stem cell based therapies for Parkinson disease. Int J Mol Sci 21:8060.
[Crossref] [Google Scholar] [PubMed]
- Brown KS, Mahendra SR, Brown HL (2019) The future of state of newborn stem cell banking. J Clin Med 8:117.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Jaganathan BG (2021) Stem cell therapy for retinal degeneration: The evidence to date. Biol: Targets Ther 15:299-306.
[Crossref] [Google Scholar] [PubMed]
- Debich M, Bill T (2022) Biotechnology and stem cell technology overview. J Appl Biotech Bioeng. 9:57-60.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi