Research Article, J Spine Neurosurg Vol: 12 Issue: 2
Single Stage Revision of Infected Posterior Lumbar Fixation Using Nano-Silver Coated Pedicle Screws and Rod System
Abdelrady Mahmoud and Ahmed Nageeb Mahmoud*
Department of Orthopedic Surgery, Ain Shams University Hospital for Surgery, Cairo, Egypt
*Corresponding Author: Ahmed Nageeb Mahmoud
Department of Orthopedic Surgery, Ain Shams University Hospital for Surgery, Cairo, Egypt
Tel: +201006281260
E-mail: Anmahmoud@med.asu.edu.eg
Received date: 28 November, 2022, Manuscript No. JSNS-22-81445;
Editor assigned date: 01 December, 2022, PreQC No. JSNS-22-81445 (PQ);
Reviewed date: 15 December, 2022, QC No. JSNS-22-81445;
Revised date: 13 April, 2023, Manuscript No. JSNS-22-81445 (R);
Published date: 20 April, 2023, DOI: 10.4172/2325-9701.1000149
Citation: Mahmoud A, Mahmoud AN (2023) Single Stage Revision of Infected Posterior Lumbar Fixation Using Nano-Silver Coated Pedicle Screws and Rod System. J Spine Neurosurg 12:2.
Abstract
Objectives: Silver coated implants have been used in human due to its known antimicrobial properties and biocompatibility. With scarcity of similar studies in the spine, the purpose of this study is to evaluate the early clinical and radiological outcomes of nano-silver coated titanium pedicle screw system in single stage exchange of infected posterior spinal fixation.
Methods: This is a retrospective study of sixteen consecutive cases who had an established posterior spinal infected fixation and were managed with surgical debridement and single stage revision fixation using Silver coated titanium screws and rods system. Patients were 9 males and 7 females with a mean age of 45 years (range 23 years-74 years)
Results: After a mean follow up of 19 months (range 14 months-25 months), 14 cases (87.5%) had successful revisions with resolution of infection and stable spinal fixation. One case had transient neurological deficit and two other cases had persistent wound infection that necessitated two stage revision surgery. The mean VAS score for pain has improved from 7.18 ± 1.83 preoperatively to 2.62 ± 2.8 at final follow up.
Conclusion: Single stage revision of infected spinal fixation using silver coated screws system along with debridement may be a viable option. Comparative studies, with larger patient number and longer follow up are required to properly assess the longer-term efficacy and cost benefit.
Keywords: Infection; Lumbar; Spine; Fixation; Silver; Pedicle; Screws
Introduction
Despite the recent surgical techniques and modern implants, postoperative spine implant infection remains one of the most common complications [1]. This high incidence is associated with the increased use of implants in spine surgery, and it is reported to reach 6%-18% when implants were used despite the use of antibiotics. Staphylococcus aureus remains the most common causative organism [2].
Some attempts have been tried to diminish the infection risk by improving the implant technology and biomechanical models. Of these advances, owing to the antimicrobial characteristics of silver that have been experimentally proven, and after successful applications in dentistry and cardiac implants silver coated implants have extensively been used in orthopedics with reported safety and efficacy to diminish the incidence or manage postoperative implant related infections [3]. In the spine field, only one study reported the use of silver components in primary cases of spinal fixation with good outcomes as regard to absence of infection in 50 patients. The aim of this retrospective study is to report the results of single stage revision for infected posterior lumbar spine fixation using nano-particle silver coated spinal fixation system, all performed by the same surgeons at a single institution. We have postulated that the use of silver coated pedicle screws may be also a viable option in revision cases for infected spinal fixation cases, which has not been reported before in any previous study [4].
Materials and Methods
Institutional review board approval was obtained before commencing this study to review the available databases for included patients. All the patients have signed informed consents before the surgery. After reviewing existing databases, the data of sixteen patients, been 9 males and 7 females has been extracted and included in this study. Patients’ mean age was 45 years (range 23 years-74 years) at time of presentation. The mean time since fixation surgery was 4.8 months (range 2.7 months-12.5 months) at presentation. Primary posterior lumbar fixation surgery performed for trauma in 11 cases, and for degenerative spine conditions in 5 cases.
Six patients gave a history of persistent wound discharge after the first surgery that healed and reappeared as a sinus at the time of presentation while the remaining 10 patients had reported no wound complications after the primary surgery. Seven patients had already performed at least one unsuccessful surgical debridement before the reference surgery and all of them were on antibiotics with no improvement. Causative organisms have been isolated from seven cases before the reference surgery (gram negative Bacillus were isolated from 3 cases, MRSA from 2 patients, Acinetobacter in 1 case and 1 patient had combined organisms). Cases presented with either persistent wound discharge, wound inflammation or dehiscence, sinus tract or exposed implants along with pain and limitation of ambulation. All the cases have elevated preoperative ESR and CRP at presentation, with mean values of 85 and 50 respectively. Two cases had Frankel grade D neurological deficits at presentation. Clinical outcomes were evaluated using the Visual Analog Scale (VAS) score for pain.
Decision of implants revision was due to chronicity of infection, obvious radiological implant loosening and/or failure of eradication of infection despite previous debridement. Pre-operative radiographs and MRI were routinely performed in all cases and CT was done in all trauma cases to assess radiological union. Before surgery, antibiotics have stopped for at least 72 hours. All the cases have been (re)revised using the Norm Silver® nano-silver coated titanium spinal system (Norm Tubitak, Ankara, Turkey). After incision, excision of the previous scar tissue and removal of the old implants, thorough debridement with at least 2 liters of saline is performed. Pedicle walls were curetted and irrigated carefully. Any persistent bone graft was removed and replaced with newly harvested graft if needed (one case). Multiple fluid and soft tissue biopsies were obtained and sent for microbial culture and sensitivity. The retrieved implants sizes were detected after removal and a new sterile surgical set were used, including surgical gloves and gowns, before inserting the new Silver coated implants with larger diameters by 1 mm-1.5 mm and 5 mm-10 mm lengths under C-arm control to verify the screws position. Extension of fixation was required in thirteen cases due to loosening of fixation in the upper or lower segment. Surgical drains were inserted in all cases.
No intraoperative complications have occurred, and patients were discharged after 7 days-18 days postoperative. Follow up, clinically and with ESR and CRP was done every 2 weeks for 3 months then every 3 months. Serial kidney and liver functions were obtained every three months postoperative, and serum Ag level was performed only in 9 cases at 6 months postoperative due to unfeasibility of the test, and all values were less than 1 ng/ml. As reported by the implant manufacturer, the maximal total amount of silver coating in the norm implants is 2 mg-5 mg, which much lower than the allowed limits. Tissue cultures taken intra-operative were positive in 12 patients (4 MRSA, 3 Klebsiella, 1 A cinetobacter, 2 E. coli and 2 mixed organisms). Post operative antibiotics were administered for 8 weeks-18 weeks, depending on the serial ESR and CRP values.
Results
After a mean follow up of 19 months (range 14 months-27 months), Fourteen patients (87.5%) had stable implants with resolution of surgical wound infections. ESR and CRP returned to normal values at an average duration of 34.5 days (range 23-52) after surgery. Two patients had preoperative neurological deficits and all of them had recovered to Frankel, et al. at three months clinical follow up. One another case had postoperative right leg pain that persisted for 10 weeks postoperatively before complete resolution. The fourteen successful cases had resolution of pain and wound swelling after three months and all of them had stable implants at the final follow up with no signs of implant loosening or failure (Figures 1 and 2). Two patients (12.5%) had persistent wound discharge and elevation of ESR and CRP and both underwent an unsuccessful debridement before implants were taken out for two stage revision Figure 3. The mean VAS score for pain for all patients, including the complicated two cases has improved from 7.18 ± 1.83 preoperatively to 2.62 ± 2.8 at final follow up.
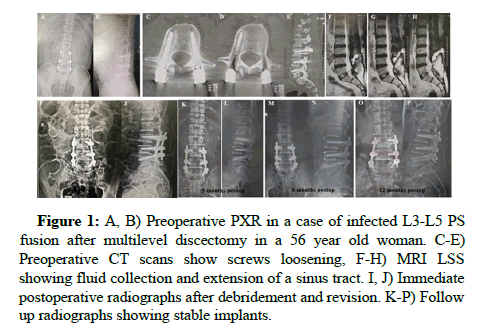
Figure 1: A, B) Preoperative PXR in a case of infected L3-L5 PS fusion after multilevel discectomy in a 56 year old woman. C-E) Preoperative CT scans show screws loosening, F-H) MRI LSS showing fluid collection and extension of a sinus tract. I, J) Immediate postoperative radiographs after debridement and revision. K-P) Follow up radiographs showing stable implants.
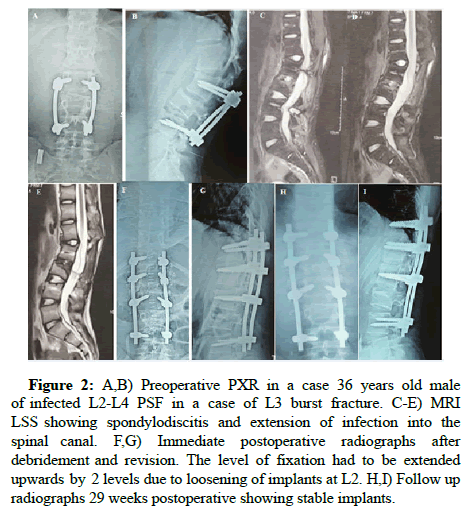
Figure 2: A,B) Preoperative PXR in a case 36 years old male of infected L2-L4 PSF in a case of L3 burst fracture. C-E) MRI LSS showing spondylodiscitis and extension of infection into the spinal canal. F,G) Immediate postoperative radiographs after debridement and revision. The level of fixation had to be extended upwards by 2 levels due to loosening of implants at L2. H,I) Follow up radiographs 29 weeks postoperative showing stable implants.
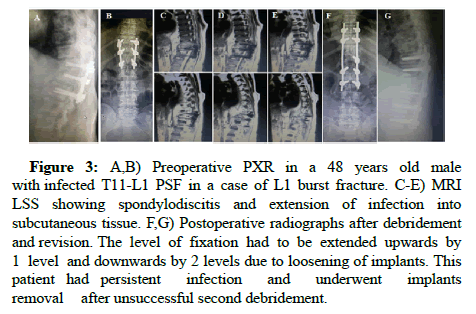
Figure 3: A,B) Preoperative PXR in a 48 years old male with infected T11-L1 PSF in a case of L1 burst fracture. C-E) MRI LSS showing spondylodiscitis and extension of infection into subcutaneous tissue. F,G) Postoperative radiographs after debridement and revision. The level of fixation had to be extended upwards by 1 level and downwards by 2 levels due to loosening of implants. This patient had persistent infection and underwent implants removal after unsuccessful second debridement.
Discussion
This retrospective study reports the results of silver coated implants in revision posterior lumbar fixation for infection. After a mean follow up of 19 months, fourteen cases (87.5%) showed successful eradication of infection with stable implants while two cases necessitated two stage revision.
Implant related infections occurring after instrumental spinal surgery are among the most difficult problems for which there remains no proven solution. Antibiotic treatment alone is not sufficient in nearly 50% of the patients, and a revision surgical procedure is inevitable. The formation of bacterial biofilm is the keystone of microbial resistance to antibiotics, and it is responsible of attachment of microorganisms to implant surfaces. Biofilm matrix, composed of polysaccharides and proteins protects the bacteria from various defence systems against immunity and antibiotics and allows for bacterial proliferation and colonization [5]. Due to the high ability of microorganisms to adhere to the implants passive surface, biofilm formation flourishes in presence of implants and, when formed, the minimal antibiotic concentration needed to inhibit bacteria has to be increased by about 50 times-500 times to inhibit bacterial proliferation in presence of biofilm [6].
Silver coated implants, by eluding silver ions have been known to have bacteriostatic and antifungal properties by various mechanisms through inactivation of sulfhydryl enzymes [7]. Ag ions affect the permeability of cell membrane, stop mitosis in prokaryotes, decrease the ability of proteolytic bacteria to replicate by directly binding bacterial DNA and RNA. Due to these characteristics, there has been increasing interest to use silver coated implants in surgery and highlight their results particularly as regard to decreasing formation of biofilm and reducing the incidence of postoperative infections.
In vitro laboratory or animal based studies have mostly shown successful outcomes in decreasing biofilm formation. Several laboratory studies showed also that silver coating, whether combined with Hydroxyapatite coating or not, has significant antibacterial properties against many organisms as Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Methicillin Resistant Staphylococcus aureus (MRSA), with increased efficacy when combined with vancomycin in vitro [8]. It may worth mentioning, too that other in vitro study has not shown significant advantage of silver coated implants in reduction of bacterial colonization.
As regard results in human in vivo studies, several clinical or commercial reports were published about the use of silver or silver HA coated implants. In orthopedics, particularly in upper and lower limbs tumor mega prostheses or hip protheses, many non-comparative studies or case series claimed its success in the management of infected cases or in reduction of infection risk in primary cases [9]. Despite that, the results in non-orthopedic clinical applications such as urinary catheters or cardiac implant or topical uses have been highly diverse and controversial while being of higher evidence than that reported orthopedic implants. Many studies reported the efficacy of silver treated implants to manage or decrease infections [10], while other studies did not find significant difference, or even reported increased the incidence of bacterial proliferation [11]. This should raise the interest to run a randomised controlled study testing the outcomes of silver coated implants in orthopedic field.
In spine surgery, on the other hand, the only available published clinical reports were of Secinti, et al., Morimoto, et al., and Secinti, et al. studied the use of silver coated implants in 50 spine patients regarding their efficacy in inhibiting infections and safety on human tissues. After 1 year follow up, none of their patients developed postoperative surgical implant infection and the detected serum silver ion concentration was normal and there were no effects on liver and/ or kidney functions. Morimoto, et al. reported an undergoing clinical trial that studies a recently developed and commercialized silver-HA coated interbody fusion cage yet there were no available clinical data yet. To our knowledge, our study is the first to use silver coated implants in revision of infected spinal fixation cases and to report the concept of single stage revision fixation in spinal fixation infection in general [12].
As regard to the safety of using silver coated implants in humans, several studies were published and set a maximum limit of the totally used silver or the serum silver levels. Based on several previous studies, Secinti, et al., reported a maximum serum Ag level to be 5 mg/L. Several studies reported the use of total silver amounts ranging from 0.1 mg to 2.89 gm # without any risk of Argyria, elevation of serum Ag or occurrence of kidney or liver problems. The mount of silver that is being used in teeth filling amalgam may reach 2.6 gm for example. In our study, the maximal silver load in our used system is 2-5, as reported by the manufacturer. Even if all this amount was absorbed by the tissues, it would be safe on organ functions. This was partially confirmed by absence of affection of liver or kidney functions in our patients and the low serum Ag in tested patients. As for orthopedic biocompatibility, silver has been shown to be biocompatible with fibroblasts and osteoblasts, which rationalize its successful use in orthopedic implants. Considering the results shown, the use of silver coated implants may be a viable option to decrease the incidence of infection in primary cases and decrease the need for two staged revisions in infected cases [13-18].
This study has several limitations. First, being nonrandomized nor controlled. Second, the few cases and the short follow up. Ideally a randomised controlled study is needed to properly assess the outcomes and the long-term results regarding anti-infective properties and the biomechanical integrity. Third, we did not measure the postoperative levels of serum silver in all patients due to test unfeasibility, depending on the reported safety studies. Moreover, many confounding factors exist and could contribute to the outcomes, including causative organisms’ virulence and patients’ comorbidities. In light with these several limitations, the results of this study should be considered with caution, when running necessary further research [19,20].
Conclusion
Single stage revision of infected spinal instrumentation using silver coated implants may be considered as a viable option to decrease the need for two stage revision and hence improve morbidity and mortality. Further research is required to prove the clinical efficacy and the cost benefit.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval
IRB approval was obtained before commencing this study.
References
- Hazer DB, Ayhan S, Palaoglu S (2015) Neurosurgical approaches to spinal infections. Neuroimaging Clin N Am 25:295-308.
[Crossref] [Google Scholar] [PubMed]
- Kasliwal MK, Tan LA, Traynelis VC (2013) Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int 29:392-403.
[Crossref] [Google Scholar] [PubMed]
- Dowdell J, Brochin R, Kim J, Overley S, Oren J, et al. (2018) Postoperative spine infection: Diagnosis and management. Global Spine J 8:37S-43S.
[Crossref] [Google Scholar] [PubMed]
- Ge L, Li Q, Wang M, Ouyang J, Li X, et al. (2014) Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int J Nanomedicine 16:2399-2407.
[Crossref] [Google Scholar] [PubMed]
- Markowska K, Grudniak AM, Wolska KI (2013) Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol 60:523-530.
[Crossref] [Google Scholar] [PubMed]
- Rai M, Kon K, Ingle A, Duran N, Galdiero S, et al. (2014) Broad spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl Microbiol Biotechnol 98:1951-1961.
[Crossref] [Google Scholar] [PubMed]
- Hazer DB, Mut M, Dincer N, et al. (2012) The efficacy of silver embedded polypropylene-grafted polyethylene glycol coated ventricular catheters on prevention of shunt catheter infection in rats. Childs Nerv Syst 28:839-846.
[Crossref] [Google Scholar] [PubMed]
- Nair LS, Laurencin CT (2008) Nanofibers and nanoparticles for orthopaedic surgery applications. J Bone Joint Surg Am 90:128-131.
[Crossref] [Google Scholar] [PubMed]
- Secinti KD, O zalp H, Attar A, Sargon MF (2011) Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. J Clin Neurosci 18:391-395.
[Crossref] [Google Scholar] [PubMed]
- Kalayci OA. Comert FB, Hazer B (2010) Synthesis, characterization, and antibacterial activity of metal nanoparticles embedded into amphiphilic comb-type graft copolymers. Polym Bull 65:215-226.
- Drasch G, Gath HJ, Heissler E, Schupp I, Roider G (1995) Silver concentrations in human tissues and their dependence on dental amalgam and other factors. J Trace Elem Med Biol. 9:82-87.
[Crossref] [Google Scholar] [PubMed]
- Seçinti KD, Attar A, Seçinti E (2016) Clinical trial using a silver coated screw rod system and one-year follow-up of the first 50 patient. J Nerv Sys Surg 6:10-21.
- Morimoto T, Hirata H, Eto S, Hashimoto A, Kii S, et al. (2022) Development of silver containing hydroxyapatite-coated antimicrobial implants for orthopaedic and spinal surgery. Medicina 58:519.
[Crossref] [Google Scholar] [PubMed]
- Anvar H, Dasgupta MK, Costerton JW (1990) Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother 34:2043-2046.
[Crossref] [Google Scholar] [PubMed]
- Gristina AG (1987) Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 237:1588-1595.
[Crossref] [Google Scholar] [PubMed]
- Secinti KD, Ozgural O, Tuna H, Attar A (2009) Antibacterial and antifungal effects of weak direct current and silver ions. Turkiye Klinikleri Tip Bilimleri Dergisi 29:577-583.
- Spadaro JA (1987) Silver anode inhibition of bacteria. In first international conference on gold and silver in medicine. 245-260.
- Modak SM, Fox CL (1973) Binding of silver sulfadiazine to the cellular components of Pseudomonas aeruginosa. Biochem Pharmacol 22:2391-2404.
[Crossref] [Google Scholar] [PubMed]
- Bragg PD, Rainnie DJ (1974) The effect of silver ions on the respiratory chain of Escherichia coli. Can J Microbiol 20:883-889.
[Crossref] [Google Scholar] [PubMed]
- Aydin M, Serin MS, Pelit A, Gunay I (1997) Silver anode induced phenotypical changes in bacteria. Ann Med Sci 6:83-87.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 
