Review Article, J Clin Exp Oncol Vol: 7 Issue: 4
Screening of Monoclonal Antibodies for Cancer Treatment
Peifeng Tang1,2, Shaoyan Liang1, Jianlin Xu3, Shaoxiong Wang1, Lijun Wang4 and Shijie Liu2*
1Department of Process Development, Mab-Venture Biopharm Co. Ltd., Shanghai, China
2Department of Paper and Bioprocess Engineering, State University of New York-College of Environmental Science and Forestry, Syracuse, New York, USA
3Biologics Process Development, Global Manufacturing and Supply, Bristol- Myers Squibb Company, Devens, Massachusetts, USA
4College of Light Industry and Food Engineering, Guangxi University, Nanning, China
*Corresponding Author : Shijie Liu
Department of Paper and Bioprocess Engineering, State University of New York-College of Environmental Science and Forestry, Syracuse, New York, USA
Tel: (315) 470-6885/470-6501
E-mail: sliu@esf.edu; petang@syr.edu
Received: August 01, 2017 Accepted: January 23, 2018 Published: January 31, 2018
Citation: Tang P, Liang S, Xu J, Wang S, Wang L, et al. (2018) Screening of Monoclonal Antibodies for Cancer Treatment. J Clin Exp Oncol 7:4. doi: 10.4172/2324-9110.1000225
Abstract
With the rapid development of cancer treatment using monoclonal antibodies (mAbs), the screening process of suitable biologics and indications attracts much attention. A general definition of ‘screening’ in the biopharmaceutical industry includes three aspects: the appropriate biologics for the specific cancers, the appropriate indications for the specific biologics and the promising biologic candidates from the pool at the pre-clinical drug discovery stage. Effective screening strategies in the biopharmaceutical industry are crucial to accelerate the drug commercialization process and select the effective biologics for patients. The current status of commercial mAbs and the global pharmaceutical market was briefly reviewed. The mechanism of commercial mAbs and the indications, as well as the current technologies for mAbs screening in the new drug discovery and cell line development stages were systematically reviewed, with an aim as a beneficial reference for screening highquality mAbs, appropriate indications with efficient technologies.
Keywords: Screening; Cancer treatment; Biomarker; Monoclonal antibodies
Abbreviations
ADC: Antibody Drug Conjugate; BLA: Biologic License Application; CMC: Chemistry Manufacturing and Control; ELISA: Enzyme-Linked Immunosorbent Assay; FMAT: Fluorometric Microvolume Assay; FACS: Fluorescence-Activated Cell Sorting; MAb: Monoclonal Antibody; M-M: Michaelis-Menten; MWC: Monod-Wyman-Changeux; QbD: Quality by Design
Introduction
Cancer is the global leading cause of death [1]. It is featured as unregulated cell division and growth [2]. Caused by genic mutation or gene expression disorder, abnormal metabolism can be observed within cells [3]. While gene therapy is still away from well accepted by FDA, monoclonal antibodies (mAbs), as one of the major parts of biologics, are currently widely recognized drugs for conservative cancer treatment. The mAbs are antibodies made by identical cells, which are all derived from a unique parent immune cell [4,5]. The history of mAbs can be traced back to 1975 when recombinant DNA technology was applied to antibody design [6]. The first mAb approved by FDA was OKT3 in 1986 [7] though it took almost three decades to the current ‘golden age’ of cancer therapies using mAbs [8]. Currently, around one hundred commercial mAbs are available in the global market.
The biopharmaceutical industry is regarded, as a matter of fact, a high risk and high revenue industry. On average, it takes $1.2 to $4 billion and 10 to 12 years for a biologic candidate to be approved and enter the market from the discovery stage. From the risk point of view, less than 0.1 % of the biologic candidates before CMC stages are able to enter into Phase I. Among those biologics, 60 % fail to pass Phase Ⅱ, while there’s another 50% failure risk at Phase Ⅲ the clinical stage. In addition, there are significantly higher risks at earlier new drug discovery stages. Thus, the efficient and successful screening of mAb candidates and corresponding indications is crucial. The word “screening” in the biopharmaceutical industry refers to three aspects:
Screening of drug or biologic candidates for specific diseases;
Screening the potential indications for specific drugs or biologics; and
Screening of promising drug or biologic candidates from the pool in the pre-clinical stage.
Undoubtedly, all the aspects are definitive for the destiny of one biologic candidate.
In this work, the current commercial mAbs and the recognized biomarkers were systematically reviewed. The principles, criteria, modeling and detection methods of biologics screening were presented and compared. This review aims at providing comprehensive screening information for cancer treatment, which is potentially beneficial for research institutes, pharmaceutical companies and patients.
Mechanism
It is known that one distinctive characteristics of cancer from other diseases is that immune cells have difficulties to distinguish tumor cells from normal cells [5]. Therefore, a process that can either assist the immune cells to identify the tumor cells, or stimulate the immune cells to be more active should exhibit potential for cancer treatment. MAbs, which are designed for distinguishing the biomarkers abnormally expressed on tumor cells or specifically expressed by immune cells, are recognized as promising biologics to annihilate tumor cells. Though the exact metabolic details of how mAbs work is awaiting better understanding, the general mechanism typically falls into two categories:
mAbs distinguish and bind the biomarkers abnormally expressed by the tumor cells, helping the immune cells to target these cells. For example, trastuzumab, which was designed to target the biomarkers HER2, is a representative commercial mAb for breast cancer.
The immune cells are activated by mAbs to attack the tumor cells. Successful commercialized examples, such as nivolumab and pembrolizumab which target PD-1 and PD-L1 biomarkers; respectively, were designed based on such mechanism.
Owing to limited understanding of mammalian cell metabolism, limited biomarkers have been detected and only with parts of them have been used for mAbs design. Table 1 listed 43 recognized biomarkers that have been successfully used for commercial mAb design. Indications that exhibited abnormal expression of these biomarkers have been well studied. The one-to-one correspondence shown in the table aims to help narrow down the screening scope of the mAbs and indications, as well as predict the clinical results and control the quality of the designed protein therapeutics, which is in compliance with the Quality by Design (QbD) principles. This information may help biopharmaceutical industry to make decisions on biologics design at early discovery stage or on indication selection at clinical stages
| Antigen Biomarkers | Indications | References |
|---|---|---|
| α-4 integrin | Multiple sclerosis | [39] |
| BLyS | Systemic lupus erythematosus | [40] |
| CCR4 | Relapsed or refractory adult T-cell leukemia/lymphoma | [41] |
| CD3 | Transplant rejection, organ | [42] |
| CD6 | Psoriasis, Arthritis, rheumatoid | [43] |
| CD19 | Precursor B-cell acute lymphoblastic leukemia | [44] |
| CD20 | Relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and multiple sclerosis | [45] |
| CD30 | Hodgkin lymphoma, and anaplastic large-cell lymphoma | [46,47] |
| CD38 | Multiple myeloma | [48] |
| CD52 | B-cell chronic lymphocytic leukemia | [49] |
| Clostridium difficile toxin B | Prevent recurrence of Clostridium difficile infection | [50] |
| Complement component 5 | Paroxysmal nocturnal hemoglobinuria | [51] |
| CTLA-4 | Metastatic melanoma | [52] |
| Dabigatran | Emergency reversal of anticoagulant dabigatran | [53] |
| EGFR | Metastatic colorectal carcinoma, and metastatic squamous non-small cell lung carcinoma | [54,55] |
| EpCAM | Malignant ascites, multiple cancers | [56] |
| F protein of RSV | Respiratory syncytial virus | [57] |
| Ganglionside P3 | Multiple cancers | [58] |
| GD2 | Pediatric high-risk neuroblastoma | [59] |
| GPIIb/IIIa | Percutaneous coronary intervention | [60] |
| HER2 | Metastatic breast cancer | [61] |
| IgE | Moderate to severe persistent asthma | [62] |
| IL12 | Plaque psoriasis | [63] |
| IL23 | Psoriatic arthritis, plaque psoriasis, and crohn's disease | [63,64] |
| IL17A / IL17RA | Plaque psoriasis | [65] |
| IL1B | Cryopyrin-associated periodic syndrome | [66] |
| IL2R | Multiple sclerosis | [67] |
| IL2RA | Prophylaxis of acute organ rejection in renal transplant | [68] |
| IL4RA | Atopic dermatitis | [69] |
| IL5 | Severe asthma | [70] |
| IL6 | Multicentric Castleman's disease | [71] |
| IL6R | Rheumatoid arthritis, and systemic juvenile idiopathic arthritis | [72] |
| IL8 | Psoriasis | [73] |
| integrin receptor | Ulcerative colitis, crohn's disease | [74] |
| PCSK9 | Heterozygous familial hypercholesterolemia, and refractory hypercholesterolemia | [75] |
| PD-1 | Metastatic melanoma, and metastatic squamous non-small cell lung carcinoma | [76-78] |
| PD-L1 | Urothelial carcinoma, metastatic non-small cell lung cancer, and metastatic Merkel cell carcinoma | [79,80] |
| PDGFRA | Soft tissue sarcoma | [81] |
| Protective antigen of Bacillus anthracis / Anthrax toxin | Inhalational anthrax | [82] |
| PSMA | Diagnostic imaging agent in newly diagnosed prostate cancer or post-prostatectomy | [83] |
| RANKL | Postmenopausal women with osteoporosis | [84] |
| SLAMF7 | Multiple myeloma | [85] |
| TNF | Rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, crohn's disease, ulcerative colitis, and plaque psoriasis | [86,87] |
| TNF α | Crohn's disease | [86] |
| VEGF | Metastatic colorectal cancer | [88,89] |
| VEGFR1 | Wet age-related macular degeneration | [90] |
| VEGFR2 | Wet age-related macular degeneration, and gastric cancer | [89,91] |
Table 1: The recognized biomarkers for biologics and the approved indications.
Design Technologies Selection
Unlike small molecule drugs that can directly enter the cells and interrupt the metabolism with poor capacity of discernment, mAbs are mild and impact indirectly on the metabolic pathways with good targeting ability on tumor cells. To improve the efficacy as well as avoid patent dispute, several advanced design technologies have been applied to improve mAb performance. The characteristics of different biologic design technologies are briefly summarized in Table 2, which were further discussed as follows:
| Design Technology | Characteristics | |||
|---|---|---|---|---|
| Manufacturing Cost | Expression (g/L) |
Purification Recovery (%) |
Efficacy | |
| Conventional | * | 2-10 | 60-80 | Normal |
| ADC | ** | 0.4-5 | 50-70 | Small and Large Molecule |
| Bi-specific | ** | 0.5-3 | <20 | Tumor and Immune Cells (Synergistic) |
| Combined Medication | *** | Depends | Depends | Synergistic Effect |
Table 2: Different biologic design technologies with their characteristics.
Antibody drug conjugate
Antibody drug conjugate (ADC) technology is one improvement strategy, which allows small molecules conjugated on an antibody molecule [9]. Via the excellent targeting ability of the protein therapeutics, the small molecule is able to directly interrupt the metabolic pathways of tumor cells with significantly improved capacity of discernment [10]. Nevertheless, challenges that ADC faces in manufacturing include:
Unstable expression level due to the variety of the link structure, especially, when the link consists of unnatural amino acids.
Low downstream yield due to the added purification steps after the conjugation of the small molecule drug to the protein molecule.
Bi-specific
Bi-specific antibody technology is another option for better biologics design. It allows one biologic molecule to recognize two biomarkers simultaneously. Theoretically, the combination of the two biomarkers should fall into one of the three types: both on tumor cells, both on immune cells and one on tumor cell and one on immune cell. Nevertheless, the third design is the major preference in the industry. By this design, the immune cells can be effectively activated, then rapidly and adequately attack the tumor cells in situ [11]. This makes the protein therapeutics exhibit synergistic effect compared to using two or more independent mAbs. This technology has the potential to enhance the efficacy while reducing the biologics dosage and side effects. However, bi-specific molecules also have their drawbacks in manufacturing, such as:
Low expression level during cell culture due to the high risks of chain mispairing and protein aggregation
Low downstream separation efficiency because of the similar physicochemical properties of the mis-pairing molecules.
Combined medication
Combined medication technology is an alternative choice to improve the efficacy. The joint usage of two or more biologics or biologics with small molecule drugs has the potential to exhibit synergistic effect on tumor cells, because different biologics and/or drugs may impact on different metabolic pathways. This technology provides one strategy to screen new indications for existing mAbs avoiding huge investment for new drug design and application. This strategy, however, has some disadvantages for commercialization, including:
Significantly high cost in manufacturing, factory operation, storage and supply chain management for different products/ molecules;
Difficulties in maintaining acceptable stability of different biologics and/or drugs if in one formulation;
Inconvenience and high cost in drug delivery if different formulations were used for different biologics/drugs.
Commercial mAbs
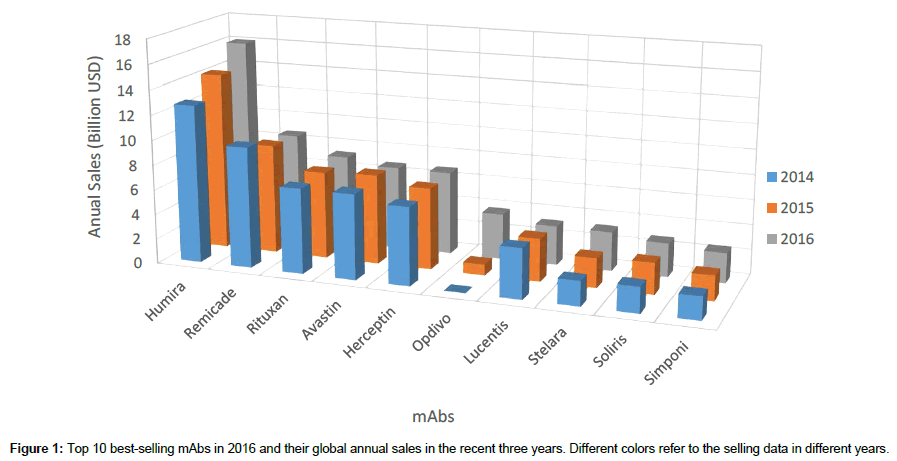
With increasing attention focused on mAbs, large amounts of investment have been attracted into the biopharmaceutical industry aiming at biologics commercialization. The study of the current commercial mAbs can benefit the biopharmaceutical industry, especially those start-up companies focusing on biosimilars, to rapidly follow up the recent trends and successfully screen the promising molecules. Figure 1 lists the top 10 best-selling mAbs in 2016 and their global annual sales in the recent three years. The data were obtained from the annual reports of these enterprises. Little ranking change was observed in Figure 1, except Opdivo was regarded as a dark-horse in the recent years due to the excellent performance of this anti-PD-1 mAb. Humira, Remicade and Rituxan kept the top three best-selling for the recent three years (Figure 1), bringing considerable revenue to Abbvie, Johnson & Johnson and Roche, respectively. In 2016, the total sales of these 10 mAbs were $ 61.2 billion, which is almost 70% of the whole global antibody market. The global oligopolistic market landscape may not be broken in the next few years due to limited number of validated biomarkers and long period of time for one biologic product to be commercialized. A list of biomarkers with corresponding commercial mAbs and patent holders were shown in Table 3. The mAbs and patent information was filtered manually from an open source tool called ‘Citeline Service’ before July 16th, 2017. Most of the biosimilars currently in research were designed based on these listed mAbs. This table also includes the major biomarkers for ADC and bi-specific antibodies, which is a trend for biologic design in the next few years.
| Antigen Biomarkers | Biologics | Patent Holders | BLA Approved |
|---|---|---|---|
| α-4 integrin | Tysabri | Biogen Idec | 11/23/2004 |
| BLyS | Benlysta | Human Genome Sciences | 03/09/2011 |
| CCR4 | Poteligeo | Amgen | 03/30/2012, Japan |
| CD6 | Alzumab | Center of Molecular Immunology | 01/07/2013, India |
| CD19 | Blincyto | Amgen | 12/03/2014 |
| CD20 | Zevalin | Spectrum Pharmaceuticals | 02/19/2002 |
| Gazyva | Genentech | 11/01/2013 | |
| Ocrevus | 03/28/2017 | ||
| Rituxan | 10/26/2009 | ||
| Arzerra | Glaxo Grp | 11/26/1997 | |
| CD30 | Adcentris | Seattle Genetics | 09/19/2011 |
| CD38 | Darzalex | Janssen Biotech | 11/16/2015 |
| CD52 | Campath | Genzyme | 05/07/2001 |
| Lemtrada | |||
| Clostridium difficile toxin B | Zinplava | Merck | 10/21/2016 |
| Complement component 5 | Soliris | Alexion | 03/16/2007 |
| CTLA-4 | Yervoy | Bristol-Myers Squibb | 03/25/2011 |
| dabigatran | Praxbind | Boehringer Ingelheim | 10/16/2015 |
| EGFR | Erbitux | ImClone Systems | 02/12/2004 |
| Portrazza | Eli Lilly | 11/24/2015 | |
| Vectibix | Amgen | 09/27/2006 | |
| BIOMAB EGFR | Biocon | 11/12/2007, India | |
| Theraloc | Oncoscience | 11/12/2003, EMEA | |
| CIMAher | Center of Molecular Immunology | 11/18/1994, Cuba | |
| CD3 | Ior-t3a | Center of Molecular Immunology | 05/15/1996, Cuba |
| Removab | Fresenius, Swedish Orphan Biovitrum, Neovii Biotech | 01/27/2011, EMEA | |
| EpCAM | |||
| F protein of RSV | Synagis | Med-Immune | 06/19/1998 |
| Ganglionside P3 | Vaxira | Recombio, Laboratorio Elea, Innogene Kalbiotech | 12/31/2012, Cuba |
| GD2 | Unituxin | United Therapeutics | 03/10/2015 |
| GPIIb/IIIa | ReoPro | Centocor | 12/22/1994 |
| HER2 | Kadcyla | Genentech | 02/22/2013 |
| Perjeta | 06/08/2012 | ||
| Herceptin | 09/25/1998 | ||
| IgE | Xolair | 06/20/2003 | |
| IL12 IL23 |
Stelara | Centocor | 09/25/2009 |
| Janssen Biotech | 09/23/2016 | ||
| IL17A | Taltz | Eli Lilly | 03/22/2016 |
| Cosentyx | Novartis | 01/21/2015 | |
| IL17RA | Siliq | Valeant | 02/15/2017 |
| IL1B | Ilaris | Novartis | 06/17/2009 |
| IL2R | Zinbryta | Biogen | 05/27/2016 |
| IL2RA | Simulect | Novartis | 05/12/1998 |
| Zenapax | Roche | 12/10/1997 | |
| IL4RA | Dupixent | Regeneron | 03/28/2017 |
| IL5 | Nucala | GlaxoSmithKline | 11/04/2015 |
| Cinqair | Teva | 03/23/2016 | |
| IL6 | Sylvant | Janssen Biotech | 04/23/2014 |
| IL6R | Actemra | Genentech | 01/08/2010 |
| 10/21/2013 | |||
| Kevzara | Sanofi | 02/01/2017, Canada | |
| IL8 | ABCreama | Yes Biotech | 07/13/2004, China |
| Integrin receptor | Entyvio | Takeda | 05/20/2014 |
| PCSK9 | Praluent | Sanofi Aventis | 07/24/2015 |
| Repatha | Amgen | 08/27/2015 | |
| PD-1 | Opdivo | Bristol-Myers Squibb | 12/22/2014 |
| 03/04/2015 | |||
| Keytruda | Merck | 09/04/2014 | |
| PD-L1 | Tecentriq | Genentech | 05/18/2016 |
| 10/18/2016 | |||
| Bavencio | EMD Serono | 03/23/2017 | |
| Imfinzi | AstraZeneca | 05/01/2017 | |
| PDGFRA | Lartruvo | Eli Lilly | 10/19/2016 |
| Protective antigen of Bacillus anthracis | Raxibacumab | Human Genome Sciences | 12/24/2012 |
| Protective antigen of the Anthrax toxin | Anthem | Elusys Therapeutics | 03/18/2016 |
| PSMA | ProstaScint | Cytogen | 10/28/1996 |
| RANKL | Prolia | Amgen | 06/01/2010 |
| Xgeva | |||
| SLAMF7 | Empliciti | Bristol-Myers Squibb | 11/30/2015 |
| TNF | Humira | Abbvie | 12/31/2002 |
| Amjevita | Amgen | 09/23/2016 | |
| Cimzia | UCB (company) | 04/22/2008 | |
| Simponi | Centocor | 04/24/2009 | |
| Simponi Aria | Janssen Biotech | 07/18/2013 | |
| Renflexis | Samsung Bioepis | 04/21/2017 | |
| Inflectra | Celltrion Healthcare | 04/05/2016 | |
| TNFα | Remicade | Centocor | 08/24/1998 |
| VEGF | Avastin | Genentech | 02/26/2004 |
| VEGFR1 | Lucentis | 06/30/2006 | |
| VEGFR2 | |||
| Cyramza | Eli Lilly | 04/21/2014 |
If the BLA was not approved by FDA, the biologics was specified with its approved location or organization
Table 3: Commercial biologics and the corresponding antigen biomarkers, patent holders and the biologic license application (BLA) approved dates.
mAbs Screening Methods
Screening criteria
After the designing of the mAbs based on the mechanisms discussed above, there could still be thousands of candidates available. This will be followed by two major screening processes to obtain best performed antigen-specific antibodies from the pool, which are:
Binding screening including specificity [12] and affinity [13]
Functional screening including cell growth, proliferation, apoptosis, endothelial tube formation, etc. [14]
While the functional assays are based on different disease models, the binding screening assays are universal in biopharmaceutical industry.
Specificity is the ability of the antibody binding to its cognate antigen and not to other targets. Affinity is the characteristic of antibody-antigen binding strength. These two criteria are crucial to ensure the efficacy, while good specificity can minimize the side-effects and good affinity is well preferred to reduce the drug dosage.
Functional activities are often the most significant characteristics of an antibody, including ability to deliver a toxin, antagonist activity, partial and full agonist activity, etc. These activities are often related to the protein allostery via the antigen-antibody specific binding [15,16].
Screening models
To quantitatively evaluate the above criteria, kinetic modeling strategies are usually applied [17]. Known models include Michaelis– Menten (M-M) model [18], Hill Equation [19-22], different types of Binding Models [23-25], Morpheein Model, Monod–Wyman– Changeux (MWC) model [26], Mechanistic kinetic description strategy [27] and empirical models derived from software such as JMP [28]. Nevertheless, different models have their advantages and drawbacks and none is appropriate in all situations.
M-M Equation has been the preferred modeling strategy in many enzyme kinetic studies due to its convenience for calculation [20,29]. M-M equation is only applicable for single domain enzymes or noninteractive oligomeric enzymes. However, most of the enzymes involved in metabolism are oligomeric. By introducing the Hill Coefficient, better simulation results can usually be generated than those using the M-M Equation [30]. However, kinetic parameters lose their mechanistic information due to the forcible introduction of the empirical Hill Coefficient. This shortcoming makes the Hill Equation more appropriate for empirical data manipulation in industry instead of mechanism research.
The Binding Models are extensions of the M-M and Hill Equations when there are more than two, typically three, molecules involved in one reaction. They were derived based on the ordered/ random molecule collision process and the second order elementary reaction mechanism [31]. These models considered multiple reaction processes simultaneously. In addition, the substrate-enzyme binding during the subsequent coenzyme and substrate binding processes for oligomeric enzymes with more than two active subunits can be important [24,31].
Morpheein Model, MWC model and Mechanistic kinetic description strategy are three modeling methods to mechanistically illustrate the molecular kinetic process by taking the interactive nature of one molecule with substrate and/or inhibitors into consideration [27,32]. More parameters are involved in the modeling which typically requires much more experimental data to support. Thus, these time- and cost-consuming methods are not the first choice in most commercial activities.
Table 4 presented a summary of the above models with their typical mathematic formula and applicable scopes. In the current biopharmaceutical researches, M-M Model and Hill Equation are the favored modeling strategies for biologics screening, due to their simplicity. While Morpheein Model, MWC Model and Mechanistic kinetic description strategy are able to well describe the kinetic properties of the molecular interactions, if the kinetic mechanism is critical to understand the biologics. Different types of Binding Models can be applied for multiple molecules involved reactions, such as bi-specific antibody involved reactions. Different strategies are selectively used based on the study purposes and research limitations.
| Type | Formula | Parameter number | Applicable Scope | References |
|---|---|---|---|---|
| M-M Model |  |
2 | non-interactive oligomeric or mono- molecular interactions | [18,23] |
| Hill Equation |  |
3 | Data manipulation for all molecular interactions | [22,92] |
| MWC model |  |
5 | Oligomeric molecular interactions | [32,93] |
| Morpheein Model |  |
5 | Oligomeric molecular interactions | [94] |
| Random Binding Model | 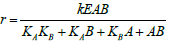 |
3 | Three molecule involved interactions | [25,95] |
| Ordered Binding Model |  |
3 | Three molecule involved interactions | [25,96] |
| Mechanistic Kinetic Description |  |
5 | Oligomeric molecular interactions | [27,94] |
Table 4: Reported kinetic modeling strategies for molecular interaction study.
Binding screening assays
Enzyme-linked Immunosorbent assay (ELISA) is one of the most popular platform technologies to identify antigen-antibody complex and both qualitatively and quantitatively evaluate the binding strength. The basic principle of ELISA based on radioimmunoassay techniques dates back top 1941 [33] and the exact method was created in 1971 [34]. Currently, it is a major detection method for biologics screening, because it is simple, quick, sensitive, specific and highthroughput [35]. Another screening technology is surface Plasmon resonance (SPR) biosensor [36]. As a gold standard for real-time and label-free monitoring technology of bimolecular interactions, it is able to determine the thermodynamic and kinetic properties of specific molecular interactions [37].
While ELISA and SPR are the common techniques for extracellular or cell-free antigen-antibody binding detection, fluorometric micro volume assay (FMAT) and fluorescence-activated cell sorting (FACS) are well-developed methods for on-cell or native binding screening [38]. The working principle based on antibodies binding to the antigen expressed on cell surface and the immunoglobulin constant region of the antibodies is detected by a fluorescently conjugated secondary antibody. As a high-throughput cell-based assay in the hybridoma screening, FMAT and FACS based technologies has significantly improved the screening efficiency and success probability.
Conclusion
Though cancers are not incurable disease due to the rapid technology development, they are still a leading threat for human health. In this paper, the recent trends and technologies of mAb development are comprehensively reviewed. The information of biomarkers, indications, commercial mAbs and the pattern status were systematically reviewed, which is beneficial for biopharmaceutical industry, research institutes and patients to make decisions. This review aims at providing a comprehensive understanding of the biomarker, indication and mAb screening strategies, which may promote further advancements in new drug discovery, novel indications of exiting drugs, as well as joint usage of mAbs and other cancer treatment methods.
Acknowledgement
The authors thank Mr. Boyuan Yin from General Electric Company, Mr. Pan Tian, Ms. Jing Zhao and Mr. Qing Dai from Mab-Venture Biopharm Co. Ltd. for their kind comments on this work.
Declaration of Interest
The authors declare no financial or commercial conflict of interest.
References
- Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S (2016) Global, regional and national comparative risk assessment of 79 behavioural, environmental and occupational and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386: 2287-2323.
- Kopeina GS, Senichkin VV, Zhivotovsky B (2017) Caloric restriction - A promising anti-cancer approach: From molecular mechanisms to clinical trials. Biochim Biophys Acta 1867: 29-41.
- Zhang X, Sun Y, Wang P, Yang C, Li S (2017) Exploration of the molecular mechanism of prostate cancer based on mRNA and miRNA expression profiles. Onco Targets Ther 10: 3225-3232.
- Beirão BC, Raposo T, Jain S, Hupp T, Argyle DJ (2016) Challenges and opportunities for monoclonal antibody therapy in veterinary oncology. Vet J 218: 40-50.
- Coulson A, Levy A, Gossellwilliams M (2014) Monoclonal antibodies in cancer therapy: Mechanisms, successes and limitations. West Indian Med J 63: 650-654
- Köhler G, Milstein C (2005) Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol 174: 2453-2455.
- Suthanthiran M, Fotino M, Riggio RR, Cheigh JS, Stenzel KH (1989) OKT3-associated adverse reactions: mechanistic basis and therapeutic options. Am J Kidney Dis 14: 39-44.
- Waldmann TA (1991) Monoclonal antibodies in diagnosis and therapy. Science 252: 1657-1662.
- Yao H, Jiang F, Lu A, Zhang G (2016) Methods to design and synthesize antibody-drug conjugates (ADCs). Int J Mol Sci 17: 194.
- Wang J (2017) Current statuses of antibody-drug conjugate bioanalysis. J Applied Bioanalysis 3: 26-30.
- Xu L, Zhang Y, Wang Q, Zhao J, Liu M (2015) Bi-specific antibodies with high antigen-binding affinity identified by flow cytometry. Int Immuno Pharma 24: 463-473.
- Liu P, Yang HT, Qiang LY, Xiao S, Shi ZX (2012) Estimation of the sensitivity and specificity of assays for screening antibodies to HIV: A comparison between the frequentist and Bayesian approaches. J Virol Methods 186: 89-93
- Pope ME, Soste MV, Eyford BA, Anderson NL, Pearson TW (2009) Anti-peptide antibody screening: selection of high affinity monoclonal reagents by a refined surface plasmon resonance technique. J Immunol Methods 341: 86-96.
- Kato M, Sasamori E, Chiba T, Hanyu Y (2011) Cell activation by CpG ODN leads to improved electrofusion in hybridoma production. J Immunol Method 373: 102-110.
- Oelmeier SA, Dismer F, Hubbuch J (2011) Application of an aqueous two-phase systems high-throughput screening method to evaluate mAb HCP separation. Biotechnol Bioeng 108: 69-81.
- Chen Y, Woolf TM, Wagner RW (2014) Antibody Screening Methods: WO. US 20140113831 A1[P].
- Gelinsky-Wersing D, Wersing W, Pompe W (2017) Bivalent kinetic binding model to surface plasmon resonance studies of antigen-antibody displacement reactions. Anal Biochem 518: 110.
- Yan X, Mager DE, Krzyzanski W (2010) Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn 37: 25-47.
- Khalilov RA, Dzhafarova AM, Dzhabrailova RN, Emirbekov EZ (2014) Analysis of the kinetic characteristics of lactate dehydrogenase from the rat brain during ischemia and reperfusion. Neurochem J 8: 265-270.
- Matoba Y, Miyasako M, Matsuo K, Oda K, Noda M, et al. (2014) An alternative allosteric regulation mechanism of an acidophilic l-lactate dehydrogenase from Enterococcus mundtii 15-1A. FEBS Open Biol 4: 834-847.
- Taguchi H, Matsuzawa H, Machida M Ohta T (1988) Allosteric and kinetic properties of L-lactate dehydrogenase from Thermus caldophilus GK24, an extremely thermophilic bacterium. Eur J Biochem 145: 283-290.
- Mijailovich SM, Li X, Griffiths RH, Geeves MA (2012) The Hill model for binding myosin S1 to regulated actin is not equivalent to the McKillop-Geeves model. J Mol Biol 417: 112.
- Alberty RA (1953) The relationship between Michaelis constants, maximum velocities and the equilibrium constant for an enzyme-catalyzed reaction. J Am Chem Soc 75: 924-926.
- Eggert MW, Byrne ME, Chambers RP (2011) Impact of high pyruvate concentration on kinetics of rabbit muscle lactate dehydrogenase. Appl Biochem Biotechnol 165: 676-686.
- Chen J, Newhall J, Xie ZR, Leckband D, Wu Y (2016) A computational model for kinetic studies of cadherin binding and clustering. Biophys J 111: 1507-1518.
- Najdi TS, Yang CR, Shapiro BE, Hatfield GW, Mjolsness ED (2006) Application of a generalized MWC model for the mathematical simulation of metabolic pathways regulated by allosteric enzymes. J Bioinform Comput Biol 4: 335-355.
- Tang P, Xu J, Oliveira CL, Li ZJ, Liu S (2017) A mechanistic kinetic description of lactate dehydrogenase elucidating cancer diagnosis and inhibitor evaluation. J Enzyme Inhib Med Chem 1: 564-571.
- Eggert MW, Chambers RP, Byrne ME, (2014) Impact of high pyruvate concentration on kinetics of rabbit muscle lactate dehydrogenase. Appl Biochem Biotechnol 165: 676-686.
- Daka NJ, Laidler KJ (1980) Temperature and pH effects on immobilized lactate dehydrogenase kinetics. Biochim Biophys Acta 612: 305-316.
- Taguchi H, Machida M, Matsuzawa H, Ohta T (1985) Allosteric and kinetic properties of L-lactate dehydrogenase from Thermus caldophilus GK24, an extremely thermophilic bacterium. Agricultural and Biological Chemistry 49: 359-365.
- Liu S (2012) Bioprocess engineering: kinetics, biosystems, sustainability, and reactor design. Elsevier
- Henry ER, Jones CM, Hofrichter J, Eaton WA (1997) Can a two-state MWC allosteric model explain hemoglobin kinetics? Biochemistry 36: 6511-6528.
- Coons AH, Creech HJ, Jones NR (1941) Immunological properties of an antibody containing a fluorescent group. Exp Biol Med 47: 200-202.
- Van Weemen BK, Schuurs AH (1971) Immunoassay using antigen-enzyme conjugates. FEBS Lett 15: 232-236.
- Wang Y, Guo J, Qiao S, Li Q, Yang J, et al. (2016) GP5 protein-based ELISA for the detection of PRRSV antibodies. Pol J Vet Sci 19: 495-501.
- Pollack SJ, Beyer KS, Lock C, Müller I, Sheppard D, et al. (2011) A comparative study of fragment screening methods on the p38alpha kinase: New methods, new insights. J Comput Aided Mol Des 25: 677-687.
- Grasso L, Wyss R, Weidenauer L, Thampi A, Demurtas D, et al. (2015) Molecular screening of cancer-derived exosomes by surface plasmon resonance spectroscopy. Anal Bioanal Chem 407: 5425-5432.
- Lee R, Tran M, Nocerini M, Liang M (2008) A high-throughput hybridoma selection method using fluorometric microvolume assay technology. J Biomol Screen 13: 210-217
- Muralidharan KK, Kuesters G, Plavina T, Subramanyam M, Mikol DD, et al. (2017) Population pharmacokinetics and target engagement of natalizumab in patients with multiple sclerosis. J Clin Pharmacol 57, 1017-1030.
- Dennis GJ (2012) Belimumab: a BLyS-specific inhibitor for the treatment of systemic lupus erythematosus. Clin Pharmacol Ther 91: 143-149.
- Sugio T, Kato K, Aoki T, Ohta T, Saito N, et al. (2016) Mogamulizumab treatment prior to allogeneic hematopoietic stem cell transplantation induces severe acute graft-versus-host disease. Biol Blood Marrow Transplant 22: 1608-1614.
- Sousa IG, do Almo MM, Simi KC, Bezerra MA, Andrade RV, et al. (2017) MicroRNA expression profiles in human CD3+ T cells following stimulation with anti-human CD3 antibodies. BMC Res Notes. 10: 124.
- K, VS (2013) Biocon successfully launches ALZUMAb for psoriasis patients in India. Curr Sci 105: 572.
- Moon H, Huh J, Cho MS, Chi H, Chung WS (2007) A case of CD45-, CD19-precursor B cell acute lymphoblastic leukemia with an atypical morphology. Korean J Lab Med 27: 253.
- Bittolo T, Pozzo F, Bomben R, D'Agaro T, Bravin V, et al. (2017) Mutations in the 3' untranslated region (3' UTR) of NOTCH1 are associated with low CD20 expression levels in chronic lymphocytic leukemia. Haematologica 102: e305-e309.
- Scott LJ (2017) Brentuximab Vedotin: A Review in CD30-Positive Hodgkin Lymphoma. Drugs 77: 1-11.
- Chen CC, Yeh SP (2016) Case report - Fatal pancreatitis occurred in a patient with refractory CD30+ anaplastic large cell lymphoma after brentuximab vedotin treatment. J Cancer Res Pract.
- Shallis RM, Terry CM, Lim SH (2017) The multi-faceted potential of CD38 antibody biomarkering in multiple myeloma. Cancer Immunol Immunother Cii 66: 1-7.
- D'Arena G, Vigliotti ML, Matera R, Musto C, Iodice G, et al. (2003) Quantitative evaluation of CD52 expression in B-cell chronic lymphocytic leukemia. Leuk Lymphoma 44: 1255-1257.
- Villafuerte Gálvez JA, Kelly CP (2017) Bezlotoxumab: anti-toxin B monoclonal antibody to prevent recurrence of Clostridium difficile infection. Expert Rev Gastroenterol Hepatol 11: 611-622.
- Holers VM (2010) The spectrum of complement alternative pathway-mediated diseases. Immunol Rev 223: 300-316.
- Bresler SC, Min L, Rodig SJ, Walls AC, Xu S, et al. (2017) Gene expression profiling of anti-CTLA4-treated metastatic melanoma in patients with treatment-induced autoimmunity. Lab Invest 97: 207.
- Pollack CV, Reilly P, Eikelboom J, Glund S, Gruenenfelder F, et al. (2016) Idarucizumab for reversal of the anticoagulant effects of dabigatran in patients in an emergency setting of major bleeding, urgent surgery or interventions. J Am Coll Cardiol 67: 664.
- Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi H, et al. (1995) Epidermal growth factor receptor (EGFr) expression in non-small cell lung carcinomas correlates with metastatic involvement of hilar and mediastinal lymph nodes in the squamous subtype. Eur J Cancer 31A: 178-183.
- Silvestris N, Tommasi S, Santini D, Russo A, Simone G, et al. (2009) KRAS mutations and sensitivity to anti-EGFR monoclonal antibodies in metastatic colorectal carcinoma: An open issue. Expert Opin Biol Ther 9: 565-577.
- Seeber A, Martowicz A, Spizzo G, Buratti T, Obrist P, et al. (2015) Soluble EpCAM levels in ascites correlate with positive cytology and neutralize catumaxomab activity in vitro. BMC Cancer 15: 372.
- Fries L, Shinde V, Stoddard JJ, Thomas DN, Kpamegan E, et al. (2017) Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun Ageing 14: 8.
- Pérez L, Estévez D, Gastón Y, Macias A (2013) Safety of racotumomab in the treatment of patients with non-small cell lung cancer. Vaccimonitor 22: 10-14.
- Görges M, West N, Deyell R, Winton P, Cheung W, et al. (2015) Dexmedetomidine and hydromorphone: A novel pain management strategy for the oncology ward setting during anti-GD2 immunotherapy for high-risk neuroblastoma in children. Pediatr Blood Cancer 62: 29-34.
- Ray MJ, Juneja M, Bett N, Walters DL (2009) A comparison of anticoagulation with bivalirudin and provisional GPIIb/IIIa inhibition with unfractionated heparin and mandatory GPIIb/IIIa inhibition during percutaneous coronary intervention in relation to platelet activation and the inhibition of coagul. EuroIntervention 5: 330-335.
- Sörensen J, Velikyan I, Sandberg D, Wennborg A, Feldwisch J, et al. (2016) Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 affibody PET/CT. Theranostics 6: 262-271.
- Busse WW, Massanari M, Kianifard F, Geba GP (2007) Effect of omalizumab on the need for rescue systemic corticosteroid treatment in patients with moderate-to-severe persistent IgE-mediated allergic asthma: a pooled analysis. Curr Med Res Opin 23: 2379-2386.
- Yawalkar N, Tscharner GG, Hunger RE, Hassan AS (2009) Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J Dermatol Sci 54: 99-105.
- Shibata S, Tada Y, Komine M, Hattori N, Osame S, et al. (2009) Anti-cyclic citrullinated peptide antibodies and IL-23p19 in psoriatic arthritis. J Dermatol Sci 53: 34-39.
- Gooderham M, Posso-De Los Rios CJ, Rubio-Gomez GA, Papp K (2015) Interleukin-17 (IL-17) inhibitors in the treatment of plaque psoriasis: A review. Skin Ther Lett 20: 1-5.
- Kuemmerle-Deschner JB, Ramos E, Blank N, Roesler J, Jung T, et al. (2011) Canakinumab (ACZ885, a fully human IgG1 anti-IL-1ß mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res Ther 13: R34.
- Su P (2010) Methods for monitoring the efficacy of anti-Il-2r antibodies in multiple sclerosis patients. US2010273204(A1)[P].
- Bumgardner Ginny L, Ramos Eleanor L (2001) Daclizumab (Humanized Anti-Il2ra Mab) prophylaxis For prevention of acute rejection in renal transplant recipients with delayed graft function1,2. Transplantation 72: 642-647.
- JR, C (2015) Method of treating atopic dermatitis or asthma using antibody to IL4RA. US8986691(P).
- Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ (2016) Benralizumab: A unique IL-5 inhibitor for severe asthma. J Asthma Allergy 9: 71-81.
- Casper C, Chaturvedi S, Munshi N, Wong R, Qi M, et al. (2015) Analysis of Inflammatory and anemia-related biomarkers in a randomized, double-blind, placebo-controlled study of siltuximab (anti-IL6 monoclonal antibody) in patients with multicentric castleman disease. Clin Cancer Res 21: 4294-4304.
- Mahmood Z, Muhammad K, Schmalzing M, Roll P, Dörner T, et al. (2015) CD27-IgD- memory B cells are modulated by in vivo interleukin-6 receptor (IL-6R) blockade in rheumatoid arthritis. Arthritis Res Ther 17: 61.
- Zhao Z, Wang S, Lin Y, Miao Y, Zeng Y, et al. (2017) Epithelial-mesenchymal transition in cancer: Role of the IL-8/IL-8R axis. Oncol Lett 13: 4577-4584.
- Hahn L, Beggs A, Wahaib K, Kodali L, Kirkwood V (2015) Vedolizumab: An integrin-receptor antagonist for treatment of Crohn's disease and ulcerative colitis. Am J Health Syst Pharm 72: 1271-1278.
- Lambert G, Petrides F, Chatelais M, Blom DJ, Choque B, et al. (2014) Elevated plasma PCSK9 level is equally detrimental for patients with non-familial hypercholesterolemia and heterozygous familial hypercholesterolemia, irrespective of low-density lipoprotein receptor defects. J Am Coll Cardiol 63: 2365-2373.
- De Wolf K, Kruse V, Sundahl N, van Gele M, Chevolet I, et al. (2017) A phase II trial of stereotactic body radiotherapy with concurrent anti-PD1 treatment in metastatic melanoma: Evaluation of clinical and immunologic response. J Transl Med 15: 21.
- Hellmann M, Rizvi N, Wolchok JD, Chan TA (2016) Genomic profile, smoking and response to anti-PD-1 therapy in non-small cell lung carcinoma. Mol Cell Oncol 3: e1048929.
- Prat A, Navarro A, Paré L, Reguart N, Galván P, et al. (2017) Immune-related gene expression profiling after PD-1 blockade in non-small cell lung carcinoma, head and neck squamous cell carcinoma and melanoma. Cancer Res 77: 3540-3550.
- Sheffield BS, Fulton R, Kalloger SE, Milne K, Geller G, et al. (2016) Investigation of PD-L1 biomarker testing methods for PD-1 axis inhibition in non-squamous non-small cell lung cancer. J Histochem Cytochem 64: 587-600.
- Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, et al. (2013) PD-L1 expression in the Merkel cell carcinoma microenvironment: Association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res 1: 54-63.
- Klug LR, Heinrich MC (2017) PDGFRA antibody for soft tissue sarcoma. Cell 168: 555.
- Kummerfeldt CE (2014) Raxibacumab: Potential role in the treatment of inhalational anthrax. Infect Drug Resist 7: 101-109.
- Chung E (2014) A state-of-the-art review on the evolution of urinary sphincter devices for the treatment of post-prostatectomy urinary incontinence: Past, present and future innovations. J Med Eng Technol 38: 328-332.
- Reyes-García R, Muñoz-Torres M, García DF, Mezquita-Raya P, García Salcedo JA, et al. (2010) Effects of alendronate treatment on serum levels of osteoprotegerin and total receptor activator of nuclear factor kappa B in women with postmenopausal osteoporosis. Menopause 17: 140-144.
- Boudreault JS, Touzeau C, Moreau P (2017) The role of SLAMF7 in multiple myeloma: impact on therapy. Expert Rev Clin Immunol 13: 67-75.
- Amano H, Matsuda R, Shibata T, Takahashi D, Suzuki S (2017) Paradoxical SAPHO syndrome observed during anti-TNF alpha therapy for Crohn's disease. Biologics 11: 65-69.
- Régent A, Mouthon L (2009) Anti-TNF alpha therapy in systemic autoimmune and/or inflammatory diseases. Presse Med 38: 761-773.
- Lieu CH, Tran H, Jiang ZQ, Mao M, Overman MJ, et al. (2013) The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS ONE 8: e77117.
- Hagstrom SA, Ying GS, Pauer GJ, Sturgill-Short GM, Huang J, et al. (2014) VEGFA and VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy: comparison of age-related macular degeneration treatments trials (CATT). JAMA Ophthalmol 132: 521-527.
- Cai M, Wang K, Murdoch CE, Gu Y, Ahmed A (2017) Heterodimerisation between VEGFR-1 and VEGFR-2 and not the homodimers of VEGFR-1 inhibit VEGFR-2 activity. Vascul Pharmacol 88: 11-20.
- Meredith EL, Mainolfi N, Poor S, Qiu Y, Miranda K, et al. (2015) Discovery of oral VEGFR-2 inhibitors with prolonged ocular retention that are efficacious in models of wet age-related macular degeneration. J Med Chem 58: 9273-9286.
- Talaiezadeh A, Shahriari A, Tabandeh MR, Fathizadeh P, Mansouri S (2015) Kinetic characterization of lactate dehydrogenase in normal and malignant human breast tissues. Cancer Cell Int 15: 19.
- Jaffe EK, Stith L, Lawrence SH, Andrake M, Dunbrack RL (2013) A new model for allosteric regulation of phenylalanine hydroxylase: Implications for disease and therapeutics. Arch Biochem Biophys 530: 73-82.
- Liu S (2015) A review on protein oligomerization process. IJPEM. 16: 2731-2760
- Nolan RP, Lee K (2011) Dynamic model of CHO cell metabolism. Metab Eng 13: 108-124.
- Wang Y, Wei L, Wei D, Li X, Xu L (2016) Enzymatic kinetic properties of the lactate dehydrogenase isoenzyme C(4) of the Plateau pika (Ochotona curzoniae). Int J Mol Sci 17: 39.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi