Research Article, J Cardiovasc Res Vol: 12 Issue: 1
Role of Copeptin in Early Detection of Acute Myocardial Infarction Patients and its Relation to the Prognosis of Mid Term
Hani Mohammed Fakhry*, Khaled Mohamed Said and Ziad Fathi Hemdan Elshaer
Department of Cardiology, Ain Shams University, Cairo, Egypt
*Corresponding Author: Hani Mohammed Fakhry
Department of Cardiology, Ain Shams University, Cairo, Egypt
Tel: 0201002554916
E-mail: drhanyfakhry@gmail.com
Received date: 03 November, 2022, Manuscript No. ICRJ-22-78905; Editor assigned date: 07 November, 2022, PreQC No. ICRJ-22-78905 (PQ); Reviewed date: 21 November, 2022, QC No. ICRJ-22-78905; Revised date: 03 January, 2023, Manuscript No. ICRJ-22-78905 (R); Published date: 10 January, 2023, DOI: 10.4172/2324-8602.1000483
Citation: Fakhry HM, Said KM, Elshaer ZFH (2023) Role of Copeptin in Early Detection of Acute Myocardial Infarction Patients and its Relation to the Prognosis of Mid Term. J Cardiovasc Res 12:1
Abstract
Objective: To evaluate copeptin's contribution to the early diagnosis of acute myocardial infarction and its impact on longterm prognosis.
Methods: Forty patients admitted to AIN shams university hospital within 4 hours of onset of chest pain of AMI were included in this research. Serum copeptin, cTn I HL, CK-Total and CK-MB levels were measured on admission and discharge
Results: Copeptin, cTn I HL, CK-Total and CK-MB levels were all high (over the cutoff threshold) in all patients at the time of admission. Males had greater serum copeptin levels at admission as compared to females, but there was no statistically significant difference. Copeptin levels at admission were significantly correlated with heart rate, cTn I HL and there was a significantly negative connection with LVEF%. The serum level of copeptin and the LVEF% had a significantly significant negative correlation at discharge. The blood level of copeptin at admission and discharge differed non-significantly between the STEMI and NSTEMI groups, however it was higher in the STEMI group. LVEF% was non-significantly greater in NSTEMI.
Conclusion: When utilised after a clinical work-up and in conjunction with troponin, copeptin plays a crucial role in the earlier and more precise diagnosis of AMI. Additionally, there is substantial evidence to support the prognostic significance of copeptin in AMI patients when it is obtained at presentation.
Keywords: Acute myocardial infarction; Copeptin; Mid-term prognosis; Serum copeptin
Introduction
In emergency medicine, the exclusion of Acute Myocardial Infarction (AMI) is a difficult technique. Only 10% of patients with internal medicine issues who go to the Emergency Department (ED) complaining of chest discomfort really have an AMI as the underlying condition, which leads to a good prognosis but comes at a high cost. Most patients are retained on Chest Pain Units (CPU) for 6 to 12 hours [1].
To guarantee prompt, effective treatment in the emergency room, correct exclusion and diagnosis are crucial. Acute Myocardial Infarction (AMI) must be diagnosed and cardiac Troponins (cTn) are the best indicators for spotting myocardial cell necrosis [2]. Due to the delayed release of cTn after a myocardial injury, there is still a troponin blind interval after the beginning of chest pain and repeat continuous cTn monitoring is necessary to distinguish between AMI and Unstable Angina Pectoris (UAP) [3].
Consequently, a biomarker that releases right away in the case of AMI is required [4]. It is hoped that this new biomarker would facilitate quick clinical practise decisions. Arginine Vasopressin (AVP), the primary hypothalamic stress hormone, is activated by AMI [5]. As a result, AVP becomes a significant marker; nevertheless, measurements of AVP are seldom repeatable due to its fragile nature and quick clearance from plasma [6].
AVP and copeptin are kept in the neurohypophyseal vesicles until they are secreted [7]. Copeptin is more measurable and stable. As a result, its level reflects the AVP production. Copeptin is therefore a highly accurate predictive indicator of AVP concentration [8].
Therefore, it is anticipated that very soon after an AMI, the novel biomarker copeptin, which is a sign of acute endogenous stress, will be raised in individuals with ACS. Therefore, it has been proposed that combining a sign of endogenous stress with a marker of cell necrosis may improve the diagnostic performance in patients with chest discomfort who report to the emergency room [9]. For AMI patients, the marker combination of troponin and copeptin will produce the best results [10].
Early on after an acute MI, there is a correlation between copeptin levels and LV dysfunction that persisted in the survivors over the follow-up [11]. In a sample of patients from the OPTIMAAL (Optimal Trial In Myocardial Infarction with Angiotensin II Antagonist Losartan) research, voors and the OPTIMAAL investigators looked at the predictive effect of copeptin in patients following acute MI [12]. They demonstrated that copeptin had an even larger prognostic value than BNP and NT-proBNP in patients with Heart Failure (HF) following acute MI, confirming the findings of Khan, et al. in a multicenter environment. Once more, the death rate was noticeably higher for individuals in the top quartile (>25.9 pmol/L). They could also show that serial copeptin measures throughout follow-up had more predictive value than a single measurement at baseline. Examining the function of copeptin in the early diagnosis of individuals with acute myocardial infarction and its relationship to long-term prognosis is the goal of this study.
Materials and Methods
Patients and methods
This investigation was planned as an observational prospective investigation. It was applied to 40 consecutive patients who had been hospitalised to the emergency room of the Ain-Shams university hospital with severe chest discomfort. The Ain-Shams university hospital's ethical guidelines were considered when this investigation was being conducted. According to ECG data, patients were divided into two groups, as indicated in Table 1.
| AMI | STEMI | NSTEMI |
|---|---|---|
| Number of patients | 23 | 17 |
| Male patients | 15 | 10 |
| Female patients | 8 | 7 |
| Age (range) | 47-71 | 49-73 |
| Age (mean ± SEM) | 58.348 ± 6.300 | 59.294 ± 5.828 |
Table 1: ECG data of the studied patients.
From the 15th of February to the 15th of September 2015, all patients hospitalised to the emergency room at Ain-Shams university with acute chest discomfort that began within 4 hours of presentation were included in the research. The following patients were not included in the study: Those with chronic heart failure, primary valve disease, prior valve surgery, trauma, major surgery within the last 4 weeks, pregnant women, anaemia (haemoglobin >10 g/dl), chronic renal illness, shock or stroke.
Every case had a thorough patient history, comprehensive clinical examination, standard 12-lead ECG and trans-thoracic echocardiography (M-mode and 2D for Dimensions, ejection fraction (ejection fraction was assessed by simpson’s method or eye-balling in patients with RWMA and by M-mode in absence of RWMA), regional wall motion abnormalities…), laboratory investigations with special emphasis on hemoglobin level, serial of total serum Creatine Kinase (CK), serum Creatine Kinase-MB fraction (CK-MB) and serum cardiac Troponin I (cTn I) and serum copeptin by ELISA technique at onset of chest pain to 4 h and at discharge in patients with MI after final clinical assessment.
Blood sampling
For the purpose of measuring the concentration of copeptin, 2 ml of venous blood from each patient was collected under aseptic conditions, without the use of an anticoagulant (serum) (at admission and at discharge). Before centrifuging the samples for 20 minutes at a rate of around 1000 g and storing them at -20°C, they were lifted for 15 minutes at room temperature. To assess the concentration of cardiac Troponin I (cTn I), two ml of venous blood were collected from each patient under aseptic conditions and after six to nine hours (two samples).
Follow-up
At 90 days, all patients were contacted and checked to see if any major adverse cardiac events had occurred, including death, coronary artery bypass grafting, AMI, acute unexpected percutaneous coronary intervention, re-hospitalization for ACS, survived sudden cardiac arrest, documented life-threatening arrhythmias or admission for HF.
Determination of serum copeptin
This enzyme immunoassay kit was developed using the competitive enzyme immunoassay technique to identify a specific peptide and its related peptides. Phoenix Pharmaceuticals Inc. of the United States delivers this kit in accordance with the porstmann and kiessig concept [13].
Principles
The immunoplate is pre-coated with secondary antibody and the nonspecific binding sites are blocked. Both biotinylated peptide and peptide standard or targeted peptide in samples will compete bind the fragment of the main antibody (peptide antibody fab). The main antibody's Fc fragment can be bound by the secondary antibody (peptide antibody). The biotinylated peptide interacts with the Streptavidin-Horseradish Peroxidase (SA-HRP), which catalyses the substrate solution. While the intensity of the yellow is directly associated with both the quantity of the biotinylated peptide-SA-HRP complex and the quantity of the peptide in standard solutions or samples, respectively. The standard peptide or samples and the biotinylated peptide compete with one another to bind to the peptide antibody, which is why (primary antibody).
Reagent preparation
Assay buffer dilution: To make a final volume of 1000 mL, the contents of the assay buffer concentrate (20 x) were diluted with 950 mL of distilled water. All other reagents in the kit and samples were diluted or reconstituted using this 1 x assay buffer solution.
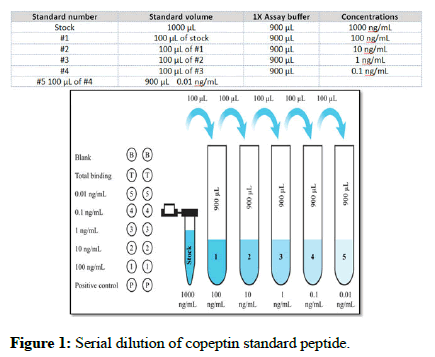
Standard peptide preparation: After centrifuging, the copeptin standard peptide was diluted with 1 mL of 1 x assay buffer. This stock solution was prepared at a concentration of 1000 ng/mL and left to remain at room temperature for 10 minutes in order to thoroughly dissolve in solution. Using this stock standard solution, a serial dilution was carried out as shown in Figure 1.
Primary antibody preparation: 5 ml of 1 x assay buffer were used to reconstitute the primary antibody, which was then given 5 minutes to dissolve fully.
Biotinylated peptide preparation: 5 ml of 1 x assay buffer were used to reconstitute the biotinylated peptide, which was then given 5 minutes to dissolve fully.
Positive control preparation: Reconstituted and centrifuged with 200 L of 1 x assay buffer, the positive control was then let to rest for at least five minutes to thoroughly dissolve.
Streptavidin-Horseradish Peroxidase (SA-HRP) preparation: Reconstituted and centrifuged with 200 L of 1 x assay buffer, the positive control was then let to rest for at least five minutes to thoroughly dissolve.
Assay procedure
Before usage, all kit components were allowed 30 minutes to acclimatise to room temperature. The A-1 and A-2 wells are empty and blank. As total binding, 50 L of 1 x assay buffer were added to wells B-1 and B-2. 50 L of pre-made peptide standards were introduced in duplicate, going from wells C-1 and C-2 to wells G-1 and G-2 in the reverse sequence of serial dilution. Wells H-1 and H-2 received 50 L of reconstituted positive control in triplicate. The proper wells received 50 mL of the samples. Each well received 25 L of rehydrated primary antibody, with the exception of the blank well. After reconstitution, add 25 L of the biotinylated peptide to all but the blank wells. The immunoplate was sealed with Acetate Plate Sealer (APS) and incubated for two hours at room temperature (23°C) using an incubator-shaking system (stat fax-2200, USA). The APS of the immunoplate was removed and the contents of the wells were discarded. The plate was completely cleaned using 350 L of 1 x assay buffer four times. 100 L of the SA-HRP solution were added to each well. An incubator shaking system (stat fax-2200, USA) was used to incubate the immunoplate for a further hour at room temperature while being shaken at 300 rpm-400 rpm. The immunoplate was cleared of the APS. 350 litres of 1 x assay buffer were used to wash the immunoplate and this process was repeated four times. TMB substrate solution weighing 100 l was poured into each well. The immunoplate was then resealed with APS and incubated for 1 hour at room temperature using an incubator shaking system (stat fax-2200, USA) at 300 rpm-400 rpm. The immunoplate was cleared of the APS. Each well received 100 L of 2 N HCl to halt the process. After the addition of the stop solution, the color of the wells was altered to yellow. In an ELISA microplate reading system (sunrise, TECAN, Austria), the absorbance in each well was measured at 450 nm (using a reference wave length of 630 nm to eliminate well defects) and the results were computed.
Results calculation
The microplate reader's output was used to measure the absorbance. On a semi-log graph paper, the standard curve was created by charting the known concentrations of the standard peptide on the log scale (X-Axis) and the Optical Density (OD) measurement (i.e., the absorbance of duplicates) at that concentration on the linear scale (Y-Axis). The OD absorbance drops as the standard concentration rises, demonstrating an inverse connection between peptide concentrations and the associated absorbance. By locating the sample's OD on the Y-Axis, drawing a horizontal line to intersect the standard curve and then drawing a vertical line from this point to intersect the X-Axis at a coordinate corresponding to the sample's peptide concentration, it was possible to calculate the concentration of peptide in a given sample. To convert all concentrations from ng/mL to pmol/L, the conversion factor was increased by 200 [14]. One method for calculating this conversion factor is as follows: Copeptin has a molecular weight of 5 kilo daltons, which is equivalent to 5000 daltons [15]. The conversion formula is as follows: Mole=weight (g)/molecular weight [16]. 200 pmol/L is equal to (1 ng/mL × 1000 mL/1 L) × (1 mol/5000 KD × 1000 pico).
Cutoff values for copeptin
In a large reference population (n=5,000), Keller, et al. [17] assessed several possible cutoff values for copeptin, with the 99th percentile cutoff value being 18.9 pmol/l, the 97.5th percentile value being 13 pmol/l and the 95th percentile value being 9.8 pmol/l. The copeptin cutoff in the majority of clinical investigations was 14 pmol/l.
The lower detection limit for the first copeptin assay was 4.8 pmol/l, the limit of quantification was 14.1 pmol/l and the functional assay sensitivity was below 12 pmol/l (lowest value with an interassay coefficient of variation below 20%), according to manufacturer's data.
In 2011, an ultrasensitive copeptin assay with lower detection limits of less than 1 pmol/l, functional assay sensitivity of less than 2 pmol/l and a 10% coefficient of variation of 2.5 pmol/l was released (manufacturer's data). This criterion is still used in the majority of studies, owing to the fact that it was first used in the early articles. The NPV for the diagnosis of AMI, on the other hand, increases with a lower threshold value. To limit the proportion of individuals who have false-negative findings, it may be advisable to utilise the 95th percentile value (10 pmol/l).
Statistical methods
Data were gathered and version 19 of the Statistical Package of Social Science (SPSS) was used to analyse them. Independent t-tests and chi-square (χ2) tests were used to compare demographic and clinical data between the two groups for continuous variables. If the P-value was less than 0.05, it was regarded as statistically significant.
Results
23 patients (57%) had a discharge diagnostic of STEMI, while 17 patients (42%), who had a ST segment depression or a normal ECG, had a discharge diagnosis of NSEMI (Table 2).
| All patients | ||
|---|---|---|
| N | % | |
| STEMI | 23 | 57.5 |
| NSTEMI | 17 | 42.5 |
Table 2: Classification of AMI patients.
The baseline characteristics of all research participants are shown in Table 3. 40 patients made up the study population; their ages varied from 47 to 73 years, with a mean age of 58.756.04 years.
Of the 40 patients, 25 (62.50%) were men and 15, (27.50%), were women. Of the total research participants, 11 individuals (27.5%) smoked.
| Range | Mean ± SD | |
|---|---|---|
| Age (years) | 47-73 | 58.750 ± 6.046 |
| BMI (Kg/m2) | 23.2-39.2 | 30.288 ± 4.185 |
| time to present in ER (h) | 4-Feb | 3.100 ± 1.008 |
| HR (bpm) | 60-93 | 78.675 ± 9.289 |
| Hemoglobin (g/dl) | 14-Nov | 12.390 ± 0.893 |
| CK-Total (IU/L) | 350-3350 | 896.500 ± 712.866 |
| CK-MB (U/L) | 60-1010 | 316.325 ± 219.260 |
| cTn I (ng/ml)A d | 0.74-1.88 | 1.288 ± 0.279 |
| cTn I (ng/ml)H L | 5.5-18.7 | 10.581 ± 3.237 |
| Copeptin (pmol/L)Ad | 40.4-113 | 55.458 ± 13.926 |
| Copeptin (pmol/L)Ds | 7.2-22.4 | 13.618 ± 3.673 |
| LVEF % | 35-56 | 42.775 ± 4.828 |
| Note: BMI: Body Mass Index, HR: Heart Rate, CK-Total: Creatine kinase Total, CK-MB:Creatine Kinase-Myocardial Band, cTn I Ad: Cardiac Troponin-I at Admission, cTn I HL: Cardiac Troponin I Highest Level, Copeptin Ad: Copeptin at Admission, Copeptin Ds: Copeptin at Discharge. | ||
Table 3: Demographic data of the study population.
Echocardiography was performed to all study patients at admission: The mean LVEF% was (42.775 ± 4.828) and regional wall motion abnormalities: Were not recorded in 3 patients (7.5%) of all study patients (all were NSTEMI) (Table 4).
| RWMA | ||
|---|---|---|
| N | % | |
| Negative | 3 | 7.5 |
| Positive | 37 | 92.5 |
| Note: RWMA: Regional Wall Motion Abnormalities | ||
Table 4: Regional wall motion abnormalities.
When compared the value of serum level of copeptin at admission in males and females it was higher in males but with no statistically significant difference (Table 5). Smoker patients had non-statistically significant higher value of copeptin at admission (P-value 0.106).
| Copeptin (pmol/L) Ad | Gender | T-Test | ||
|---|---|---|---|---|
| Male | Female | t | P-value | |
| Range | 40.4-113 | 41.3-83.8 | 0.658 | 0.515 |
| Mean ± SD | 56.588 ± 14.875 | 53.573 ± 12.446 | ||
| Note: Copeptin Ad: Copeptin at Admission | ||||
Table 5: Serum level of copeptin at admission in males and females.
All patients' serum copeptin levels upon entry are shown in Table 6. The blood level of copeptin upon admission was not significantly correlated with age, BMI, haemoglobin CK-total, CK-MB or cTn I Ad. Copeptin upon admission, heart rate and cTnI HL were found to significantly positively correlate with one another. A significant inverse relationship between the serum copeptin level at admission and the LVEF% was discovered. A non-significant connection was seen between serum copeptin levels and sex, age, BMI, haemoglobin, CK-Total, CK-MB, cTn I AD and cTn I HL at the time of discharge for all patients. At discharge, there was a highly significant (P-value= 0.002) negative correlation between the level of copeptin and the LVEF% (Table 6).
| Copeptin (pmol/L)Ad at admission | Copeptin (pmol/L)Ad at discharge | |||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Age (years) | 0.064 | 0.695 | 0.163 | 0.316 |
| BMI (Kg/m2) | 0.036 | 0.824 | 0.034 | 0.836 |
| Time topresent in ER (h) | -0.047 | 0.773 | ||
| HR (bpm) | 0.35 | 0.027* | ||
| Hemoglobin (g/dl) | 0.127 | 0.434 | -0.009 | 0.954 |
| CK-Total (IU/L) | 0.064 | 0.78 | 0.198 | 0.221 |
| CK-MB (U/L) | 0.051 | 0.757 | 0.203 | 0.21 |
| cTn I(ng/ml) Ad | 0.116 | 0.475 | 0.229 | 0.155 |
| cTn I (ng/ml) H L | 0.41 | 0.009* | 0.199 | 0.218 |
| LVEF% | -0.307 | 0.054* | -0.475 | 0.002* |
| Note: BMI: Body Mass Index, HR: Heart Rate, CK-Total: Creatine Kinase Total, CK-MB: Creatine Kinase-Myocardial Band, cTn I Ad: Cardiac Troponin-I at Admission, cTn I HL: Cardiac Troponin I Highest Level, Copeptin Ad: Copeptin at Admission, Copeptin Ds: Copeptin at Discharge, *significantat at p-value=(0.027-0.009-0.054). | ||||
Table 6: Copeptin levels at admission and discharge in relation to several research parameters.
The blood level of copeptin at admission and discharge differed non-significantly between the STEMI and NSTEMI groups, however it was higher in the STEMI group (Table 7). LVEF% was non-significantly greater in NSTEMI (Table 8).
| Copeptin (pmol/L) Ad | Al patients | T-Test | ||
|---|---|---|---|---|
| STEMI | NSTEMI | T | P-value | |
| Range | 40.6-113 | 40.4-77.5 | 1.148 | 0.258 |
| Mean ± SD | 57.622 ± 16.134 | 52.529 ± 9.948 | ||
| Copeptin (pmol/L) Ds | STEMI | NSTEMI | T | P-value |
| Range | 7.2-22.4 | 7.7-18.1 | 0.491 | 0.626 |
| Mean ± SD | 13.865 ± 4.038 | 13.282 ± 3.202 | ||
| Note: Copeptin Ad: Copeptin at Admission, Copeptin Ds: Copeptin at Discharge. | ||||
Table 7: Comparison between STEMI and NSTEMI groups with copeptin level at admission and at discharge.
| LVEF% | Alpatients | T-test | ||
|---|---|---|---|---|
| STEMI | NSTEMI | T | P-value | |
| Range | 35-56 | 38-50 | 1.211 | 0.233 |
| Mean ± SD | 43.565 ± 5.575 | 41.706 ± 3.460 | ||
Table 8: Comparison between STEMI and NSTEMI groups with LVEF%.
The difference between STEMI and NSTEMI groups regarding RSWMA is presented in Table 9.
| RSWMA | Al patients | Chi-Square | ||||||
|---|---|---|---|---|---|---|---|---|
| STEMI | NSTEMI | Total | ||||||
| N | % | N | % | N | % | X2 | P-value | |
| Negative | 0 | 0 | 3 | 17.65 | 3 | 7.5 | 4.388 | 0.036 |
| Positive | 23 | 100 | 14 | 82.35 | 37 | 92.5 | ||
Table 9: Comparison between STEMI and NSTEMI groups with RSWMA.
Patients were contacted and evaluated for the occurrence of major adverse cardiac events (mortality, AMI, survived sudden cardiac arrest, re-hospitalization for ACS, acute unexpected percutaneous coronary intervention, documented life threatening arrhythmias, coronary artery bypass grafting or admission for HF after all patients were flown up for 90 days. Three individuals experienced serious cardiac events (Table 10). These MACE patients exhibited higher copeptin levels in their blood at both admission and discharge, with P-values of 0.001 and 0.003, respectively. When comparing MACE between STEMI and NSTEMI groups, this connection was not significant (P-value=0.122) (Table 11).
| MACE | ||
|---|---|---|
| N | % | |
| Negative | 37 | 92.5 |
| Positive | 3 | 7.5 |
Table 10: Major adverse cardiac events.
| Copeptin (pmol/L) Ad | MACE | T-test | ||
|---|---|---|---|---|
| Negative | Positive | t | P-value | |
| Range | 40.4-77.5 | 77.7-113 | -6.912 | <0.001 |
| Mean ± SD | 52.535 ± 8.561 | 91.500 ± 18.868 | ||
| Copeptin (pmol/L)Ds | Negative | Positive | t | P-value |
| Range | 7.2-18.3 | 17.6-22.4 | -3.119 | 0.003 |
| Mean ± SD | 13.151 ± 3.354 | 19.367 ± 2.639 | ||
Table 11: Relation between copeptin at admission and at discharge with MACE.
Discussion
The goal of this trial was to determine whether it was possible to use pathophysiologically distinct biomarkers in combination or as separate biomarkers to aid in the early clinical identification of AMI patients who have chest pain. Copeptin served as a stand-in biomarker for the AVP mediated stress adaption axis, while cTnI and CK-MB were examined as myocardial necrotic biomarkers.
This was intended to be an observational prospective experiment. From February 15th to September 15th, 2015, forty individuals were admitted to the emergency department of Ain-Shams university hospital in Cairo, Egypt, with signs and symptoms of AMI during the 1st four hours of chest discomfort.
The cardiology department at Ain Shams university gave its approval to the study plan. Individuals with ST segment depression or a normal ECG received a STEMI discharge diagnosis in 23 patients (57%) and an NSEMI diagnosis in 17 patients (42%), according to this study. With a mean age of 58.756.04 years, patients varied in age from 47 to 73; 25 patients (62.50%) were male and 15 patients (27.50%) were female. Of the total research participants, 11 individuals (27.5%) smoked.
At the time of admission, all patients had copeptin, cTn I, CK-Total and CK-MB levels that were high (over the cutoff threshold). Copeptin levels varied from 40. pmol/L to 103 pmol/L upon admission, with a mean of 55.458 ± 13.926.
There was a non-significant difference in serum copeptin levels between the STEMI and NSTEMI groups at admission and discharge in this research, however the STEMI group's levels were higher. The quick release of AVP/copeptin following an AMI is explained by a number of theories. AVP's quick response as a component of the endocrine stress axis, which causes the release of cortisol and adrenocorticotropic hormone, is one potential explanation.
Copeptin is thought to be a quick and accurate biomarker of each person's stress reaction [18]. AVP/copeptin production from the posterior pituitary gland may result from direct injury to cardiac baroreceptors or from baroreceptor activation caused by the threat of hypotension from an AMI. The second option is backed by the observation that individuals with STEMI experience the greatest copeptin increase following AMI [19].
Copeptin has little specificity for a single illness, such as myocardial infarction, because it is raised in various clinical situations characterised by endocrinologic stress signals. Copeptin biomarkers, on the other hand, show high sensitivity for the illness state since myocardial infarctions are associated with hypothalamic stress axis activation.
Males had greater serum copeptin levels upon admission in this trial, but there was no statistically significant difference between the two genders. This was comparable to that which Duchenne, et al. described [20].
Patients who smoked exhibited non-statistically significant increased copeptin values upon admission (P-value 0.106). This was in line with what CHOPIN observed, but it differed from what Duchenne, et al. reported, with smoking patients having statistically significant higher copeptin values [21].
The serum level of copeptin upon admission was not significantly correlated with age, BMI, haemoglobin CK-total, CK-MB or cTn I Ad. Additionally, this agreed with what CHOPIN stated. Time to present in the ER had a non-significant negative connection with serum copeptin levels upon admission (h). This was comparable to what Gimenez, et al. reported [22].
Copeptin upon admission, heart rate and cTnI HL were found to significantly positively correlate with one another. This was comparable to what Silva Marques, et al. reported and it showed a strong positive connection with both heart rate and cTnI HL [23].
Similarly to what Berliner, et al. discovered, the blood copeptin level at discharge was shown to be negatively associated with LVEF% in our study, with a highly significant P-value of 0.002. Subendocardial infarction may account for the lack of RSWMA in the three individuals (7.5%) with NSTEMI [24].
Re-hospitalization for ACS, AMI, acute unexpected percutaneous coronary intervention, coronary artery bypass grafting, proved life threatening arrhythmias or admission for HF were all serious adverse cardiac events evaluated in all patients followed up for 90 days. Three people (7.5%) had serious adverse cardiac events. MACE patients showed greater copeptin levels in their blood at both admission and discharge, with highly significant P-values of 0.001 and 0.003, respectively.
All patients presenting for potential ACS have been included in the majority of research investigating the predictive significance of copeptin in chest pain. Because biomarkers are not currently used in the therapy of STEMI, the majority of studies do not include patients with ST elevation. The predictive usefulness of copeptin was confirmed by three such investigations published in 2013 [25]. Patients were monitored for 60 days in a singlecenter trial at Skane university hospital in Lund, Sweden.
When 478 consecutive patients with chest discomfort were admitted, troponin and copeptin levels were measured. The end goals were AMI, mortality and non-elective revascularization at 60 days. Only 2% of individuals with chest pain who initially reported with death were also found to have elevated copeptin levels. In the patients who had a negative copeptin, neither in the hospital nor during the 60 days follow-up, there were any fatalities. A negative copeptin was found to be an accurate predictor of 60 days survival in the research.
In a 2nd single center study, copeptin's prognostic value was compared to troponin's while patients with chest pain were followed up on for six months in Germany. The study comprised 230 consecutive individuals with suspected ACS who also had chest discomfort and 107 of these patients had an AMI diagnosis. Copeptin, when paired with troponin, was a substantial additive prognostic factor at 180 days and a strong predictor of death.
The copeptin assists in the early detection of patients with AMI experiment is the most extensive examination of the predictive usefulness of copeptin in chest pain. Approximately 2000 individuals with chest discomfort were recruited from 16 hospitals in the United States. All-cause mortality was measured at 30 and 180 days as a secondary goal. Copeptin performed exceptionally well in predicting early acute episodes at 30 days. Additionally, copeptin demonstrated a greater predictive value when combined with troponin than either one alone as a predictor of death at 6 months.
Other studies have chosen to concentrate on ACS specific diagnosis. The Leicester Acute Myocardial Infarction Peptide (LAMP) research was one of the first substantial studies evaluating the predictive usefulness of copeptin. Copeptin levels in 980 patients with post-acute myocardial infarction were monitored for a year (STEMI and NSTEMI). Copeptin levels were substantially greater in patients with heart failure who died or were readmitted than in those who did not.
According to receiver operating characteristic analysis, copeptin's predictive value was equivalent to that of Brain Natriuretic Peptide (BNP) and the two together performed even better. A crucial discovery is how copeptin affects prognosis copeptin has been connected to the degree of left ventricular remodeling after an AMI and has been found to be a reliable indication of mortality and morbidity in those who develop heart failure after an AMI [26]. These results were corroborated by the higher likelihood of increased copeptin concentrations and clinical heart failure in those people. In the future, early treatment regimens following an AMI may be selected using this risk categorization, such as the use of AVP receptor antagonists (the "vaptan" class of medications) [27-29].
Conclusion
Additionally, these findings imply that even if AMI is ruled out, further investigation into the cause of the increased copeptin level is probably necessary. A recent study of patients with heart failure treated in an outpatient clinic revealed the independent predictive information offered by using copeptin and troponin readings. According to this study, copeptin and cardiac troponin T increases are effective predictors of mortality and hospitalisation both alone and in combination. The researchers hypothesise that simultaneous examination of myocardial injury and the active vasopressin system may have prognostic value. Others have also reported on copeptin's prognostic efficacy in congestive heart failure. Copeptin testing's enhanced ability to rule out AMI earlier may improve the ED's resource management and significantly lower overall treatment costs. Our findings indicated that a dual marker technique using copeptin and cTnI had a high PPV in the early diagnosis of AMI.
Copeptin plays a critical role in the earlier and more accurate identification of AMI when used in combination with troponin following a clinical work-up. When copeptin is administered upon presentation, there is also a lot of data to suggest its prognostic relevance in AMI patients.
Limitations
It's important to recognise the following study's limitations: First, the inclusion of only a few patients from a single recruiting medical institution limits the scope of our study (single centre study). In order to get more accurate and reliable cutoff values and diagnostic impact, the results must be validated and expanded in bigger multicenter trials on a greater number of patients. Second, as no therapeutic choice or route was based on copeptin values, this study could not precisely quantify the clinical advantages brought about by the interaction of copeptin and cTnI. Therefore, further randomised interventional trials are necessary to get this data. Third, this study's copeptin was quantified utilising its commercial ELISA kits. The assay process took a long time since it took 5-6 hours to find copeptin. This method's disadvantage is that it cannot be used in clinical practise or for the early detection of AMI, when cTnI is at its peak in terms of peak, sensitivity and specificity.
Funding
None.
Conflict of Interest
Has been reported.
References
- Searle MJ, Muller R, Slagman A, Storchmann H, Oestereich P, et al. (2013) Do they relate to underlying disease and outcome? The Charite Emergency Medicine Study (CHARITEM). Eur J Emerg Med 20:103-108.
[Crossref] [Google Scholar] [PubMed]
- Goodacre S, Nicholl J, Dixon S, Cross E, Angelini K, et al. (2004) Randomised controlled trial and economic evaluation of a chest pain observation unit compared with routine care. BMJ 328:254.
[Crossref] [Google Scholar] [PubMed]
- Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, et al. (2000) Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 342:1163-1170.
[Crossref] [Google Scholar] [PubMed]
- Lidija D, Kido CE (2013) Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med 23:172-192.
[Crossref] [Google Scholar] [PubMed]
- Lotze U, Lemm H, Heyer A, Muller K (2011) Combined determination of highly sensitive troponin T and copeptin for early exclusion of acute myocardial infarction: First experience in an emergency department of a general hospital. Vasc Health Risk Manag 7:509-515.
[Crossref] [Google Scholar] [PubMed]
- Thygesen K, Mair J, Giannitsis E (2013) How to use high sensitivity cardiac troponins in acute cardiac care. Eur Heart J 33:2252-2257.
[Crossref] [Google Scholar] [PubMed]
- Christian HN, Bingisser R, Nils G (2012) The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med 10:7.
[Crossref] [Google Scholar] [PubMed]
- Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, et al. (2011) Correlation of plasma copeptin and vasopressin concentrations in hypo, iso and hyperosmolar states. J Clin Endocrinol Metab 96:1046-1052.
[Crossref] [Google Scholar] [PubMed]
- Piyanuttapull S (2013) Correlation of plasma copeptin levels and early diagnosis of acute myocardial infarction compared with troponin- T. J Med Assoc Thai 96:13-91.
[Google Scholar] [PubMed]
- Thelin J, Borna C, Erlinge D, Ohlin B (2013) The combination of high sensitivity troponin T and copeptin facilitates early rule-out of ACS: A prospective observational study. BMC Cardiovasc Disord 13:42.
[Crossref] [Google Scholar] [PubMed]
- Bohyn E, Dubie E, Lebrun C, Jund J, Beaune G, et al. (2014) Expeditious exclusion of acute coronary syndrome diagnosis by combined measurements of copeptin, high-sensitivity troponin and GRACE score. Am J Emerg Med 32:293-296.
[Crossref] [Google Scholar] [PubMed]
- Khan S, Dhillon O, OBrien R, Struck J, Quinn P, et al. (2007) C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation 115:2103-2110.
[Crossref] [Google Scholar] [PubMed]
- Voors AA, Von Haehling S, Anker SD, Hillege HL, Struck J, et al. (2009) C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: Results from the OPTIMAAL study. Eur Heart J 30:1187-1194.
[Crossref] [Google Scholar] [PubMed]
- Do they relate to underlying disease and outcome? The Charite Emergency Medicine Study (CHARITEM).
- Mastropietro W, Mahan M, Valentine M, Clark A, Hines C, et al. (2012) Copeptin as a marker of relative arginine vasopressin deficiency after pediatric cardiac surgery. Intensive Care Med 38:2047-2054.
[Crossref] [Google Scholar] [PubMed]
- Morgenthaler N, Struck J, Alonso C, Bergmann A (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52:112-119.
[Crossref] [Google Scholar] [PubMed]
- Keller T, Tzikas S, Zeller T, Czyz E, Lillpopp L, et al. (2010) Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol 55:2096-2106.
[Crossref] [Google Scholar] [PubMed]
- Katan M, Morgenthaler N, Widmer I, Puder J, Konig C, et al. (2008) Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett 29:341-346.
[Google Scholar] [PubMed]
- Duchenne J, Mestres S, Dublanchet N, Combaret N, Marceau G, et al. (2009) Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 361:858-867.
[Crossref] [Google Scholar] [PubMed]
- Duchenne J, Mestres S, Dublanchet N, Combaret N, Marceau G, et al. (2013) Diagnostic accuracy of copeptin sensitivity and specificity in patients with suspected non-ST-elevation myocardial infarction with troponin I below the 99th centile at presentation. BMJ Open 4:e004449.
[Crossref] [Google Scholar] [PubMed]
- Maisel A, Mueller C, Neath S, Christenson R, Morgenthaler N, et al. (2013) Copeptin helps in the early detection of patients with acute myocardial infarction. J Am Coll Cardiol 62:150-160.
[Crossref] [Google Scholar] [PubMed]
- Gimenez RM, Twerenbold R, Reichlin T, Wildi K, Haaf P, et al. (2014) Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J 35:2303-2311.
[Crossref] [Google Scholar] [PubMed]
- Afzali D, Erren M, Pavenstadt HJ, Vollert JO, Hertel S, et al. (2013) Impact of copeptin on diagnosis, risk stratification and intermediate-term prognosis of acute coronary syndromes. Clin Res Cardiol 102:755-763.
[Crossref] [Google Scholar] [PubMed]
- Kelly D, Squire IB, Khan SQ, Quinn P, Struck J, et al. (2008) C-terminal provasopressin (copeptin) is associated with left ventricular dysfunction, remodeling and clinical heart failure in survivors of myocardial infarction. J Card Fail 14:739-745.
[Crossref] [Google Scholar] [PubMed]
- Tentzeris I, Jarai R, Farhan S, Perkmann T, Schwarz MA, et al. (2011) Complementary role of copeptin and high-sensitivity troponin in predicting outcome in patients with stable chronic heart failure. Eur J Heart Fail 13:726-733.
[Crossref] [Google Scholar] [PubMed]
- Alehagen U, Dahlstrom U, Rehfeld JF, Goetze JP (2011) Association of copeptin and N-terminal proBNP concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. JAMA 305:2088-2095.
[Crossref] [Google Scholar] [PubMed]
- Neuhold S, Huelsmann M, Strunk G, Stoiser B, Struck J, et al. (2008) Comparison of copeptin, B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: Prediction of death at different stages of the disease. J Am Coll Cardiol 52:266-272.
[Crossref] [Google Scholar] [PubMed]
- Peacock WF, Nowak R, Christenson R, DiSomma S, Neath SX, et al. (2011) Short-term mortality risk in emergency department acute heart failure. Acad Emerg Med 18:947-958.
[Crossref] [Google Scholar] [PubMed]
- Maisel A, Xue Y, Shah K, Mueller C, Nowak R, et al. (2011) Increased 90 days mortality in patients with acute heart failure with elevated copeptin: Secondary results from the Biomarkers in Acute Heart Failure (BACH) study. Circulation Heart Fail 4:613-620.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi