Research Article, J Plant Physiol Pathol Vol: 11 Issue: 6
Response of Different Salts as Alternative Nitrogen Source for Ginger (Zingiber officinal rosc.) In Vitro Regeneration
Genene Gezahegn1*, Tileye Feyissa2 and Yayis Rezene1
1Department of Agriculture, Agricultural Research Institute, Hawassa, Ethiopia
2Department of Biotechnology, Addis Ababa University, Addis Ababa, Ethiopia
*Corresponding Author: Tileye Feyissa
Department of Biotechnology, Addis Ababa University, Addis Ababa, Ethiopia
Tel: 251913818867
E-mail: genuetu2005@gmail.com
Received date: 04 November, 2022, Manuscript No. JPPP-22-79054; Editor assigned date: 07 November, 2022, PreQC No. JPPP-22-79054 (PQ); Reviewed date: 22 November, 2022, QC No. JPPP-22-79054; Revised date: 13 February, 2023, Manuscript No. JPPP-22-79054 (R); Published date: 20 February, 2023, DOI: 10.4172/2329-955X.1000364
Citation: Gezahegn G, Feyissa T, Rezene Y (2023) Response of Different Salts as Alternative Nitrogen Source for Ginger (Zingiber oficinal rosc.) in vitro Regeneration. J Plant Physiol Pathol 11:2
Abstract
In Ethiopia, ginger is mainly produced in southern parts of the country specifically in some districts of Wolayta and Kambata Tambaro administrative zones. Its production is being challenged primarily due to bacterial wilt disease eruption as of 2012 production season. Use of disease free tissue culture generated seed rhizome as part of integrated management was considered as best option to reduce the disease impact. On the other hand, major media components like ammonium nitrate are not available for large scale in vitro propagation. To enhance disease free ginger in vitro propagation; experiment was designed with the aim to select alternative sources of nitrogen to replace unavailable ammonium nitrate due to its explosive nature and safety issue. Three nitrogen salts at different level were evaluated as part of MS medium for in vitro regeneration of Volvo ginger cultivar. Three alternative salts tasted (NH4Cl KNO3 and urea) were observed as potential replacements of NH4NO3 in MS medium supplemented with 2.0 mg/l BAP and 1.0 mg/l Kinetin. The highest mean shoot number (9.33) of shoots from media containing 1.0 g/l NH4Cl followed by mediums containing 1.9 g/l KNO3 and 4.5 g/l urea with record of mean shoot numbers 7.33 and 7.00 respectively. Whereas, the lowest explants survival and proliferation was observed on medium containing 2.0 g/l and 1.65 g/l NH4Cl. Root formation and survival after acclimatization were also affected negatively at elevated levels of NH4Cl. The highest mean number of roots was observed on medium supplemented with 1.0 g/l NH4Cl followed by normal MS media. In contrary, the lowest root number (4) was observed on medium containing 2.0 g/l NH4Cl. Survival after acclimatization was found to be 98% for plants derived from medium containing 4.5 and 1.9 g/l urea and KNO3 respectively followed by 95% for plants derived from medium containing 1.0 g/l NH4Cl. This experiment revealed that the three nitrogen salts can be used as nitrogen source. But urea (3 g/l to 4.5 g/l) was the first option for ginger in vitro propagation which ensures availability and low cost tissue culture technique.
Keywords: Ginger; Bacterial wilt; Disease free; Low cost; Urea
Introduction
The cultivation and utilization of ginger in Ethiopia started during 13th Century when Arabs introduced it from India to East Africa [1]. Ginger has been used for many purposes and become a major spice in both the local and export markets. In terms of area harvested and total production Ethiopia stood 10th and 14th, respectively in 2011 among the 36 countries engaged in ginger production globally [2]. Its production at large scale was mostly limited in the hot humid areas. Rhizome is the most commonly used planting material for production which needs large amount per unit area of land from 2-3 tons per hectare of land in 40 cm × 15 cm spacing in Ethiopia [3]. Moreover, ginger wilt disease complex that erupted in 2012 production season has devastated the crop and halted its production and marketing. The use of disease free clean planting material associated with a clean planting medium has been suggested to be one of the best options to restore ginger production in Ethiopia. Many workers have reported rapid clonal propagation of ginger on Murashige and Skoog (MS) growth medium with varying concentration of plant growth regulators. Most of the tissue culture media types including modified MS media are characterized by high level of nitrogen in the form of two nitrate salts; Ammonium Nitrate (NH4NO3) and Potassium Nitrate (KNO3) supplementing each other. These nitrogen and potassium are macro nutrients required in large amount in plant tissue culture media for successful regeneration and mass propagation of plantlets. However, the explosive nature of ammonium nitrate makes it to be banned from market and this resulted in unavailability and unaffordability due to high cost up to $400/Kg in Ethiopia. This is recently challenging tissue culture research and large scale propagation of strategic crops including ginger. Studies focused on effect of nitrogen source in the form of ammonium nitrate and potassium nitrate indicate that MS media with nitrogen only from potassium nitrate but omitted ammonium nitrate resulted in significantly high number of shoots per explants [4]. MS medium with high ammonium nitrate has been also reported to be inhibitory to the tuberization process in D. alata [5]. Atim and her colleagues in 2013 reported banana plantlets in vitro regenerated from MS medium supplemented with high concentration of potassium and nitrogen significantly reduced bacterial wilt disease incidence and increased incubation period of the disease. Recent study carried out to substitute NH4NO3 with urea in potato plantlets regeneration media reported maximum shoot and leaf numbers with healthy and robust plantlets of potato over media with NH4NO3 [6]. Hence, this research article focuses on performance of three different nitrogen salts as alternative source of nitrogen nutrient in MS media instead of NH4NO3 for efficient ginger plantlets in vitro propagation.
Materials and Methods
Plant material: Plantlets of Volvo cultivar were in vitro generated as per protocol developed by Ayenew and his colleagues in 2012 and optimized as per Areka tissue culture laboratory condition were used as experimental materials.
Shoot induction and multiplication: Explants of 2 cm-3 cm length from ginger rhizomes sprouted on sterile sand were collected for surface sterilization and initiation. Surface sterilization was done after thorough washing by tap water and home detergents. Then explants were deepen to 70% ethanol for 10 minutes accompanied by shaking in intervals. After discarding ethanol 3 times washing with sterile distilled water was followed before exposing to 2% chlorox (Gion berekina) for 15 minutes and similar washing by distilled four times. Then each explant was excised to 1 cm-1.5 cm length before transferring to initiation MS media supplemented with 2 mg/l BAP and 1 mg/l kinetine keeping one explant per jar. For multiplication, well initiated explants with growing shoots and active nodes at the bottom of the explants on initiation media were transferred to multiplication MS media similar in growth hormones combination in initiation. Apical dominance was carefully removed to induce multiple shoot growth before transfer to this stage. Successive sub-culturing to fresh same media was done in average 30 days interval.
Three nitrogen salts (NH4Cl, KNO3 and urea (N2H4CO) at four concentration levels for each constituting 14 treatments including standard MS medium supplemented with different concentrations of growth regulators and growth regulator free as control were used. Culture jars of 250 ml capacity were randomly arranged on growth shelves after explants were transferred to 40 ml medium. Five in vitro shoots as explants were cultured per jar in six replication for each treatment at all stages. In treatments where KNO3 is omitted or minimized comparable amount of KH2PO4 (1.9 g and 0.95 g/l for full and half strength media respectively) was added to the media during preparation for the treatments in order to keep K+ level constant. Sucrose (table sugar) at 30 g/l as carbon source and solidifying agent; agar at 6 g/ l were used across the experiments. The pH was adjusted to 5.8 using either 1N of NaOH or HCl before addition of agar. Moreover, all media prepared as per each treatment were supplied with 2 mg/l Benzyl Aminopurine (BAP) and 1 mg/l Kinetin for shooting.
Rooting and acclimatization: Rooting stage was bypassed as per protocol in use at Areka plant tissue culture research laboratory in which simultaneous rooting was experienced at multiplication medium. For acclimatization, well established plantlets with shoots, leaves and roots were carefully pulled out from media using scalpels. Clean tap water was used to wash out media and very long roots were trimmed to 5 cm-6 cm before planting in pots containing soil mix. Soil mix for acclimatization was prepared from decomposed coffee husk, red ash and forest soil, mixed well in 2:1:1 ratio respectively. The soil mix was sterilized in barrel given fire at the bottom for 2 hours to avoid any nematodes and worms.
Data collection for both in vitro and in vivo plantlets performance of each treatment; number of explants survival, days to shoot induction, number of shoots, number of roots, shoot and root length, shoot fresh weight, number of plants survived in acclimatization were done. Data collected for most parameters was subjected to ANOVA and mean of each treatment was compared using LSD at 5% significance level using SAS version 9.3. Whereas, data of explants survival in vitro and plants survived in acclimatization was stated as percentage.
Cost benefit analysis was also computed based on the moment market price of salts used. Cost saved by different alternative N2 sources used in this study was calculated with the formula;
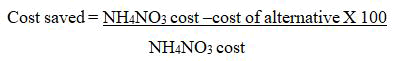
Results and Discussion
Surface sterilization and rate of initiation
Surface sterilization with ethanol alone has not controlled the entophytic contaminants; hence 0.1 % of mercuric chloride tried later has led to 50% explants free of contamination. 90% of contamination free explants initiated average of 2.5 shoot in MS initiation medium with 2 mg BAP and 1 mg/l Kinetin.
Contamination free plantlets transferred for multiplication to fresh media performed well with record mean number of plantlets 8.5 per explant. High loss of plantlets due contamination was minimized to 10% in average by clean lines control, subsequent sub-culturing. Berihu in 2018 reported multiplication factor of 8 for unspecified variety using MS medium with 2.5 mg/l BAP and 0.5 kinetin to which the output of this experiment also coincides.
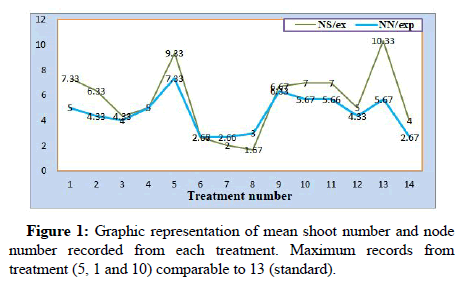
The ANOVA result for the in vitro regeneration variables among the 14 treatments showed that there is highly significant difference among treatment means for all the six measurements. Three treatment combinations to NH4NO3 replacement were observed as promising sources of nitrogen nutrient as compared to standard MS media (13th treatment). MS medium with omitted NH4NO3 and replaced by 1 g/l NH4Cl resulted in statistically none significant number of shoots and length with standard MS media containing 1.65 g/l NH4NO3. Average shoots in number (9.33) for NH4Cl supplied media and 10.00 shoots per explant was recorded for standard MS media indicating there is no significant difference among the two treatment means. MS medium supplemented with 3.8 g/l KNO3 to compensate N2 from ammonium nitrate showed the second highest mean number of shoots (7.33) per explant. For the third alternative tested urea, which is the common inorganic nitrogen fertilizer medium containing 3 g/l and 4.5 g/l produced 7.0 mean numbers of shoots per explant. Another in vitro performance parameter recorded is number of active buds on micro rhizome under shoot clumps which can grow to new shoots in next sub-culturing. The highest mean number (7.33) for this was also recorded for medium of 1 g/l NH4Cl followed by 4.5 g/l urea in which 5.67 number of buds per explants achieved (Table 1).
| Trt | Treatment combination | NS/ex | NN/exp | SL (cm) | LN | RN | RL |
|---|---|---|---|---|---|---|---|
| 1 | MS-NH4NO3+KNO3 | 7.33 | 5.00 | 6.23 | 4.33 | 10.33 | 10.00 |
| 2 | MS-50% NH4NO3+50% KNO3 | 6.33 | 4.33 | 7.90 | 3.67 | 11.33 | 8.96 |
| 3 | MS-50% NH4NO3–50% KNO3 | 4.33 | 4.00 | 4.60 | 4.33 | 7.33 | 8.13 |
| 4 | MS-50% NH4NO3 | 5.00 | 5.00 | 5.60 | 4.33 | 8.33 | 7.66 |
| 5 | MS-NH4NO3+1 gm/l NH4Cl | 9.33 | 7.33 | 8.67 | 5.00 | 19.66 | 12.56 |
| 6 | MS-NH4NO3+1.325 gm/l NH4Cl | 2.66 | 2.67 | 3.26 | 1.67 | 6.33 | 7.33 |
| 7 | MS-NH4NO3+1.65 gm/l NH4Cl | 2.00 | 2.66 | 2.60 | 1.66 | 5.33 | 4.73 |
| 8 | MS-NH4NO3+2 gm NH4Cl | 1.67 | 3.00 | 2.06 | 2.00 | 4.00 | 3.13 |
| 9 | MS-NH4NO3+1.5 gm urea | 6.67 | 6.33 | 7.00 | 4.33 | 10.00 | 8.36 |
| 10 | MS-NH4NO3+3 gm urea | 7.00 | 5.67 | 7.36 | 4.67 | 11.67 | 9.23 |
| 11 | MS-NH4NO3+4.5 gm urea | 7.00 | 5.67 | 5.67 | 4.10 | 14.67 | 11.73 |
| 12 | MS-NH4NO3+6 gm urea | 5.00 | 4.33 | 5.00 | 3.5 | 9.00 | 11.70 |
| 13 | MS standard | 10.00 | 5.67 | 8.56 | 5.00 | 16.00 | 12.86 |
| 14 | MS HF control | 4.00 | 2.67 | 8.46 | 4.00 | 10.00 | 6.10 |
| CV (%) | 19.17 | 22.77 | 14.54 | 24.89 | 14.69 | 10.26 | |
| LSD (5%) | 1.805 | 1.75 | 1.42 | 1.56 | 2.52 | 1.5 | |
| Note: NS/exp: Number Shoots Per Explant, NN/explant: Number of Active Node Per Explant, SL: Shoot Length, LN: Leaf Number Per Shoot RN: Root Number Per Shoot and RL: Root Length. | |||||||
Table 1: Mean shoot number per explant on medium containing different nitrogen sources.
The analysis result also revealed that there is significant difference among other treatments for number of active nodes which indicated shoot proliferation continues with similar trend in subsequent sub-culturing as the number of nodes is directly proportional to shoot number. The highest mean shoot number is recorded on medium containing 1 mg/l NH4Cl from the alternatives tasted followed by 1.9 g/l KNO3 and urea at 3 g/l and 4.5 g/l media. On the other hand, non-significant number of shoots and nodes, very short shoots and roots were recorded on medium containing high amount of NH4Cl (>1 g/l ) and urea more than 4.5. Moreover, it was observed that supplying ammonium chloride more than 1 g/l and urea above 4.5 g/l resulted in limited root induction and growth followed by shoot drying after three weeks of culturing. This might be due to toxic effect of high chlorine to plants which prevents uptake of nutrients by the explants. Number of leaves per shoots is the least affected growth parameter as there is no significance difference among most treatments except for treatments with high NH4Cl and urea. From the result observed three treatments; 1, 5 and 10 at specified levels were observed as potential alternative sources of N2 which can replace NH4NO3 for ginger micropropagation (Figure 1).
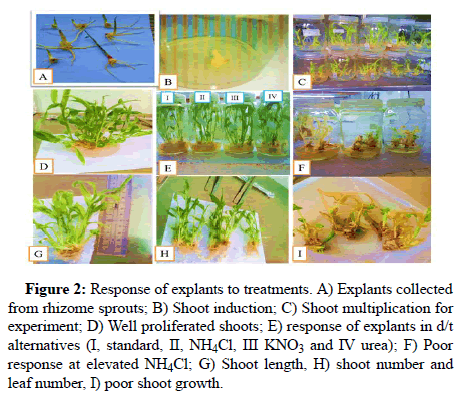
Study conducted on ginger by using double amount (3.8 g/l) of KNO3 in MS media as sole source of nitrogen indicated that nitrogen in the form of KNO3 significantly improved proliferation rate of ginger in vitro, in both full and half strength media. Leaf growth and root formation was also reported better in media devoid of NH4NO3 [7]. Another experiment conducted to substitute NH4NO3 by urea for in vitro regeneration of potato reported that 5 g/l urea resulted in better proliferation and regeneration of potato plantlets which is basically in line with our finding even if the crops are different [8]. It is obvious that ammonium nitrate was recently banned in many countries including Ethiopia. Hence tissue culture researchers are globally looking for alternative sources as replacement especially to compensate ammonium part (Figure 2). Our experiment which included ammonium chloride was the first in its kind to the best of our knowledge which can be adopted to other crop in vitro regeneration protocols.
Figure 2: Response of explants to treatments. A) Explants collected from rhizome sprouts; B) Shoot induction; C) Shoot multiplication for experiment; D) Well proliferated shoots; E) Response of explants in d/t alternatives (I, standard, II, NH4Cl, III KNO3 and IV urea); F) Poor response at elevated NH4Cl; G) Shoot length, H) Shoot number and leaf number, I) Poor shoot growth.
Root growth and acclimatization
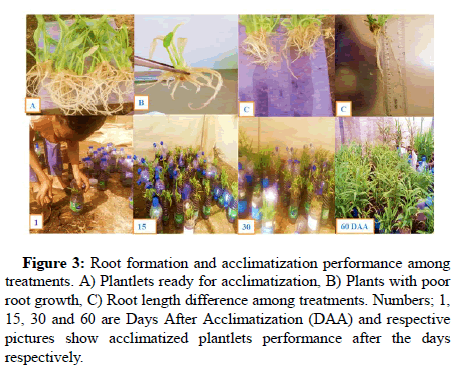
The shoots developed roots spontaneously at multiplication stage on all medium combinations of salt types and growth regulators. The ANOVA showed that there is highly significant difference among treatments for root number and length. The highest mean number of roots and length were recorded from medium of KNO3 at 1.9 g/l concentration followed by standard MS medium with 1.65 g/l NH4NO3 and 10. Whereas, the lowest mean number and length of roots were recorded from medium in which shoot growth and development was also limited. Media containing 1.325 g/l to 2 g/l of NH4Cl recorded limited root growth with no significant difference. Similar trend was also observed in root length mean decreases when amount of NH4Cl increases from 1.325 g/l to 2 g/l. The low number and length of roots has affected the growth of shoots in vitro and also affected acclimatization survival percentage of plants acclimatized from each medium. The root and shoot growth inhibition effect observed was directly proportional to increase in ammonium chloride amount which is probably due to accumulation of NH4+ and chlorine free ions that make the media acidic and hence affected nutrient up take and also can act as toxic to plant shoots (Figure 3). Study conducted before revealed that uptake of nitrate ions by plant cells leads to a drift towards an alkaline pH, while NH4+ uptake results in a more rapid shift towards acidity and makes balance of PH in plants [9]. These previous work reports and observations in this experiment tells us elevated amount of NH4Cl and urea makes the media more acidic which finally limits shoot and root growth in in vitro ginger plantlets production.
Figure 3: Root formation and acclimatization performance among treatments. A) Plantlets ready for acclimatization, B) Plants with poor root growth, C) Root length difference among treatments. Numbers; 1, 15, 30 and 60 are Days After Acclimatization (DAA) and respective pictures show acclimatized plantlets performance after the days respectively.
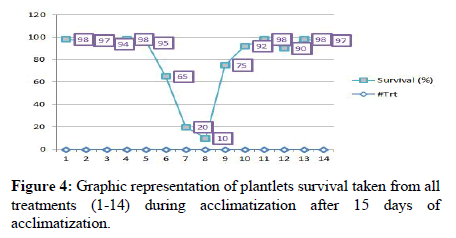
Acclimatization was done after six weeks of culturing directly from multiplication stage as the plantlets have developed roots simultaneously on multiplication medium. Root development in multiplication medium of ginger was also reported previously due to enough endogenous auxin hormone to induce roots [10]. Plantlets grown together as shoot clumps are detached first after washing out media and acclimatized. Plants with average of root number six and above per shoot and medium to large (>7 cm) root and shoot length had resulted in 96% survival after 15 days of acclimatization (Figure 4). Whereas, plants with short shoot, few roots and root length were failed to achieve enough survival after fifteen days of acclimatization. In this regard plants from medium with 1.65 g/l and 2 g/l NH4Cl resulted in 15 percent of acclimatization survival due to their weak regeneration. On the other hand, plantlets from 1.325 g NH4Cl and 1.5 g/l urea resulted in 65% and 75% acclimatization survival after 15 days of acclimatization respectively. Plantlets from the remaining 10 media combinations resulted in average of 94% survival after 15 days. These mediums from which plants are with the highest survival rate were nearly equal with MS standard with and without growth regulators used as standard checks. Research reports for acclimatization survival rate of ginger in vitro regenerated plants are nearly similar with our findings. Study conducted using two cultivars to optimize protocol for Ethiopian ginger reported average of 83.5% acclimatization survival [11]. The acclimatization survival rate achieved was also in line with recent published protocol which reported average of 95% plantlets ex vitro survival for ginger micro-propagation in standard MS media with different growth regulators [12].
Cost benefit analysis for alternatives used
The cost of alternative nitrogen sources has saved costs for in vitro regeneration of ginger with minimum number of plantlets penalty per culture which can be compensated by purchasing some additional amount of specific alternative salt type [13]. Among the three alternatives tested potassium nitrate followed by ammonium chloride is costly and urea is the cheapest and easily available alternative source of nitrogen (Table 2). Urea at $0.88/kg moment price to use 3 g/l amount in one litter of medium has saved 99.75% from equal amount of NH4NO3 at pick price of $392 during experiment time in 2021. Maximum plantlets penalty (30%) per batch culture was also resulted from medium of urea at selected amount 3 g/l which can be compensated by subsequent sub-culturing as it is easily available with very low cost. Whereas, a kg of ammonium nitrate rarely obtained by high cost ($392/Kg) has only 30% plantlets advantage over urea, 26.7 over KNO3 and 6.7% to NH4Cl medium. The second better alternative, NH4Cl from which the highest mean shoot number and better growth was with medium cost and also required in small amount (1 g/l) resulted in statistically non-significant shoot number as compared to standard MS medium.
| Item | Alternative salts | Unit cost ($/kg) | Cost saved (%) | Average plants/exp | #Plants penalty (%/exp) |
|---|---|---|---|---|---|
| 1 | NH4NO3 | 392 | 0 | 10 | 0 |
| 2 | KNO3 | 47 | 88 | 7.33 | 26.7 |
| 3 | NH4Cl | 27.45 | 93 | 9.33 | 6.7 |
| 4 | Urea | 0.88 | 99.75 | 7 | 30 |
Table 2: Cost and plantlets comparison of alternative sources.
Conclusion
From the experiment conducted and extensive observations analysis at different stages and rounds it was concluded that ammonium nitrate which has been major source of nitrogen in both nitrate and ammonium can be replaced with one of the three alternatives tasted. Among the tasted three salt types; urea which is common inorganic nitrogen fertilizer for efficient mass propagation of disease free ginger plantlets was best. That can also ensure low cost tissue culture for ginger. This work is first in its kind in Ethiopia and Africa to the best of our knowledge which can be adopted for other crops in vitro regeneration media.
References
- Asfaw K, Derbew B (2021) Leveraging early dry season planting of ginger under irrigation to enhance production from bacterial wilt infected seed rhizome. Afr Crop Sci J 29:259-275.
- Asfaw K, Merga J, Genene G, Abukiya G (2021) Technical guideline for management of ginger bacterial wilt and leaf spot diseases in Ethiopia. Farm Africa, Ethiopia. 1-76.
- Atim M, Beed F, Tusiime G, Tripathi L, van Asten P (2013) High potassium, calcium and nitrogen application reduce susceptibility to banana Xanthomonas wilt caused by Xanthomonas campestris pv. musacearum. Plant Dis 97:123-130.
[Crossref] [Google Scholar] [PubMed]
- Ayenew B, Wondifraw T, Kassahun B (2012) In vitro propagation of Ethiopian ginger (Zingiber officinale rosc.) cultivars: Evaluation of explant types and hormone combinations. Afr J Biotechnol 11:3911-3918.
- Bashar MA, Hoque ME, Siddique AB, Syfullah K, Sheuly KN, et al. (2021) Potentiality of urea as a substitute for ammonium nitrate in tissue culture media for potato plantlet regeneration. Plant Cell Biotechnol Mol Biol 22:118-126.
- Berihu M (2018) In vitro diseases cleaning, micro-propagation and field establishment of ginger (Zingiber officinale rosc.) cultivars. Int J Pharm Sci 3:8-11.
- Cecilia CV (2010) Influence of media strength and sources of nitrogen on micropropagation of ginger, Zingiber officinale rosc. Int J Sci Res 2:150-155.
- Endrias G, Asfaw K (2011) Production, processing and marketing of ginger in Southern Ethiopia. J Hortic Forestry 3:207-213.
- Genene G, Tigist M, Mekbib M (2019) Protocol adoption, in vitro regeneration of bacterial wilt free ginger (Zingiber officinale rosc.) plantlets and seed rhizome underprotected conditions. Proceeding of AGP-II completed crop research 1:76-78.
- Hyndman, Hasegawa PM, Bressan RA (1982) The role of sucrose and nitrogen in adventitious root formation on cultured rose shoots. Plant Cell Tissue Organ Cult 1:229-238.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:219-223.
- Tariku H, Kassahun S, Gezahegn G (2016) First report of ginger (Zingiber officinale) Bacterial wilt disease in ethiopia. Res J Agric 4:5-9.
- Zahid NA, Jaafar HZ, Hakiman M (2021) Micropropagation of ginger (Zingiber officinale rosc.) ‘bentong’ and evaluation of its secondary metabolites and antioxidant activities compared with the conventionally propagated plant. Plants 10:630.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi