Review Article, J Pharm Drug Deliv Res Vol: 13 Issue: 3
Regulatory Pathway and Application Preparation of 510(K) as Per USFDA
Silpa S, Pallavi Misra, Teshini Suthahar and S. Nagalakshmi*
Department of Pharmaceutics, Sri Ramachandra Faculty of Pharmacy, Sri Ramachandra Institute of Higher Education and Research (DU), Porur, Chennai 116, India
- *Corresponding Author:
- Nagalakshmi S
Department of Pharmaceutics,
Sri Ramachandra Faculty of Pharmacy,
Sri Ramachandra Institute of Higher Education and Research (DU),
Porur,
chennai 116,
India;
E-mail: nagalakshmi.s@sriramachandra.edu.in
Received date: 17 April, 2023, Manuscript No. JPDDR-23-96190;
Editor assigned date: 20 April, 2023, PreQC No. JPDDR-23-96190 (PQ);
Reviewed date: 04 May, 2023, QC No. JPDDR-23-96190;
Revised date: 17 June, 2024, Manuscript No. JPDDR-23-96190 (R);
Published date: 24 June, 2024, DOI: 10.4172/2325-9604.1000275
Citation: Silpa S, Misra P, Suthahar T, Nagalakshmi S (2024) Regulatory Pathway and Application Preparation of 510(K) as Per USFDA. J Pharm Drug Deliv Res 14:3.
Abstract
The medical device has been increasing dramatically in the health care industry. To safeguard human life and deliver a high quality, secure, and useful product, it needs to be strictly regulated. Certain nations strictly adhere to regulations to satisfy the above stated requirement. Among these, US are one of the most regulated nations in the world. There is a special committee in the US to monitor and control the medical device industry. But all the medical devices don’t need to get approval from USFDA for certain type of device we can just notify the regulatory body. It achieved by comparison of the device with already available device in market.
Keywords: 510(K); Pre-market notification; USFDA medical device; Regulatory pathway; Application preparation
Introduction
There are two different kinds of medical devices: In vitro medical devices and out of in vitro medical devices. Based on the safety and risk of the device, these are divided into three categories: High, medium, and low risk. The majority of the time, high risk devices require permission before going on the market. In contrast, low or medium risk devices may submit a 510(K) (i.e., market notification) application in accordance with regulatory body recommendations.
Types of application for USFDA medical device submission
There are three types of applications.
• Pre-Market Approval (PMA).
• De-novo pathway.
• 510(K) pre-market notification.
510(K) pre-market notification
510(K) pre-market notification: 510 (K) pre-market notification is an application which will be submitting to the regulatory authority (i.e., USFDA) to prove that the marketing device is safe and effective and demonstrate the substantially equivalent to the predicate device which is already approved by FDA [1].
Steps involved in 510(K)
Pre-Request Designation (PRD): This PRD is helpful to place the product in the division if we are unsure on where to put a product, such as a drug, biologic, medical device, or combination product. An applicant submits a Pre-RFD to the OCP in order to request from the FDA that the product be classified as a drug, device, biological product, or combination product, or as a non-combination product, depending on whether the regulatory divisions CBER, CDER, or CDRH oversee that classification. The preliminary classification or reason for the submitted application will be provided by OCP. The review process takes 60 days. If there are any questions, the review team will get in touch with the applicant. If there are any other questions about the review process or product clarifications, the applicant can get in touch with the OCP team. The team will inform the applicant of the extended review period if it is unable to finish the evaluation within 60 calendar days. OCP feedback is not applicable if any changes (to the component or the indication) were made after the PRD was submitted. We can consult OCP for advice in this situation. If the assignment query involves many items, OCP advises submitting a new PRD. The summary of steps involved in 510(K) is given in the Figure 1.
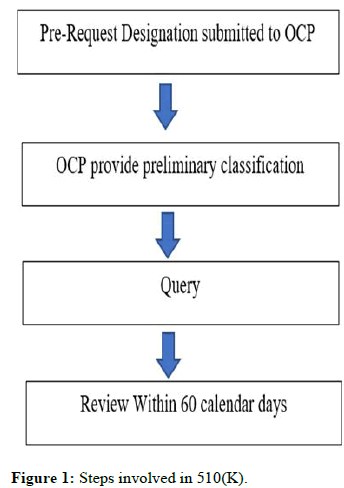
Figure 1: Steps involved in 510(K).
Materials and Methods
513(g) notification for confirmation on regulatory judication
Sources to obtain information about device:
• Database for product classification.
• Database on 510 (K).
• PMA database.
• Device guidance.
• E-mail to DICE@cdrh.fda.gov.
• E-mail to combination@fda.gov.
• Class I and class II devices exempt from 510(k) requirements.
• General biological products standards.
• Additional standards for diagnostic substances for laboratory tests.
• Clinical chemistry and clinical toxicology devices.
• Hematology and pathology devices.
• Immunology and microbiology devices.
• Anesthesiology devices.
• Cardiovascular devices.
• Dental devices.
• Ear, nose, and throat devices.
• Gastroenterology-urology devices.
• General and plastic surgery devices.
• General hospital and personal use devices.
• Neurological devices.
• Obstetrical and gynecological devices.
• Ophthalmic devices.
• Orthopedic devices.
• Physical medicine devices.
• Radiology devices.
Content included in 513 (g)
• Cover letter.
Cover letter should contain
• Date of the letter requested.
• Name of the device.
• The clarification related to classification of device.
• Applicant name, address, phone number, fax number and email.
• Applicant signature.
Description
The detail description includes
• Composition includes list of material.
• Schematic drawing, picture, or proposed device.
• Summary of device operational principles.
• Type and amount of energy delivered from device.
• Similar device description marketed in US.
Intended use
Intended use includes
• The disease or condition device used.
• Prescription over-the-counter use.
• Where the device interacted with.
• Frequency of use.
• Physiological purpose (e.g., removes water from blood, transports blood, etc.).
• Population used.
• Any other labeling information.
Proposed label
The proposed label of the device, Brochure or package insert or promotional label for similar device which is marketed in US. If no such label is available both proposed and for similar device this should include in cover letter [2].
Submission of 513(g)
Electronic copy (or).
• One complete paper copy to.
• U.S. food and drug administration.
• Center for devices and radiological health.
• Document Control Center (DCC)-WO66-G609.
• 10903 new Hampshire avenue.
• Silver spring, MD 20993-0002. The steps involved is shown in Figure 2
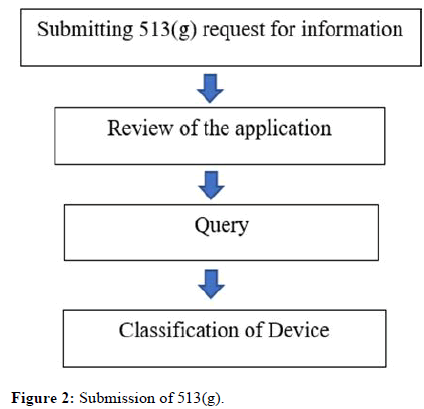
Figure 2: Submission of 513(g).
Technical documentation requirement for 510 (k) submission
Design History File (DHF)
Design history file is prepared accordance with “21 CFR 820 subpart C 820.30”.
Design controls
General
• Each manufacturer of a class III, class II, or class I device, including a class I device described in this section's paragraph (a) (2), should establish and maintain procedures to control the design of the device to ensure compliance with the applicable design standards.
• The following class I devices are subject to design controls devices automated with computer software and it is listed in the following Table 1.
Table 1: Class I devices subjected to design controls and their appropriate sections.
Design and development planning: Each manufacturer is required to create and maintain plans that outline design and development procedures, make references to them, and specify who is in charge of carrying them out. The relationships between different organisations and activities that influence or contribute to the design and development process must be identified and described in the plans. As the process of design and development progresses, plans must be evaluated, modified, and authorized.
Design input: Every manufacturer must establish and maintain procedures to guarantee that a device's design features, taking the needs of the user and patient into account, are adequate for its intended usage. The methods must deal with unclear, conflicting, or insufficient requirements. The defined design input requirements must be evaluated and approved by a designated individual(s). The approval must be documented, and that documentation must include the approver(s)' signature and the date.
Design output: Every manufacturer must create and uphold standards for categorizing and defining design output so that it may be evaluated for compliance with design input requirements. Design output techniques must ensure that the design outputs that are essential to the device's functionality are recognised and must either include acceptance criteria or make reference of them. The output of a design must be documented, reviewed, and authorized before it is made public. The approval must be noted, as well as the date and signature of the individual or individuals who provided their consent.
Design review: Each manufacturer must create and enforce standards to guarantee that formal, organised reviews of the design outcomes are conducted at the relevant points in the development of the device's design. Every design review must involve a person or individuals who are not directly in control of the design stage being examined, as well as specialists as required. There must also be representatives from every function involved in the design phase that is being examined. The design history file must contain the outcomes of a design review along with information about the design, the date, and the reviewer(s) (the DHF).
Design verification: Each manufacturer must establish and maintain methods for verifying the device design. Design output conformance with design input specifications should be shown through design verification. The DHF must contain the results of the design verification together with details on the design, method(s), date, and person(s) doing the verification.
Design validation: Each producer must establish and maintain procedures for confirming the device design. Under specific operational conditions, design validation must be performed on the initial manufacturing units, lots, or batches, or their equivalents. Testing of manufacturing units in real-world or simulated usage environments is a part of design validation, which verifies that devices fulfil their intended functions and meet predetermined user needs. Where appropriate, design validation also includes software validation and risk assessments. The DHF must contain the findings of the design validation as well as details regarding the design, method(s), date, and person(s) doing the validation.
Design transfer: In order to ensure that the device design is accurately translated into production needs, each manufacturer must establish and uphold policies.
Design changes: Before a design change is implemented, each manufacturer must set up and maintain processes for identifying, capturing, validating or, as needed, verifying, reviewing, and approving it.
Design history file: A DHF must be established and upheld by each manufacturer for each type of equipment. The DHF shall contain or refer to the records necessary to verify that the design was produced in accordance with the approved design plan and the requirements of this section.
Biocompatibility studies
Biocompatibility study was carried out as per ISO 10993. The ISO (International Organization for Standardization) is a global federation of national standards agencies (ISO member bodies). International standards are often created by technical bodies within ISO. Any member body interested in a subject for which a technical committee has been established has the right to be represented on it. International governmental and non-governmental organisations work with ISO to fulfil the assignment. ISO collaborates closely with the International Electrotechnical Commission (IEC) on all matters pertaining to electrotechnical standards [3].
Results and Discussion
ISO 10993-biological evaluation of medical device
• Part 1: Evaluation and testing within a risk management process.
• Part 2: Animal welfare requirements.
• Part 3: Tests for genotoxicity, carcinogenicity, and reproductive toxicity.
• Part 4: Selection of tests for interactions with blood.
• Part 5: Tests for cytotoxicity.
• Part 6: Tests for local effects after implantation.
• Part 7: Ethylene oxide sterilization residuals (Table 2).
• Part 9: Framework for identification and quantification of potential degradation products.
• Part 10: Tests for irritation and skin sensitization.
• Part 11: Tests for systemic toxicity.
• Part 12: Sample preparation and reference materials.
• Part 13: Identification and quantification of degradation products from polymeric medical devices.
• Part 14: Identification and quantification of degradation products from ceramics.
• Part 15: Identification and quantification of degradation products from metals and alloys.
• Part 16: Toxicokinetic study design for degradation products and leachable.
• Part 17: Establishment of allowable limits for leachable substances.
• Part 18: Chemical characterization of materials.
• Part 19: Physico-chemical, morphological, and topographical characterization of materials (technical specification).
• Part 20: Principles and methods for immunotoxicology testing of medical devices (technical specification) [4].
Table 2: Biological effect of the medical devices.
Substantial equivalence evaluation: A comparison between a new device and a predicate device that should be equal in terms of its intended application, activity, performance, etc. is referred to as "substantial equivalence. Although the device has a unique technological feature, the data it has produced is essentially identical to that of the predicate device, thus it should be equally safe and effective and shouldn't give rise to any safety or effectiveness issues when compared to the predicate device. The distinguishing technical aspect.
The FDA review considers both the safety and effectiveness factors. The FDA must first prove that the device's intended application and its foundation are "the same." It is possible that a device's intended use may not necessarily align with other indications for usage, such as the target audience or the condition it is meant to treat. When variations influence (or have the potential to influence) the safety and/or effectiveness of the new device relative to the predicate device and the variations cannot be sufficiently assessed under the comparative criteria of substantial equivalence, the changes result in a new intended use.
In order to determine whether a new device and a predicate device share "the same technological characteristics" or whether a "significant change in the materials, design, energy source, or other features of the device" does not raise new safety and efficacy concerns, the FDA must first determine whether the two devices are technologically equivalent. The FDA must also decide whether the new device is just as safe and effective as a device that has been legally marketed [5].
Even though the 510(k) process compares a new device to a predicate device rather than requiring an independent demonstration of the new device's safety and effectiveness, as is required for approval of a PMA, the FDA's review decision in both cases reflects a determination of the level of control required to provide a "reasonable assurance of safety and effectiveness." The evidence standard, however, is unique. The 510(k) context is frequently one of the factors on which FDA makes its findings regarding the predicate device's reasonable assurance of safety and effectiveness. Manufacturers are frequently obliged to provide descriptive information to demonstrate the essential similarities between a new product and a predicate piece of equipment, such as a comparison of specifications, materials, and technology [6]. On the other hand, the FDA frequently evaluates the variations between the new device and the predicate device to ascertain their effect on safety and efficacy. If the alterations have a significant difference between the new product and the predicate device, there must be more proof to prove substantial equivalence impact on or have the potential to have a significant impact on, safety or effectiveness. The review process of the medical devices is given as flow chart in Figure 3.
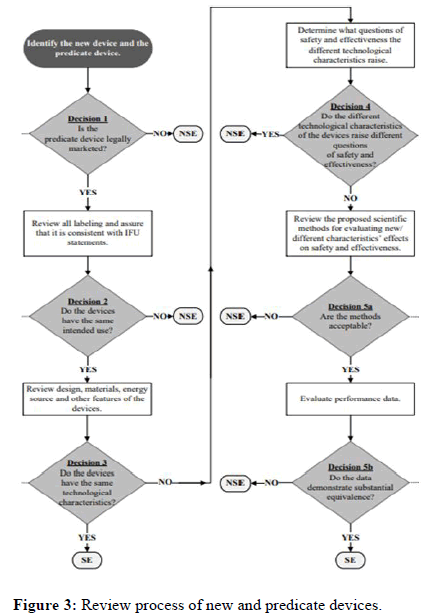
Figure 3: Review process of new and predicate devices.
Format of 510(K)
• The 510(K) should contain the following documents.
• Medical device user fee cover sheet (form FDA 3601).
• CDRH premarket review submission cover sheet (FDA form 3514).
• 510(k) cover letter.
• Indications for use statement (FDA form 3881).
• 510(k) summary or 510(k) statement.
• Truthful and accuracy statement.
• Class III summary and certification.
• Financial certification or disclosure statement.
• Declarations of conformity and summary reports.
• Device description.
• Executive summary/predicate comparison.
• Substantial equivalence discussion.
• Proposed performance testing-clinical.
Submission and review of 510(K)
Step 1: Login and acknowledgment: One electronic copy of a 510(K) submission should be sent to the CDRH or CBER document control centre [7]. DCC assigns a single, special control number, also known as a 510(K) number or K number, after receiving an application. The letter "K" is followed by a 6-digit number to start the 510(K) number. The first two digits represent the current year, while the following four represent the submission number for that year.
DCC then do the two-step verification
a) Proper user fee payment.
b) E-copy validation.
DCC sent an email with a Hold letter within 7 working days if the user fee and electronic copy were invalid. After receiving the hold letter, the applicant has 180 days to fix the problem if it is not, the application is deemed withdrawn and removed from the reviewing system. The DCC will send an acknowledgment copy to the contact person if the correct user fee and e-copy are completed. The Acknowledgment includes the submission's 510(K) number, the date it was received, and the appropriate user fee.
Step 2: Acceptance review: DCC routes the 510(k) submission to the appropriate OHT after issuing the confirmation, taking into account the type of device and medical expertise specified in the 510(k) submission. Once received at the office, the 510(k) is given to the appropriate department before being given to a senior evaluator [8-14]. The lead reviewer uses the relevant acceptance checklist to perform the acceptance assessment while referring to the disapproval policy for section 510(k) of the FDA guidelines. During the acceptance review, the review lead determines whether application submitted satisfied it will be move to next procedure. The applicant receives the notification within 15 working dates [15].
The acceptance review result will be one of the following
• Accepted.
• Not accepted for review or.
• Under substantive review.
If rejected for review the applicant has 180 calendar days to clarify the defect listed in the RTA hold. If not, it’s considered revoked and removed from review system. If the original is removed, applicant needs to submit new application. After being granted, a 510(k) moves on to the substantive review.
Step 3: Substantive review: Lead reviewers perform a thorough review of 510(k) submissions during content review. The lead reviewer also interacts with the applicant during an in-depth review, which must take place within 60 working days.
The substantive interaction communication is typically
• An email stating that FDA will proceed to resolve any outstanding deficiencies interactive review.
• An Additional Information (AI) request which places the submission on hold.
Step 4: Interactive review: The lead reviewer communicate with the applicant during this process it applicant need to provide the valid e-copy during the interaction.
Step 5: Additional Information (AI) request: If the primary reviewer makes an AI request, submission will be paused. The sender has 180 calendar days from the date the AI request sends a detailed response. DCC must receive a response within 180 days of the AI request, in case you forget. Extensions longer than 180 days are not allowed. In the event that FDA does not receive an adequate response to all errors in the IA's request within 180 days of the IA's request, the request will be reviewed for withdrawal and removed from the review system ours (Figure 4). If the original 510(k) is withdrawn, the 510(k) applicant must file a new 510(k) for marketing clearance by the FDA. The sender must send a response along with a valid electronic copy to DCC. The answer must be:
• Include the submitter’s name.
• List the 510(k) number.
• Identify the submission as Additional Information (AI) to the 510(k).
• List the date of FDA's request for additional information and,
• Provide the requested information in an organized manner.
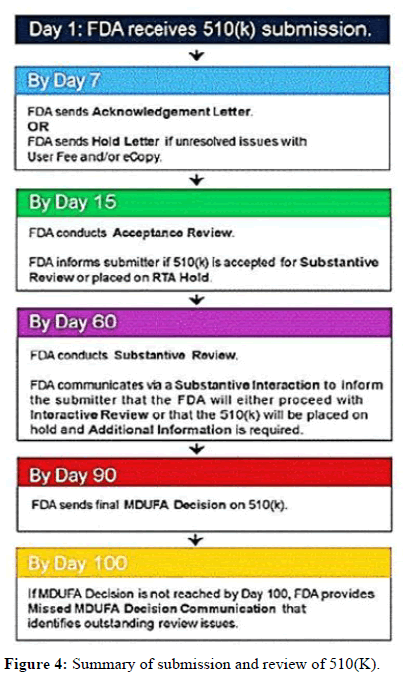
Figure 4: Summary of submission and review of 510(K).
Conclusion
To protect public health, strict laws and regulations for medical devices are essential. These regulators are responsible for ensuring that companies follow laws and regulations to protect public health. The ease of filling equipment in India is gradually increasing, and the production and import of equipment is strictly regulated. Despite India becoming a 'global pharmaceutical hub', its regulator is still struggling to keep up with the growth of the global economy. FDA section 510(k) requires device manufacturers to register at least 90 days before placing medical devices on the market to notify FDA. FDA may determine if a medical device is substantially identical to a commercially available device from this PMN, also known as a 510(k) notice or pre-market notice. Whenever the material, design, chemical composition, manufacturing process, power source or intended use of a medical device is changed, notice must be given prior to sale.
Acknowledgments
The authors would like to express their gratitude to faculty of pharmacy, SRIHER, Porur, Chennai, Tamil Nadu, India for providing the support to carry out the review work.
Conflicts of Interest
The authors have no conflict of interest.
References
- Food and Drug Administration (2018) How to Prepare a Pre-Request for Designation (Pre-RFD). Guidance for Industry.
- US Food and Drug Administration (2019) FDA and industry procedures for section 513(g) requests for information under the federal food, drug, and cosmetic act. Guidance for Industry and Food and Drug Administration Staff.
- How to Prepare a Pre-Request for Designation (Pre-RFD).
- US Food and Drug Administration (2020) Biological evaluation of medical devices-part 1: Evaluation and testing within a risk management process. Guidance for Industry and Food and Drug Administration Staff.
- Deloitte and Nat Health Healthcare Federation of India (2016) Medical devices: Making in India–A Leap for Indian healthcare. Healthcare Industry.
- Kumar D, Yadav V, Mathewson N (2016) A new regulatory paradigm for medical devices in India. Regulatory Focus.
- Brolin S, Zhang L (2008) Global regulatory requirements for medical devices. Academy for Sustainable Society and Technical Development, Eskilstuna.
- Charu S, Anjan B (2016) Medical devices: Making in India a leap for Indian healthcare. Healthcare Industry.
- Arogya Legal (2020) All medical devices in India to be regulated as “drugs”–medical devices (amendment) rules. Medical devices.
- Rott P (2019) Certification of medical devices: Lessons from the PIP scandal. Certification-trust, accountability, liability. Springer. 189-211.
- Central Drugs Standard Control Organization (2020) Medical device definition. Department of Health and Family Welfare.
- US Food and Drug Administration (2020) How to determine if your product is a medical device. Silver Spring.
- US Food and Drug Administration (2021) Guidance documents (medical devices and radiation-emitting products). Silver Spring, Hampshire.
- US Food and Drug Administration (2021) Biocompatibility assessment resource centre.
- US Food and Drug Administration (2020) Guidance document for biocompatibility of certain devices in contact with intact skin. Draft Guidance for Industry and Food and Drug Administration Staff, Rockville. Maryland.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 
