Research Article, J Mol Biol Methods Vol: 6 Issue: 4
Regeneration and Reuse of Transwell-type Culture Inserts for Blood-Brain Barrier Modelling
Margarita Shuvalova1,2,3* and Georgii Nosov2,3
1Department of Molecular and Cellular Biology, Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry of Russian Academy of Sciences, Moscow, Russia
2Federal Center of Brain Research and Neurotechnologies’ of the Federal Medical and Biological Agency, Moscow, Russia
3Department of Molecular and Cellular Biology, Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Pirogov Russian National Research Medical University, Moscow, Russia
*Corresponding Author: Margarita Shuvalova,
Department of Biology, Shemyakin and
Ovchinnikov Institute of Bioorganic Chemistry of Russian Academy of Sciences, Moscow,
Russia
E-mail: margarita22zelenskaya@gmail.com
Received date: 18 November, 2023, Manuscript No. JMBM-23-120440;
Editor assigned date: 21 November, 2023, PreQC No. JMBM-23-120440 (PQ);
Reviewed date: 05 December, 2023, QC No. JMBM-23-120440;
Revised date: 12 December, 2023, Manuscript No. JMBM-23-120440 (R);
Published date: 19 December, 2023, DOI: 10.4172/jmbm.1000147
Citation: Shuvalova M (2023) Regeneration and Reuse of Transwell-type Culture Inserts for Blood-Brain Barrier Modelling. J Mol Biol Methods. 6:4.
Abstract
Culture inserts of Transwell type are widely used in laboratories worldwide to model non-cerebral vessels, the Blood-Brain Barrier (BBB), and other blood-tissue barriers, and also for the study of chemo taxis and transmigration. However, the use of inserts can generate plastic waste that has an environmental impact due to their one-off design and massive consumption. Thus, it is important to develop a method that can reduce the utilization of inserts but not affect research efficiency. In this study, we propose using a 1:1 (v:v) mix of 30% hydrogen peroxide and 99% sulfuric acid (“piranha solution”) to completely remove cell debris and matrix from culture inserts. Blood-brain barrier modeling models with inserts regenerated using piranha solution have barrier properties comparable to those of fresh inserts. We show that piranha solution is an effective reagent and allows for the reuse of Transwell-type inserts for blood-brain barrier modeling up to 5 times. Therefore, the use of this method greatly reduces the production of laboratory waste and benefits numerous laboratories worldwide.
Keywords: Transwell; Blood-Brain Barrier (BBB); Culture insert; Cell synthesis; Immunology
Abbreviations
Blood-Brain Barrier (BBB); Dulbecco’s Modified Eagle Medium (DMEM); Fetal Bovine Serum (FBS); Lucifer Yellow (LY); P-the apparent permeability coefficient; Phosphate-Buffered Saline (PBS); Polydimethylsiloxane (PDMS); Radioimmunoprecipitation Assay (RIPA); Standard Error of The Mean (SEM); Transendothelial Electrical Resistance (REER).
Introduction
The Blood-Brain Barrier (BBB) is an interface between the blood and the brain that limits the entry of potentially harmful substances, cells, and pathogens into brain tissue, while also providing delivery of nutrients and removal of waste products. The BBB is primarily composed of micro vessels surrounded by astrocytic processes. Endothelial cells line the vessel wall and are directly responsible for the barrier properties. Pericytes, which regulate the vessel lumen, are located around the endothelial cells, surrounded by a basal membrane. Astrocytic processes are adjacent to the endothelial cells and pericytes on the brain side [1].
Given the complexity of studying brain vasculature, the development of in vitro BBB models has been necessary. The first experiments involving in vitro modeling of the BBB were conducted by Cancilla and colleagues in 1980. In these studies, endothelial cells were cultured on a micro porous polycarbonate filter [2]. Currently, there are various in vitro BBB models available, which differ in their configuration, cell types used, presence of fluid flow, and other parameters. The diversity of in vitro BBB models is described in several reviews [3-5].
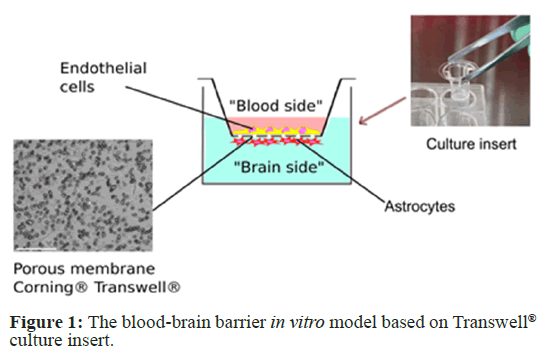
The most widely used “classic” in vitro BBB model is based on the Boyden chamber assay, originally introduced by Boyden for the analysis of leukocyte chemotaxis [6]. The Boyden chamber consists of two medium-filled compartments separated by a microporous membrane: an upper compartment, which can be considered the “blood” side, and a lower compartment, which is the “side of the brain” [7] (Figure 1).
Now this type of culture inserts is widely known under the commercial name “Transwell”. Membranes are usually made of polycarbonate or polyethylene terephthalate with pore sizes ranging from 0.4 μm to 8.0 μm. A number of different Boyden chamber devices are commercially available and used in BBB models [8]. Among them are Trans wells®, ThinCert®, ChemoTx®, Ibidi, and others. Benefits of the models based on the Boyden chamber include ease of use and establishing cultures, moderate scalability, and the ability to quickly and non-destructively quantify barrier integrity by measuring Transendothelial Electrical Resistance (TEER) or permeability coefficient [9,10]. Cells can be separated and harvested for further study (proteomic and genomic analysis) and are available in a variety of pore sizes and membranes to suit a variety of experimental requirements. Moreover, Transwell inserts are widely used not only for BBB modeling, but also for modeling peripheral micro vessels, and other blood-tissue barriers, as well as for studying chemotaxis and transmigration [11].
However, commercial Transwell-type inserts are made for one- time use, are expensive for most laboratories worldwide, and generate plastic waste that can have a negative impact on the environment. Thus, the goal of this study was to provide a method that can reduce the utilization of commercial inserts while not affecting research efficiency.
To date, several reagents have been provided for the regeneration of Transwell-type inserts. Among them are Trypsin-EDTA, 6M urea, and Radio Immune Precipitation Assay (RIPA) buffer [12]. Despite their effectiveness in removing cells, they are not able to completely remove collagen coverage, which is widely used in BBB modeling. Therefore, it is necessary to develop a method that would allow for the effective removal of not only cells but also collagen remnants and other debris.
In this study, we propose the use of a 1:1 (v:v) mix of 30% hydrogen peroxide and 99% sulfuric acid (“piranha solution”) to completely remove cell debris and matrix from culture inserts. The result of the mixture of H2O2 and H2SO4 gives rise to a strong oxidizing agent called per-hexa-sulfuric acid (H4SO6) that is used to clean organic residues off substrates. Polycarbonate and Polydimethylsiloxane (PDMS) are resistant against strong oxidizing agents; therefore, theoretically, regeneration using this substance should not affect the membrane and holder of the insert.
Materials and Methods
Cell cultures
BEnd.3 endothelial cell line (ATCC® CRL-2299) was cultivated in a full Dulbecco’s Modified Eagle Medium (DMEM) medium (DMEM medium (PanEco Ltd.) supplemented with 10% FBS (Gibco), GlutaMAX (Gibco) and PenStrep (PanEco Ltd.)), and the medium was changed twice a week. Cells were passaged using a standard trypsinization protocol. The cells were washed with Versene solution (PanEco Ltd.), incubated with a 0.25% trypsin solution (PanEco Ltd.) for 5-10 minutes, suspended in full DMEM to stop the reaction, centrifuged for 5 minutes at 300 g, and then suspended in full DMEM.
Primary astrocytes were obtained as described previously. Briefly, C57BL/6 mice (P4-P6) were decapitated, brains were isolated and washed with cold Phosphate-Buffered Saline (PBS), olfactory bulbs, cerebella, and brain stem were removed, and the cortices were cut in pieces. Cortices were incubated in TrypLE™ Express (Gibco), at 37◦C for 30 minutes, and centrifuged for 5 min at 300 x g to pellet cortex pieces. Cortex pieces were dissociated into a single cell suspension by pipetting. The cell suspension was plated in a T-75 culture flask coated with poly-D-lysine at the rate of 4 cortexes on the one T-75 flask and cultured in a full DMEM medium. The medium was changed twice a week [13]. Cells were passaged using a standard trypsinization protocol. The cells were washed with Versene solution (PanEco Ltd.), incubated with a 0.25% trypsin solution (PanEco Ltd.) for 5-10 minutes, suspended in full DMEM to stop the reaction, centrifuged for 5 minutes at 300 g, and then suspended in full DMEM.
In vitro BBB model construction
In vitro BBB models were constructed as described previously [14]. The BBB models were constructed using a Corning Transwell culture insert for a 24-well plate with polycarbonate membrane with a pore size of 3 μm (Corning® Transwell® Inserts, Corning Incorporated,
Corning, NY, USA). Briefly, the apical side of the porous membrane of the insert was coated with collagen Ι from the rat tail (5 mg/mL) (Gibco). 100 μl suspension with 10^5 of primary murine astrocytes was placed on the lower side of the membrane of the inverted insert and left for 3 hours for attachment. Then the insert was flipped to the normal position and placed in a 24-well culture plate with a full DMEM medium. Then 10^4 of bEnd.3 cells were seeded on the apical side of the porous membrane. The models were maintained in full DMEM, medium was changed twice a week.
Measurement of barrier properties
The barrier integrity of the in vitro BBB model was evaluated by measurement of permeability for the fluorescent dye Lucifer Yellow (LY) [10]. Measurement of permeability for LY is a standard method for evaluating the barrier properties of in vitro BBB models. For permeability analysis, all medium from the upper and lower compartments was aspirated. 200 μl of 1mM LY in PBS was added to the upper chamber of a culture insert. 1 ml of PBS was added to the lower chamber. The culture plate was placed into a CO2 incubator at 37°C for 60 min. Then solutions from the upper and lower compartments were aspirated and the fluorescent signal was measured using Varioskan LUX multimode microplate reader (Thermo Scientific™). The following equation was used to calculate the apparent permeability coefficient (P):
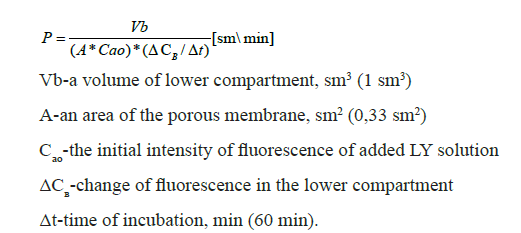
Fluorescence of the PBS solution was considered as background and was subtracted from Сао and ΔCв values.
Regeneration of transwell inserts
All manipulations with hydrogen peroxide and sulfuric acid were carried out in a fume hood, following general safety precautions for working with corrosive and toxic substances. A 100 mL glass chemical beaker was used to add 25 mL of 30% hydrogen peroxide, followed by 25 mL of 99% sulfuric acid. Do not use a smaller container or pour a larger volume of liquid, as gasses will be released during the reaction, which can cause liquid splattering. The used inserts were immediately immersed in the beaker using tweezers, ensuring that they were fully submerged in the solution. The inserts were left in the piranha solution until the active gas release ceased, which usually took 5-10 minutes. When the piranha solution interacts with organic substances, heat is released, so it is not recommended to touch the beaker with bare hands to avoid burns. After 5-10 minutes, the inserts were removed from the beaker using tweezers and rinsed three times in sterile distilled water (ddH2O). They were then left in sterile ddH2O for 10-15 minutes to completely remove any residue of the piranha solution. Further manipulations were carried out under sterile conditions using a laminar flow hood. To sterilize the inserts, they were left in 70% ethanol for at least one hour and then rinsed twice in sterile ddH2O. Before further use, it was important to check the inserts under a microscope for any breaks in the membrane.
Cell viability assay
To test the potential influence of insert regeneration on cell viability, staining with Trypan blue solution (0.4%) was used. The culture inserts with growing cells were washed with Versene solution, then incubated with trypsin solution (0.25%) (1 mL in the lower compartment and 200 μL in the upper compartment). The cells were then collected from both compartments, centrifuged, suspended in 100 μL of PBS and stained with Trypan blue. Cells collected from the lower compartment were astrocytes detached from the bottom side of the membrane. Cells from the upper compartment were endothelial cells that grew on the top side of the membrane. Cell counting was performed using a countess™ automated cell counter.
Statistics
The data were analyzed using Prism GraphPad software with a 1-way Analysis of Variance (ANOVA) and Bonferroni’s multiple comparisons test. The difference between means was considered statistically significant when p<0.05. The data are expressed as the mean ± Standard Error of the Mean (SEM).
Results
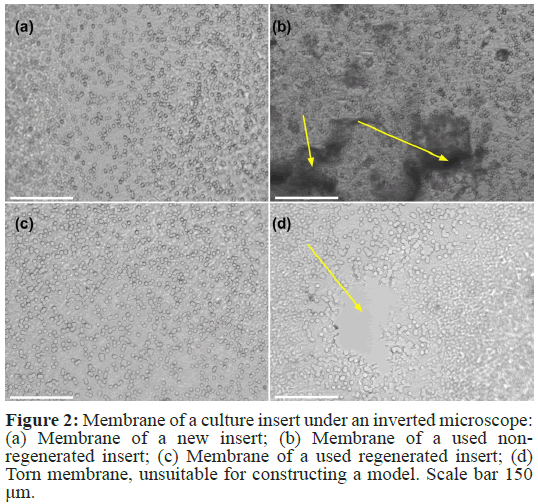
First, we examined the membranes of new inserts, used non- regenerated inserts, and used regenerated inserts under an inverted microscope. Used non-regenerated inserts showed remnants of collagen coating and cellular debris. New and regenerated inserts did not differ in appearance (Figure 2).
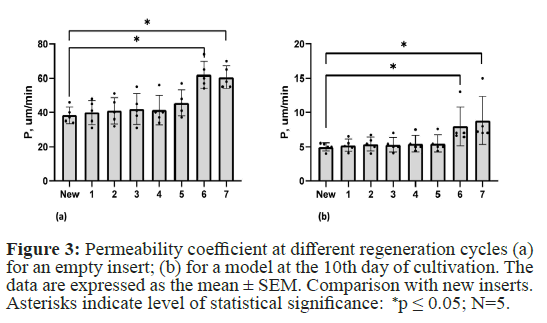
To determine the impact of piranha solution treatment on the barrier properties of BBB models and the culture inserts themselves, we measured the permeability coefficient for the fluorescent dye Lucifer Yellow. We measured the barrier properties for new inserts as well as after 1-7 regeneration cycles. Inserts with visible membrane damage under the microscope were not used. It is worth noting that after each regeneration cycle; approximately 10% of the membranes were damaged. The permeability of both the model inserts and the empty inserts coated only with collagen did not change compared to the new inserts until the fifth regeneration cycle. However, starting from the sixth cycle, statistically significant differences were observed in both the empty inserts and the BBB models. Therefore, up to 5 regeneration cycles, there is no statistically significant difference in the permeability coefficient for both the empty insert and the BBB model constructed on it (Figure 3).
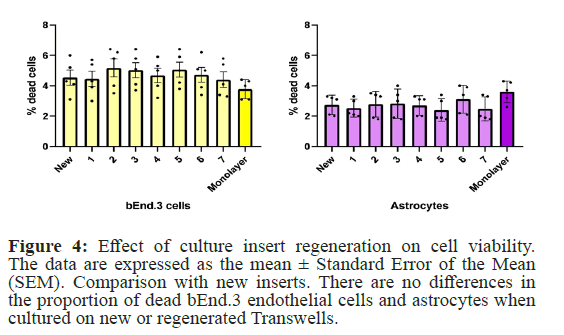
To test whether the regeneration of cultured inserts using Piranha solution affects cell viability, the proportion of dead cells was determined by staining with Trypan Blue. Analysis did not reveal any differences in the proportion of dead cells when cultured on new or regenerated Transwells. The proportion of dead endothelial cells was higher than that of dead astrocytes. This can be explained by the fact that dead astrocytes are more effectively removed during medium changes, while dead endothelial cells mostly remain in the upper compartment, which cannot be completely removed to avoid mechanical damage to the membrane. Thus, it can be concluded that the regeneration of Transwells using Piranha solution does not affect the viability of cells in the BBB model (Figure 4).
Figure 4: Effect of culture insert regeneration on cell viability. The data are expressed as the mean ± Standard Error of the Mean (SEM). Comparison with new inserts. There are no differences in the proportion of dead bEnd.3 endothelial cells and astrocytes when cultured on new or regenerated Transwells.
Discussion
We have proposed a simple and reliable method for regenerating Transwell culture inserts with polycarbonate membrane. The method involves treating the inserts with a piranha solution, which completely removes organic residues, including the collagen coating that is difficult to remove with other methods. We used the inserts for constructing BBB models, but our protocol can also be applied to other uses of these inserts-for instance, this method potentially allows reuse Transwells after chemotaxis and transmigration assays. We found that up to 5 regeneration cycles, there were no statistically significant differences in the barrier functions of the BBB models compared to new inserts. However, after the 6th cycle, the barrier properties started to deteriorate. This could be due to two reasons. Firstly, the insert material is weak but still subjected to the piranha treatment. And secondly, it could be related to mechanical damage to the membrane during insert manipulation. We noticed that the manipulation of the inserts themselves (using tweezers) can cause membrane breaks, most commonly at the attachment site to the holder. Thus, it can be hypothesized that the increased permeability of BBB models during regeneration cycles is not solely due to the piranha solution treatment, but rather due to the mechanical properties of the insert itself. When using a regenerated insert, it is essential to check its membrane integrity under a microscope.
In summary, Transwell culture inserts with polycarbonate membranes can be reused up to 5 times after cell and collagen coating removal with piranha solution. The use of this method greatly reduces the production of laboratory waste and benefits numerous laboratories worldwide.
Conclusion
In conclusion, the regeneration and reuse of Transwell-type culture inserts present a promising approach for establishing robust and cost-effective Blood-Brain Barrier (BBB) models. Through careful optimization of cleaning and sterilization protocols, these inserts can be efficiently recycled, thereby reducing experimental costs and environmental impact associated with single-use culture systems. Furthermore, the ability to reuse inserts maintains consistency in experimental conditions, ensuring reproducibility and reliability of BBB model studies. However, it is imperative to validate the integrity and functionality of regenerated inserts to confirm their suitability for continued use in BBB research. Collaborative efforts between researchers and manufacturers are essential for developing standardized protocols and guidelines to support the widespread adoption of regenerated Transwell inserts in BBB modeling applications. Overall, the implementation of regeneration and reuse strategies for Transwell inserts holds significant promise in advancing research on the blood-brain barrier and its role in neurological diseases and drug delivery.
Acknowledgements
We thank Professor Vsevolod Belousov and the “Federal Center for Brain and Neurotechnology” for the provided funding and the opportunity to carry out this work.
Author Contributions
M.S. conceived and designed the experiments, M.S. performed the experiments, M.S. analyzed the data, G.N. contributed reagents/ materials/analysis tools, M.S. wrote the paper.
References
- Daneman R, Prat A (2015). The blood-brain barrier. Cold Spring Harb 7(1):a020412.
- DeBault LE, Cancilla PA (1980). γ-Glutamyl transpeptidase in isolated brain endothelial cells: Induction by glial cells in vitro. Science 207(4431):653-655.
- Williams-Medina A, Deblock M, Janigro D (2021). in vitro models of the blood-brain barrier: Tools in translational medicine. Front Med Technol 2:623950.
- Shah B, Dong X (2022). Current status of in vitro models of the blood-brain barrier. Curr Drug Deliv 19:1034-1046.
- Jagtiani E, Yeolekar M, Naik S, Patravale V (2022). in vitro blood brain barrier models: An overview. J Control Release 343:13-30.
- Boyden S (1962). The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115(3):453-466.
- Wilhelm I, Fazakas C, Krizbai I (2011). in vitro models of the blood-brain barrier. Acta Neurobiol Exp 71(1):113-128.
- Chen HC (2005). Boyden chamber assay. Methods Mol Biol 294:15-22.
- Vigh JP, Kincses A, Ozgür B, Walter FR, Santa-Maria AR, (2021). Transendothelial electrical resistance measurement across the blood-brain barrier: A critical review of methods. Micromachines 12(6):685.
- Zhao W, Han L, Bae Y, Manickam DS (2019). Lucifer yellow-a robust paracellular permeability marker in a cell model of the human blood-brain barrier. J Vis Exp 150:e58900.
- Sivandzade F, Cucullo L (2018). In-vitro blood-brain barrier modeling: A review of modern and fast-advancing technologies. J Cereb Blood Flow Metab 38(10):1667-1681.
- Kato T, Mikami Y, Sun L, Rogers TD, Grubb BR (2021). Reuse of cell culture inserts for in vitro human primary airway epithelial cell studies. Am J Respir Cell Mol Biol 64(6):760-764.
- Shuvalova ML (2023). Protocol for isolation of primary murine astrocytes.
- Xue Q, Liu Y, Qi H, Ma Q, Xu L, (2013). A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int J Biol Sci 9(2):174.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi