Case Report, J Liver Disease Transplant Vol: 12 Issue: 3
Primary Leiomyosarcoma of Liver Mimicking Liver Abscess in a 40 Year Female
Shikha Chopra*
Department of Pathology, Deen Dayal Upadhyay Hospital, New Delhi, India
*Corresponding Author: Shikha Chopra
Department of Pathology, Deen
Dayal Upadhyay Hospital, New Delhi, India
E-mail: drshikhachopra@gmail.com
Received date: 20 April, 2023, Manuscript No. JLDT-23-96680;
Editor assigned date: 24 April, 2023, PreQC No. JLDT-23-96680 (PQ);
Reviewed date: 02 May, 2023, QC No. JLDT-23-96680;
Revised date: 20 June, 2023, Manuscript No. JLDT-23-96680 (R);
Published date: 27 June, 2023, DOI: 10.36648/2325-9612.100236
Citation: Chopra S (2023) Primary Leiomyosarcoma of Liver Mimicking Liver Abscess in a 40 Year Female. J Liver Disease Transplant 12:3.
Abstract
A 40-year-old female came with complaints of pain in right hypochondrium. She had no history of liver disease or alcohol abuse. Liver function tests were mildly elevated and all tumor markers were normal. USG abdomen was suggestive of hepatomegaly with large thick walled cystic lesion showing thick internal septations? Liver abscess PET-CT revealed intensely FDG avid large well circumscribed heterogeneous lesion in segment IV, VIII of liver extending to segment V, showing necrosis and large enhancing solid component and few enhancing septations within a non-enhancing area, highly suspicious for neoplastic etiology. No scan evidence of abnormal hypermetabolism elsewhere in the body. A liver biopsy of the lesion was done and a diagnosis of spindle cell tumor with nuclear atypia was given, which is followed by extended right hepatectomy resection. Macroscopically, there was a breach in the capsule, and the liver had been replaced by a tumor with a maximum diameter of 16 cm with necrotic and hemorrhagic areas. In microscopic findings, the tumor consisted of the proliferation of spindle cells arranged in fascicles and storiform patterns with large areas of necrosis and hemorrhage. Moderate nuclear atypia was observed with increased mitosis (>19/10 HPF) making it FNCLCC grade 2 (score 5). Immunohistochemical staining for Smooth Muscle Actin (SMA), Vimentin, and h-caldesmon showed diffuse cytoplasmic positivity in all tumor cells with 45% Ki-67 along with negative staining for C-kit, S100, CD34, CD31, CD117, EMA, PanCk, Hep-par1, L-arginase, HMB45, MDM2 and DOG1, informing a final diagnosis of primary hepatic leiomyosarcoma.
Keywords: Leiomyosarcoma; Primary hepatic leiomyosarcoma; Liver abscess; Chemotherapy; Immunohistochemical staining
Introduction
Leiomyosarcomas are mesenchymal tumors arising from smooth muscle. The primary sarcomas of the liver are angiosarcomas, epithelioid hemangioendotheliomas, or undifferentiated embryonal sarcoma constituting nearly 70%. Primary hepatic leiomyosarcoma is an extremely rare tumor, accounting for 0.2%-2% of all primary hepatic malignancies. The diagnosis requires high level of suspicion and exclusion of metastasis from other primary site of origin of other hepatic and extrahepatic tumor with spindle cell morphology. So far, less than 100 cases have been reported in the literature. Here we discuss a case of primary hepatic leiomyosarcoma in an adult female patient with detailed discussion and exclusion of other possible differentials.
Case Presentation
A 40-year-old female patient presented to our hospital with complaints of pain in right hypochondrium since 2 months. She had no history of any liver disease, blood transfusion and alcohol abuse. She had no significant personal or family history. The patient’s laboratory findings on admission were as follows:
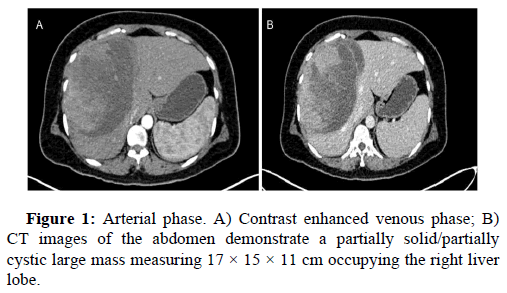
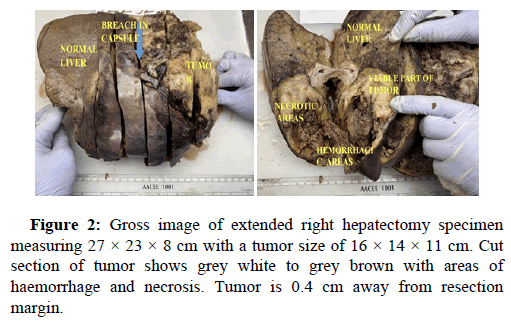
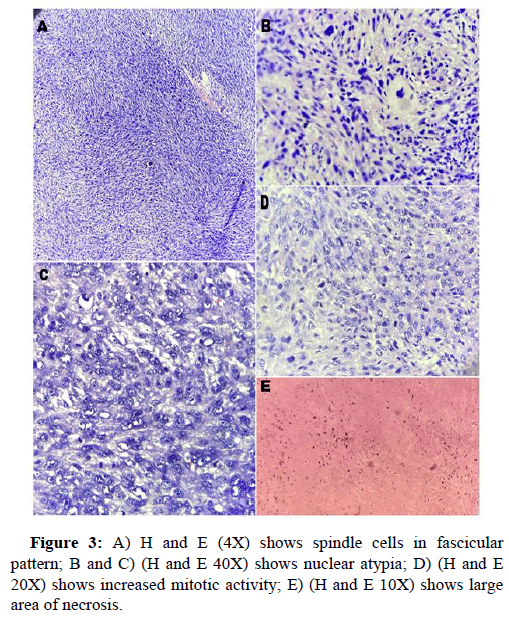
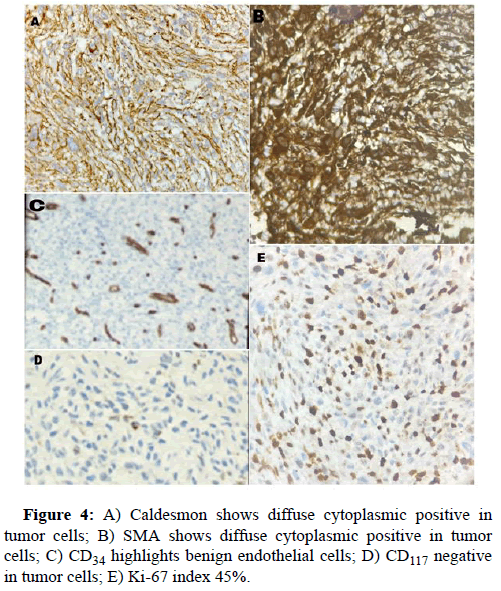
White blood cell count, 17900/μl; hemoglobin, 12 g/dl; platelet count 156, total protein 3.5 g/dl; serum albumin 2.0 g/dl; serum creatinine, 0.80 mg/dl; and HbA1c 5.4%. Liver function tests revealed total bilirubin 5.2; ALT-1510, AST-3132, ALP-183, total bilirubin-5.2, Blood urea-29, prothrombin time with INR-28.7. Antibody tests were negative for both hepatitis B surface antigen and hepatitis C virus. The tumor markers like AFP, Ca19.9, CEA, Ca125 were within normal range. USG abdomen done at our hospital suggestive of hepatomegaly with large thick walled cystic lesion showing thick internal septations, internal echoes and few solid components in right lobe of liver abscess. Contrast enhanced CT and PET CT scan Figure 1 revealed intensly FDG avid large well circumscribed heterogeneous lesion in segments IV and VIII of liver extending into segment V, showing large non enhancing/necrotic area and relatively large enhancing solid component with few thick enhancing septations, highly suspicious for neoplastic aetiology. No scan evidence of abnormal hypermetabolism elsewhere in the body. A liver biopsy of the lesion was done with clinical suspicion of liver abscess. The entire core was composed of spindle cells with minimal native liver parenchyma. So a diagnosis of spindle cell tumor with nuclear atypia was given. Later extended right hepatectomy was performed to prevent tumor rupture. Macroscopically, there was a breach in the capsule, and the liver had been replaced by a tumor with a maximum diameter of 16 cm with large necrotic and hemorrhagic areas (Figure 2). Light microscopy revealed the tumor consisted of the proliferation of spindle cells arranged in fascicles and storiform patterns with large areas of necrosis (25% to 30%) and hemorrhage. Moderate to severe nuclear atypia were observed, and nuclear fission images were demonstrated in >19/high power field (Figure 3). A diagnosis of Leiomyosarcoma was given with FNCLCC grade 2 and score 5. Immunohistochemical staining for tumor cells revealed caldesmon, α-Smooth Muscle Actin (αSMA) and Vimentin to be diffusely positive, while CD117, S100, desmin, CD34, CD31, EMA, PanCk, Hep-Par1, L-arginase, MDM2, HMB45 and DOG1 were negative. Ki-67 labeling index of the main tumor was 45 %. Figure 4 these findings favour smooth muscle differentiation and informing a final diagnosis of primary hepatic leiomyosarcoma. No other neoplastic lesion was detected on PET-CT, so the tumor was considered to be primary, not metastatic liver tumor. The patient had an uncomplicated post-operative course and was discharged on seventh post operative day. During follow up, patient was started on chemotherapy (AIM regime) and was healthy till date (2 months post diagnosis).
Results and Discussion
The mesenchymal malignancies contribute 1%-2% of the primary hepatic malignancies, whereas hepatocellular carcinoma and cholangiocarcinoma forming the major bulk [1]. Most of the primary hepatic mesenchymal malignancies include angiosarcomas, epithelioid hemangioendotheliomas, or undifferentiated embryonal sarcoma constituting nearly 70% of the malignancies [2]. Primary hepatic leiomyosarcoma is a rare cause of primary hepatic malignancies, accounting for less than 2% of all primary liver malignancies. As mentioned in the literature, PHL usually arises from intrahepatic vascular structures, bile ducts, or ligamentum teres, however underlying pathogenetic mechanisms of the disease have not yet been identified. Few studies reported its association with AIDS, EBV, Hodgkin’s lymphoma, immunosuppression after organ transplantation etc. [3,4]. Matthaei, et al.; Shamseddine et al. and few other authors demonstrated adult patients with primary hepatic leiomyosarcoma without any known predisposing conditions [5,6]. The most common cause of hepatic leiomyosarcomas is metastasis from a primary in the lungs, gastrointestinal tract, uterus and peritoneum [7,8]. Hence exclusion of metastasis is important before making a diagnosis of a primary leiomyosarcoma of liver.
The non-specific symptoms including the lack of serological markers make the diagnosis of hepatic leiomyosarcoma very challenging. Clinical manifestations, laboratory and imaging examinations too have limited value for diagnosis and differential diagnosis. In this scenario, histological examination is the only way to achieve the definite diagnosis. The morphological differentials are wide, so our approach should include the commoner entities and hepatic tumors that have spindle cell morphology. Along with that relevant immunohistochemistry is also required to rule out other possibilities. The spindle cell type Gastrointestinal Stromal Tumor (GIST) should be the first differential diagnosis and must be ruled out before giving a confident diagnosis of leiomyosarcoma. The subtle morphological clues of leiomyosarcoma could be blunt ended nuclei with deep eosinophilic cytoplasm instead of relatively pale eosinophilic cytoplasm and spindle shaped nuclei seen in GIST. CD117 (c-kit) and DOG1 positivity suggests a diagnosis of GIST and SMA (Smooth Muscle Actin) positivity and negative CD117 favors the possibility of smooth muscle origin tumor. Angiosarcoma is the commonest primary hepatic sarcoma which shows variable shaped vascular spaces and solid areas lined by atypical endothelial cells that express CD34, CD31. These malignant endothelial cells can show spindle to epithelioid morphology, so immunohistochemistry is often required. Solitary Fibrous Tumor (SFT) arising from the adjacent soft tissue and can infiltrate the liver. CD34 positivity and SMA negativity is the key immunological feature for diagnosis of SFT. Malignant Peripheral Nerve Sheath Tumor (MPNST) shows fascicular arrangement of spindle cells with kinky nuclear morphology and pale cytoplasm. S100 is positive in almost 50% of the MPNST and SMA is almost always negative. Hepatic angiomyolipoma, which is a benign mesenchymal tumor of the liver, but can be malignant, also needs exclusion. The spindle cells in HAML shows immunopositivity for Melan-A, rules out the possibility of HAML in our case.
The non-mesenchymal liver tumors like Primary Sarcomatous Hepatocellular Carcinoma (PSHCC) and metastatic malignant melanoma can mimic a leiomyosarcoma. Immunohistochemistry with heppar-1, L-arginase, melan A and S-100 negativity rules out these differentials in our case.
However, PHL has aggressive metastatic potential and is usually diagnosed as locally advanced or metastatic disease [9]. In our case the tumor was located in the liver, we were able to perform a surgical resection, obtaining a tumor free margin. Hepatic resection is considered the only potentially curative treatment for tumors without distant metastasis. The role of chemotherapy and radiotherapy regimens has not yet been established. There is no standard chemotherapeutic regimen but few authors have reported addition of chemotherapeutic regimens like Ifosamide and Mesna, mitoxantrone, cisplatin, and fluorouracil, doxorubicin based therapy [10]. Our patient was treated with BCCA protocol for sarcomas with intact liver functions, with Adriamycin, Ifosfomide, Mesna and is on follow up [11].
Conclusion
Primary hepatic leiomyosarcoma is a rare malignant tumor with delayed diagnosis and poor prognosis. Gastroenterologist and pathologist should be aware of this rare entity as early diagnosis requires a high suspicion due to atypical clinical presentation, nonspecific imaging features and adequate immunohistochemistry is required for a confident diagnosis. Further studies are required for better understanding of this uncommon clinical entity and for establishing standardised treatment strategies of these patients.
References
- Shivathirthan N, Kita J, Iso Y (2011) Primary hepatic leiomyosarcoma: Case report and literature review. World J Gastrointest Oncol 3:148-152.
[Crossref] [Google Scholar] [PubMed]
- Mitra S, Rathi S, Debi U, Dhiman RK, Das A (2018) Primary hepatic leiomyosarcoma: Histopathologist's perspective of a rare case. J Clin Exp Hepatol 8:321-326.
[Crossref] [Google Scholar] [PubMed]
- Zhu KL, Cai XJ (2019) Primary hepatic leiomyosarcoma successfully treated by trans catheter arterial chemoembolization: A case report. World J Clin Cases 7:525-531.
[Crossref] [Google Scholar] [PubMed]
- Yugawa K, Kinjo N, Imai D, Sanefuji K, Kawata K, et al. (2020) Large surgically resected leiomyosarcoma of the liver: A case report. Surg Case Rep 6:168.
[Crossref] [Google Scholar] [PubMed]
- Matthaei H, Krieg A, Schmelzle M, Boelke E, Poremba C, et al. (2009) Long-term survival after surgery for primary hepatic sarcoma in adults. Arch Surg. 144:339-344.
[Crossref] [Google Scholar] [PubMed]
- Shamseddine A, Faraj W, Mukherji D, El Majzoub N, Khalife M, et al. (2010) Unusually young age distribution of primary hepatic leiomyosarcoma: Case series and review of the adult literature. World J Surg Oncol 8:56.
[Crossref] [Google Scholar] [PubMed]
- More RB, Shera IA, Patel SK, Setya AK, Raj V (2017) Primary hepatic leiomyosarcoma a space occupying lesion in the liver: An enigma for diagnosis. Gastroenterol Pancreatol Liver Disord 4:1-3.
- Cioffi U, Quattrone P, de Simone M, Bonavina L, Segalin A, et al. (1996) Primary multiple epithelioid leiomyosarcoma of the liver. Hepatogastroenterology 43:1603.
[Google Scholar] [PubMed]
- Garrido I, Andrade P, Pacheco J, Rios E, Macedo G (2022) Not all liver tumors are alike an accidentally discovered primary hepatic leiomyosarcoma: A case report. World J Hepatol 14:860-865.
[Crossref] [Google Scholar] [PubMed]
- Lv WF, Han JK, Cheng DL, Tang WJ, Lu D (2015) Imaging features of primary hepatic leiomyosarcoma: A case report and review of literature. Oncol Lett 9:2256-2260.
[Crossref] [Google Scholar] [PubMed]
- Lin YH, Lin CC, Concejero AM, Yong CC, Kuo FY, et al. (2015) Surgical experience of adult primary hepatic sarcomas. World J Surg Oncol 13:87.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi