Research Article, J Trauma Stress Disor Treat Vol: 8 Issue: 2
Prefrontal Dysfunction in Girls with Post-Traumatic Stress Disorder Secondary to Child Sexual Abuse, and Its Relation to Basal Cortisol Levels
Araceli Sanz-Martin*, Sofía Preciado-Mercado, Olga-Inozemtseva and Ibza América García-León
Instituto de Neurociencias, CUCBA, Universidad de Guadalajara, Guadalajara, México
*Corresponding Author: Araceli Sanz-Martin
Laboratorio de Estrés y Neurodesarrollo, Instituto de Neurociencias
CUCBA, Universidad de Guadalajara, Francisco de Quevedo 180
Col. Arcos Vallarta, Guadalajara, Jalisco, Z.C. 44130, México
Tel: 521(33)37771150
E-mail: araceli.sanz.martin@gmail.com
Received: December 13, 2018 Accepted: January 18, 2019 Published: January 25, 2019
Citation: Sanz-Martin A, Preciado-Mercado S, Olga-Inozemtseva, García-León IA (2019) Prefrontal Dysfunction in Girls with Post-Traumatic Stress Disorder Secondary to Child Sexual Abuse, and its Relation to Basal Cortisol Levels. J Trauma Stress Disor Treat 7:3.
Abstract
Child sexual abuse is a stressful event that is often associated with Post-traumatic Stress Disorder (PTSD), alterations in the hypothalamic-pituitary-adrenal axis and such brain structures as the prefrontal cortex, which is related to the executive functions and other higher order processes. Though it has been reported that child sexual abuse survivors with PTSD have higher baseline cortisol levels, and that this is associated with cognitive decline, the relation between it and cognitive performance in victims of child sexual abuse with PTSD is not clear. Therefore, the objective of this research was to determine the relation between basal cortisol levels and the cognitive functions related to the prefrontal cortex in female adolescents with PTSD secondary to child sexual abuse. Twenty 12-15-year-old girls with PTSD secondary to child sexual abuse and 20 control adolescents with no history of abuse were evaluated. The groups were matched according to age, IQ, socioeconomic status and schooling. Cognitive processes related to the prefrontal cortex were evaluated using BANFE-2, BRIEF and the STOP-IT task. Also, three saliva samples were collected from each participant during two consecutive days.
Findings show that the PTSD group had lower performance on most of the BANFE-2 tasks, more executive problems according to BRIEF, and higher cortisol levels than the control group. Finally, there was a correlation between basal cortisol levels and the indexes of the test that evaluated prefrontal cognitive functions; thus, it is feasible to suggest that the cognitive deficiencies observed are related to anatomo-functional alterations of the prefrontal cortex, since this structure has a high density of glucocorticoid receptors.
Keywords: Adolescents; Child sexual abuse; Cortisol; Executive functions; Post-traumatic stress disorder
Keywords
Adolescents; Child sexual abuse; Cortisol; Executive functions; Post-traumatic stress disorder (PTSD)
Introduction
Prefrontal dysfunction in female adolescents with posttraumatic stress disorder secondary to child sexual abuse, and its relation to basal cortisol levels
The adolescent brain undergoes both progressive and regressive changes that are regionally-specific and serve to refine that organ’s functional connectivity [1,2]. During typical adolescence, cortical development is characterized by the progressive thinning of the gray matter that begins well before adolescence in cortical regions involved in basic sensory and motor functions and continues in the prefrontal cortex (PFC) throughout this period. The amygdale also shows agerelated changes in functional connections with other cortical regions, specifically the medial prefrontal cortex, insula, superior temporal sulcus, parahippocampal gyrus and posterior cingulate [3]. In addition to these extensive connections, the amygdala contains a significant number of sexual hormone receptors [4] and suffers volume variations and hyperreactivity to emotional stimuli during adolescence [5,6]. The amygdala’s hyper-reactivity to emotional stimuli, together with the immaturity of the prefrontal cortex, might make adolescents more vulnerable to developing post-traumatic stress disorder (PTSD) in response to stressful events, such as child sexual abuse (CSA).
CSA has been related to psychiatric [7,8], hormonal, neuroanatomical, cognitive and behavioral consequences [9]. Child abuse survivors often present a deregulation of the activity of the hypothalamic-pituitary-adrenal (HPA) axis, which could induce an increase in the release of basal cortisol [10,11]. Excess cortisol could interfere with neural transmission and affect subsequent behavioral performance [12,13], while under prolonged exposure, it could have more long-lasting and distal effects via neuronal death [14], delays in myelination, developmental abnormalities such as neuronal pruning, inhibition of neurogenesis and gliogenesis, or brain growth factors [15- 18], especially in brain regions with a high density of glucocorticoid receptors that are involved in late postnatal development [19], such as the hippocampus, amygdala and prefrontal cortex [19-24].
Increased and prolonged cortisol exposure can also contribute to the development of pathologies [25,26] such as post-traumatic stress disorder (PTSD) [9,27] and cognitive impairments, like those related to the PFC, which include abstraction, emotional regulation, self-monitoring and the executive functions. The latter form a set of mental capacities necessary to formulate a plan and execute the steps to carry out the task according to that plan [28]. While there is no consensus on the processes that make up the executive functions, most authors agree that working memory, inhibitory control, mental flexibility, planning and decision-making are all involved [28-30].
In relation to PTSD and CSA, studies report using isolated tasks that have revealed poor performance in attention, abstract reasoning/executive functions [31], and working memory [32,33]. In addition, participants with these conditions have manifested increased cognitive interference on a color-word Stroop task [34,35], and have failed to maintain the criteria for successful classification on the Wisconsin Card-Sorting Test [36]. Other research has focused on abused subjects regardless of the PTSD condition. For example, female victims of repeated CSA showed increased variability in response latency and diminished inhibitory capacity on a go/no-go/ stop vigilance task designed to assess motor inhibition [37]. Mueller et al. (2010), meanwhile, found that adolescents with a history of child maltreatment (including sexual abuse) showed lower performance than their peers with no traumatic history on a modified version of the Stop-Signal task, which also evaluates motor inhibition.
The aforementioned research supports the presence of vulnerability to cognitive processes related to the prefrontal cortex in adolescents exposed to CSA with PTSD due, among other factors, to the hyper-secretion of cortisol. However, for victims of early stress no studies have yet explored the relationship between this stress hormone and cognitive performance, or evaluated comprehensively the set of cognitive processes related to the PFC in female adolescents with PTSD secondary to CSA, much less how their executive performance may be affected in daily life.
Considering that PTSD secondary to CSA has been associated with anatomical and functional alterations in the PFC, cognitive impairments, and increased cortisol levels, the aim of the present study was to determine the relation between baseline cortisol levels and cognitive functions related to the PFC in female adolescents with PTSD secondary to CSA. We hypothesized that there will be a correlation between basal cortisol levels and the scores on tests designed to measure the cognitive and behavioral functions related to the PFC, and that the adolescents with CSA-related PTSD will present –compared to their healthy peers with no history of trauma–deficient performance on cognitive functions related to the PFC, executive impairments in early life, and higher basal cortisol levels in their saliva.
Method
Subjects
Twenty 12-15-year-old female adolescents with sexual abuserelated PTSD (PTSDG) and twenty 12-15-year-old healthy female adolescents (controls) were evaluated. All the PTSD participants came from foster homes where they had resided for between six months and five years. Participants in the control group (CG) were recruited from a public secondary school. They all lived with their families and had no history of child maltreatment according to a semi-structured interview designed in our laboratory. All participants had normal IQs according to the brief form of the WISC-IV-R, [38] (Table 1), were regular students, and did not have Attention Deficit Disorder, according to the DSM-V [39] criteria.
In terms of schooling, reports by teachers and parents stated that no participant in either group had failed more than one school grade. All participants were healthy, right-handed and had no prior history of neurological disorders, learning disabilities, drug abuse or chronic illness. Participants in CG were matched with those in the PTSDG by age, handedness, socioeconomic status and IQ scores.
We confirmed that the PTSDG participants presented histories of intra-familiar CSA by reviewing files held at the foster homes and through semi-structured interviews. Eleven of the twenty adolescents had been sexually-abused by their stepfathers, one by her stepbrother, seven by their fathers, and one by a brother. The girls in PSTDG had been raped, except for six cases in which they had been “fondled” and seduced.
To select participants, we interviewed each girl and her parents or advisors, and applied the WISC-IV, the child PTSD symptom scale (CPSS) [40], and a child abuse screening tool (ICAST-C) [41]. The latter allowed us to determine the presence and intensity of PTSD and the kind of abuse experienced by each girl. In addition, the children’s depression inventory (CDI) [42] and Spence Children’s Anxiety Scale (SCAS by Spence) were applied to determine the presence of symptoms of depression and anxiety.
All procedures involved in this research were approved by the Ethics Committee of the Institute of Neuroscience (registration number ET112015-208) in accordance with the ethical standards laid down in the 1964 Helsinki Declaration. All participants and their parents or guardians gave their informed consent prior to inclusion in the study.
Evaluation of the cognitive functions
To assess the cognitive functions (working memory, motor and interference inhibitory control, mental flexibility, planning and problem-solving), the Battery for Evaluating the Executive Functions-2 [43], Flores et al., 2014), and a Stop-Signal task (STOPIT) [44] were used. In addition, in order to evaluate executive functioning in daily life, the Behavior Rating Inventory of Executive Function [45] was applied.
BANFE-2
Battery for Evaluating the Executive Functions-2 (BANFE-2) includes a series of tests designed to measure several cognitive functions related to the PFC. These tasks are divided based on anatomo-functional criteria: a) functions related to the orbitofrontal cortex; b) functions related mostly to the anterior prefrontal cortex (frontopolar or rostral); and c) processes related to the dorsolateral prefrontal cortex. These tasks form three broad indexes –orbital, dorsolateral and anterior– and generate an overall result called the global executive score [46]. Subsequently, the various BANFE tasks used and their broad scores were analyzed and will be arranged and presented according to the cognitive function they assessed.
Working memory
a) Visuospatial working memory: on a sheet of paper with figures of everyday objects, participants had to reproduce the sequences of touches in the same order shown by the examiner. This task consisted of 4 lists that increased the number of figures from 4-7 elements. Two scores were recorded: the maximum sequence of touches that the participants could make without error, and order mistakes when they indicated a figure did not match the original sequence.
b) Self-directed signaling: a self-directed working memory test that utilizes a sheet with figures of objects and animals. Participants are asked to point to all the figures without exception. The rules stipulate that she must not point to a figure that is around the one pointed before (either up, down, left, right or diagonal). The score obtained was the number of hits.
c) Alphabetical ordering of words: participants were shown 3 lists, each with 5 or 6 words that could begin with either a vowel or consonant. They had to order the words mentally and then say them in correct alphabetical order. The score obtained was the number of attempts required to reproduce each list correctly (up to 5 trials).
d) Subtraction and consecutive addition: this task evaluates the ability to perform simple calculation operations in reverse sequence. On the consecutive subtraction test, participants had to subtract a quantity consecutively (3 by 3 or 7 by 7, for example) until they reached he minimum number (e.g. 2 or 1). The consecutive sum task used the same instruction, but instead of subtracting, participants had to add. The scores obtained on both tasks were for the number of correct answers and execution time.
Flexibility
Card classification: this task uses a deck of cards with 4 different geometric figures (circle, cross, star, triangle), 4 numbers and 4 colors (purple, brown, red, light blue). Subjects are given 64 cards and asked to place them under one of the 4 corresponding base cards that are shown on a sheet according to a criterion that they must generate, based on color, shape or number. The correct decision is established by an arbitrary criterion determined by the evaluator (in accordance with the sequence established by the test). The scores obtained were for the number of hits (matches between the criteria of subject and evaluator), the number of perseverations (sum of immediate and deferred perseverations), the number of completed categories, and maintenance errors.
Inhibition
Stroop forms A and B: consist of sheets composed of columns each with six color words. There are two conditions: one neutral, the other conflictive. In the neutral condition, participants are only asked to read the printed word (the word is printed in that color to create a word-color relationship effect). In the conflictive condition, they must pronounce the color in which the word is printed. This is conflictive because the word-color relationship is broken. The test consists of two parts. In Stroop-A, subjects are asked to read the written word except when it is underlined, in which case they must say the name of the color in which the word is printed. In Stroop-B, the evaluator points to the different columns and asks the subject to say what is written, except when she/he says “color”, then the participant must say the color in which the words are written. The scores obtained on these tests consist in the number of Stroop errors (i.e., when a color is misnamed), and execution times.
Planning
a) Mazes: this task includes five mazes with increasing levels of difficulty. It is designed to evaluate the participant’s ability to respect limits and plan motor execution to reach a goal. They are asked to solve each maze in the shortest time possible while following certain rules; namely, they cannot touch or cross the walls, nor lift the pencil once the task has begun. The scores obtained were the number of times that the subject entered a road with no way out, and execution time.
b) Tower of Hanoi: this test evaluates the ability to plan several actions in a specific order as a way to reach a goal. The test presents a base with three stakes placed vertically and 3 or 4 different size discs. Participants must obey the following rules: they can move only one disk at a time and cannot place a larger disc on top of a smaller one. The objective of the task is to move the original arrangement from the first stake to the third. The score obtained is the number of movements required to reach the goal.
Decision-making
Card game: this test assesses participants’ ability to perform an exercise under conditions of uncertainty, and to learn risk-benefit relationships. They are told that the objective is to obtain the highest possible result. There are groups of cards that participants choose one-by-one. Depending on the number on the card chosen, the evaluator opens a corresponding card, which indicates the points won, or punishments received, in each case. The cards that subjects choose are numbered from 1-5. Those marked 1, 2 and 3 stipulate mild punishments and appear less often, while numbers 4 and 5 give more points but are more frequent and impose harsher punishments. The scores obtained are: total points obtained, and the percentage of risk cards.
Abstraction
Semantic classifications: this test assesses the ability to analyze a series of animal figures and group them into as many semantic categories as possible. A picture with 30 animal figures is shown and participants must generate all the categories that occur to them in a 5-minute period. The score obtained was the number of abstract categories (e.g. mammals, domestic, marine, etc.).
Fluency
Verbal fluency evaluates the ability to select and reproduce efficiently as many verbs in the infinitive form as possible during one minute. The score obtained was the number of hits.
Stop-signal task
The STOP-IT computer program developed by Verbruggen et al. [44] was used. This task includes two phases: the first consists of 32 practice tests, while the second is composed of three blocks of 64 tests each. The task shows a primary stimuli 75% of the time and the subject’s performance is measured by typing “1” when a white circle stimulus appears in the middle of the screen against a black background), but “2” when a square stimulus appears in the same place with the same color characteristics. A “stop” stimulus is shown 25% of the time randomly during the task. When it appears, a primary stimulus is followed by an auditory one called he stop-signal. At that point, subjects must suppress their primary response which is to press “1” or “2” and refrain from pressing any key; that is, they have to inhibit the learned motor behavior. In addition, the length of the stop-signal delay (SSD) changes during the task according to each subject’s effectiveness, such that if the inhibition is correct SSD is increased by 50 milliseconds (ms), but if unsuccessful, it is decreased by 50 ms. The scores normally obtained on this test are the average SSD, average reaction time (RT), percentage of correct inhibitions, and the stop signal reaction time (SSRT), which was calculated by subtracting SSD from RT. However, in the present study, only SSRT was analyzed, since it is the most sensitive variable for measuring inhibition capacity.
Rating Inventory of Executive Functions (BRIEF) is an inventory for parents and teachers of school-age children that assesses executive function behaviors in the home and school environments. The forms for the parent and teacher each contain 86 items that measure different aspects of executive functioning: inhibition, shift, emotional control, initiation, working memory, planning/organizing, organization of materials, and monitoring. These scales are then used to form two broader indexes –behavioral regulation and metacognition and an overall score called the global executive composite. In this study, we used only the parent’s form, which was applied to each adolescent’s parents (CG) or caregivers (PTSDG). The broad scores were analyzed.
Cortisol measurement
We were only able to analyze saliva samples from 15 participants in each group since some had gum bleeding, canker sores or an infectious disease (e.g., flu). Three saliva samples were taken from them on two consecutive days between day 4 and 7 after menstruation. On each day, the first sample was taken in the morning as soon as the girl awoke, the second half an hour later (before eating or brushing her teeth), and the third at night, just before going to bed. Cortisol levels were measured by the enzyme-linked immunosuppressive assay (ELISA), a competition-based analysis that measures the interaction of an unknown amount of antigen present in the sample and a fixed amount of enzyme-labeled antigen. The antigens in the sample compete for the binding sites of the antibodies that line the wells. After the substrate reaction, the wells are washed to stop the competition reaction. The intensity of the color that develops is inversely proportional to the amount of antigen in the sample (i.e., the more intense the color, the lower the cortisol level measured in μg/mL, and vice versa). Results can be determined directly using the standard curve by performing an immunological assay for the in vitro quantitative determination of free cortisol in human saliva. The ALPCO Kit was used for the quantitative determination of cortisol in these samples. For the statistical analysis, the cortisol levels from the two days were averaged to obtain two values: one for the morning and one for the evening.
Statistical analysis
First, the Levene test was run to determine if the data presented equal variances. Based on the results of this analysis, Student’s t analyses for independent groups were applied to test for betweengroup differences in the IQ results, psychopathological data, BANFE-2 global indices, and Stop-Signal scores. In addition, because some of the BANFE-2 and BRIEF scores did not meet the homoscedasticity criterion, the Mann-Whitney U test was applied to compare the groups. Broad scores from the neuropsychological tests were analyzed, except for the BANFE-2 global indices, where standardized scores were used. To determine the differences between groups and saliva samples, split-plot ANOVAS (2 groups x 3 samples) were performed, followed by Bonferroni’s ex-post analysis to ascertain the meaning of the differences. The size effects were calculated in all cases.
Finally, to determine whether there was a relationship between cortisol levels and executive functioning, either a Pearson or Spearman correlation analysis was performed between the cortisol levels from each sampling day and the BANFE and BRIEF global indices, together with the SSRT from the Stop-Signal task.
For the Student’s t and Mann-Whitney U tests, the significance level was corrected according to the Bonferroni criteria by dividing 0.05 by thenumber of dependent variables (DV) in each parameter. In this way, the significance for the BANFE-2 global indices was p<0.0125 (DV=4), for the BANFE-2 tasks, 0.002 (DV=26), for the Stop-Signal task, 0.05 (DV=1), for the BRIEF global indexes,<0.017 (VD=3), and for the BRIEF scale scores, <0.006 (VD =8). All statistical analyses were performed with IBM SPSS V19.
Results
Characteristics of the groups
As Table 1 shows, the Student’s t analyses found no significant differences between the groups in terms of age or IQ, though PTSDG showed a higher intensity of such PTSD symptoms as depression and anxiety.
| PTSDG | CG | Comparisons | NCS | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t38 | p | r | PTSD/GC | |
| Age | 12.75 | 0.91 | 13.08 | 0.91 | 0.0001 | 1 | 0.0001 | |
| IQ | 83 | 5.8 | 83 | 4.28 | -0.74 | .46 | 0.1192 | |
| PTSD | 44 | 12.34 | 6 | 4.61 | 13.2 | .001* | 0.9061 | 20/0 |
| Depression | 55 | 6.17 | 41 | 7.01 | 7.08 | .001* | 0.7542 | 2/0 |
| Anxiety | 59.7 | 7.96 | 47.25 | 6.78 | 5.324 | .001* | 0.6563 | 6/0 |
| Age at the last episode of sexual abuse | 9.4 | 2.95 | ||||||
| Institutionalization time | 2.95 | 1.580 | ||||||
Columns four, five and six present the tfg values, significance (p) and size effect (r) of the t tests conducted to compare the group values for each variable. The last column shows the number of cases that presented significant, clinically-psychopathological symptoms (NCS) in each group; *significant differences; N=40.
Table 1: Characteristics and matching criteria of the PTSD and control (CG) groups, with statistical comparisons.
BANFE-2
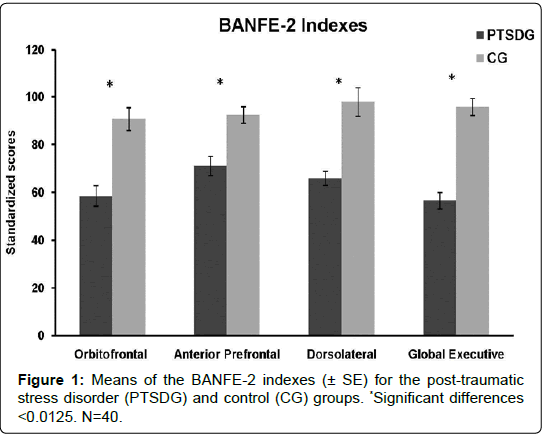
According to the Student’s t analyses, PTSDG presented a significantly lower score than CG in the orbital (t38=-5.003, p˂0.001, r=.6302), dorsolateral (t38=-7.018, p˂0.001, r=.7513), anterior (t38=- 4.082, p˂0.001, r=.5521) and global executive composites (t38=-7.954, p˂0.001, r=.7904) (Figure 1).
The Mann-Whitney U analysis showed that PTSDG had lower performance than CG on the tasks that make up BANFE-2, except for the mazes, the card game, fluency, and the visuo-spatial (order errors) and Tower of Hanoi tasks, though there was no significant tendency on the last two assessments (Table 2).
| EF | Task | PTSDG | CG | Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Range | Mean | SD | Median | Range | U | p | r | ||
| Working Memory | Visuo-spatial | |||||||||||
| Maximum sequence | 3.15 | 0.813 | 3 | 2 | 2.56 | 0.83 | 2.5 | 3 | 140 | 0.108 | -0.382 | |
| Order errors+ | 4.3 | 2.75 | 4.5 | 11 | 2.15 | 2.37 | 1 | 7 | 95.5 | 0.004 | -0.429 | |
| Self-directed signaling | ||||||||||||
| Hits | 19.25 | 3.09 | 20 | 12 | 21.7 | 2.43 | 23 | 6 | 63.5 | .000* | -0.835 | |
| Alphabetical ordering of 5 words+ | ||||||||||||
| Number of attempts | 2.75 | 1.07 | 2.5 | 4 | 1.75 | 0.851 | 2 | 3 | 89 | .002* | -0.717 | |
| Alphabetical ordering of 6 words+ | ||||||||||||
| Number of attempts | 4.55 | 0.61 | 5 | 2 | 3.25 | 1.11 | 3 | 4 | 57.5 | .000* | -0.907 | |
| Subtraction from 3 in 3 | ||||||||||||
| Time+ | 140.35 | 79.03 | 151 | 250 | 81.85 | 68.3 | 53.5 | 275 | 80.5 | .001* | -0.723 | |
| Hits | 10.3 | 2.84 | 11 | 11 | 12.15 | 1.57 | 13 | 6 | 111 | 0.015 | -0.582 | |
| Subtraction from 7 in 7 | ||||||||||||
| Time+ | 228.35 | 96.38 | 297 | 251 | 186.4 | 96.57 | 181 | 259 | 47 | .000* | -0.451 | |
| Hits | 7.75 | 3.77 | 7 | 11 | 10.6 | 3.56 | 12 | 14 | 100 | 0.006 | -0.61 | |
| Addition | ||||||||||||
| Time+ | 121.1 | 43.17 | 123.5 | 165 | 67.05 | 30.12 | 53 | 130 | 47 | .000* | -0.926 | |
| Hits | 17.85 | 2.21 | 18.5 | 6 | 19.85 | 0.366 | 20 | 1 | 85 | .001* | -0.788 | |
| Flexibility | Card classification: | |||||||||||
| Hits | 34.9 | 7.09 | 36 | 24 | 45.2 | 7.09 | 48.5 | 23 | 61 | .000* | -0.843 | |
| Perseverations | 15.55 | 6.12 | 9.4 | 7.52 | 15 | 30 | 7.5 | 19 | 96.5 | 0.004 | -0.627 | |
| Maintenance errors | 0.9 | 0.85 | 1 | 2 | 1.15 | 1.424 | 1 | 6 | 187 | 0.738 | -0.084 | |
| Inhibition | Stroop A | |||||||||||
| Stroop errors | 5.2 | 5.13 | 3.5 | 17 | 1.65 | 1.23 | 1 | 4 | 88.5 | .002* | -0.687 | |
| Time+ | 117.45 | 39.46 | 112 | 194 | 86 | 14.69 | 81 | 62 | 58.5 | .000* | -0.869 | |
| Stroop B | ||||||||||||
| Stroop errors | 5.45 | 3.75 | 6 | 14 | 1.4 | 1.79 | 0.5 | 6 | 51 | .000* | -0.877 | |
| Time+ | 102.55 | 21.71 | 99 | 99 | 80.65 | 18.22 | 75.5 | 55 | 55 | .000* | -0.928 | |
| Planning | Mazes | |||||||||||
| Road with no way out | 1.8 | 1.54 | 1.5 | 5 | 0.9 | 0.79 | 0 | 1 | 133 | 0.072 | -0.429 | |
| Time+ | 29.3 | 7.84 | 28 | 26 | 24.55 | 10.44 | 22.5 | 34 | 143 | 0.127 | -0.345 | |
| Tower ofHanoi, 3 discs | ||||||||||||
| Movements+ | 20.8 | 12.47 | 16.5 | 40 | 11.7 | 4.89 | 11.5 | 18 | 118.5 | 0.026 | -0.495 | |
| Tower ofHanoi, 4 discs | ||||||||||||
| Movements+ | 116.1 | 72.66 | 38.5 | 116 | 65.5 | 54.94 | 25 | 37 | 105.5 | 0.009 | -0.572 | |
| Decision-making | Card game | |||||||||||
| Percentage of risk cards+ | 31.9 | 12.71 | 34 | 62 | 33.15 | 9.75 | 35 | 29 | 190.5 | 0.799 | -0.058 | |
| Points obtained | 19.65 | 23.93 | 21.5 | 126 | 25.65 | 12.65 | 27 | 38 | 160 | 0.289 | -0.242 | |
| Abstraction | Semantic classification | |||||||||||
| Number of abstract categories | 0.9 | 1.2 | 0 | 3 | 3.35 | 1.18 | 3 | 4 | 38 | .000* | -1,014 | |
| Fluency | Verbal | |||||||||||
| Hits | 11.05 | 4.31 | 11 | 15 | 13.3 | 3.96 | 14 | 15 | 143 | 0.127 | -0.346 |
+ Scores where higher values mean worse execution; *significant differences< .002. N=40.
Table 2: Broad scores of the BANFE-2 tasks used. The means, standard deviations, medians and ranges of each group (post-traumatic stress disorder [PTSDG] and control [CG]) are shown with statistical comparisons between groups (Mann-Whitney U, p and size effect ‘r’).
Stop-signal
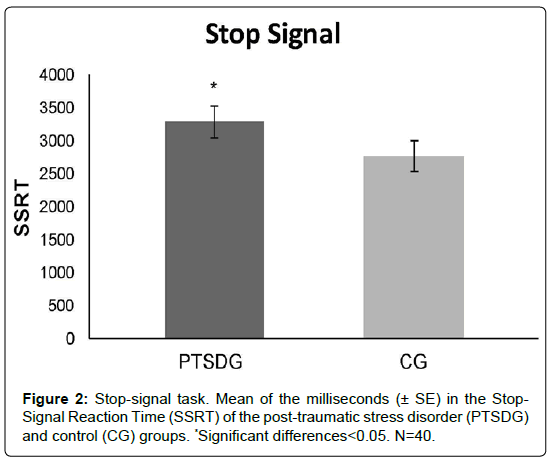
According to the Student’s t analyses, PTSDG had a higher SSRT than CG on the Stop-Signal task (t38=1.108, p=.042, r=.324) (Figure 2).
BRIEF
Here, the Mann-Whitney U analysis showed that PTSDG had more executive impairments in daily life than CG, since the former obtained higher scores on the behavioral regulation (U=29.5,p=.000, r=-1.115) and metacognition indexes (U=16, p=.000, r=-1.192) and the global executive composite (U=3, p=.000, r=0.627) (Figure 3). The same result was observed in all BRIEF scales, except the organization of materials and shift, where no significant tendency appeared (p =.009) (Table 3).
| PTSDG | CG | Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Scales | Mean | SD | Median | Range | Mean | SD | Median | Range | U | p | r |
| Inhibition | 19.8 | 5.75 | 19.5 | 18 | 10.75 | .851 | 11 | 3 | 10 | .000* | -1,163 |
| Shift | 14.7 | 4.05 | 14.5 | 13 | 11.45 | 2.48 | 11 | 7 | 104 | .009 | -0,585 |
| Emotional control | 19.1 | 5.36 | 18.5 | 19 | 12.55 | 2.33 | 12 | 10 | 54 | .000* | -0,888 |
| Initiate | 14.8 | 4.89 | 13 | 15 | 10 | 1.56 | 10 | 6 | 41.5 | .000* | -0,968 |
| Working memory | 16.7 | 5.88 | 15 | 20 | 10.95 | 1.54 | 10 | 6 | 45 | .000* | -0,957 |
| Planning/organizing | 20.3 | 5.77 | 18 | 19 | 13.9 | 1.68 | 14 | 6 | 25.5 | .000* | -1,065 |
| Organization of materials | 8.6 | 3.8 | 7 | 12 | 7.95 | 1.43 | 8 | 5 | 171 | .445 | -0,179 |
| Monitoring | 15.7 | 9.8 | 15 | 15 | 52.6 | 4.38 | 10 | 6 | 24 | .000* | -1,072 |
*Significant differences<.006. N=40.
Table 3: Broad scores of the BRIEF scales.The means, standard deviations, medians and ranges of each group (post-traumatic stress disorder [PTSDG] and control [CG]) are shown with statistical comparisons between groups (Mann-Whitney U,p and size effect “r”).
Cortisol levels
The ANOVA showed the existence of principal effects for group (F1,28=86.154, p=.000, ɳ2=.755) and sample (F1,2=6.305, p=.006, ɳ2=318), but no interaction between them. PTSDG presented higher cortisol levels than CG in all three samples. Post hoc compassions indicated that only PTSDG showed a significant decrease in the nighttime sample compared to both morning samples (Figure 4).
Correlations between cognition and cortisol
As Table 4 shows, there were significant correlations between the three cortisol samples and all BRANFE-2 and BRIEF indexes, but none with SSRT.
| Analysis | Scores | Upon awakening | Morning | Night | |||
|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||
| Pearson | BANFE-2 | ||||||
| Orbitofrontal Index | -.582 | .001* | -.517 | .003* | -484 | .007* | |
| Anterior Prefrontal Index | -.496 | .005* | -.372 | .043* | -492 | .006* | |
| Dorsolateral Index | -.507 | .004* | -.471 | .009* | -.460 | .010* | |
| Global Executive | -.619 | .000* | -.576 | .001* | -.510 | .004* | |
| SSRT | .285 | .127 | .342 | .064 | -.177 | .350 | |
| Spearman | BRIEF | ||||||
| Behavioral Regulation Index | 705 | .000* | .715 | .000* | .505 | .004* | |
| Metacognition Index, | .572 | .001* | .724 | .000* | .456 | .011* | |
| Global Executive Composite Index | .644 | .000* | .742 | .000* | .456 | .011* | |
N=30.
Table 4: Correlations (Pearson or Spearman) and significance between cortisol levels in saliva and the BANFE-2 indexes, Stop-Signal Reaction Time (SSRT) and BRIEF indexes.
Discussion
The results obtained in the present study show that the group of adolescents with PTSD secondary to sexual abuse had deficiencies in many cognitive processes related to the PFC. Also, they had higher basal levels of salivary cortisol compared to the healthy adolescents with no history of trauma. In addition, the levels of this hormone showed a significant correlation with prefrontal cognitive functioning.
These findings further revealed that the adolescent girls with PTSD secondary to sexual abuse presented significantly lower scores on the BANFE-2 global index. While CG achieved standardized score values that fell within the low normal range (80-100), PTSDG’s scores were below 70 (i.e., two standard deviations below the mean), which could indicate the presence of a prefrontal dysfunction. These results agree with those obtained in the BRIEF’s Global Executive Composite and the Behavioral Regulation and Metacognition indexes, where PTSDG had higher scores, which suggest executive impairment in daily life. This finding was related to both the ability to (1) shift a cognitive set and modulate emotions and behavior via appropriate inhibitory control, and (2) initiate, plan, organize and sustain future-oriented problem-solving in working memory.
Unlike previous studies, we evaluated a wide range of cognitive processes related to the prefrontal cortex and executive functioning in daily life, so ours constitutes a more ecological approach to the problem. When analyzing the raw scores of the tasks that assessed the different cognitive processes examined, we found that PTSDG consistently presented lower performance than CG, except on the decision-making tasks, but including the BANFE-2 task, which assessed working memory. These results agree with those reported in earlier studies which found that young adult women with a history of sexual abuse have lower scores on tasks related to working memory [33,37]. Similar findings have been reported in people who have experienced stressful events and suffered PTSD [47]. Moreover, our BANFE-2 findings agree with PTSDG’s higher scores on the BRIEF working memory scale. In fact, the caregivers of the PTSDG girls describe them as having a weak working memory, difficulty in remembering things even for a few seconds, losing track of what they are doing as they work, or forgetting what they are supposed to obtain when sent on errands.
Mental flexibility is understood as the ability to modify one’s behavior according to environmental contingencies. PTSDG also presented lower scores than CG for this cognitive process; especially a lower number of correct answers and a higher number of perseverations compared to CG on the card classification task. Likewise, PTSDG’s higher scores on the BRIEF shift scale indicated that they have difficulty in moving freely from one situation, activity or aspect of a problem to another as the circumstances demand. In this regard, our results are similar to those reported by other authors who usedt he classic Wisconsin Card-Sorting Test (WCST), which evaluates several cognitive functions related to flexibility and categorization [48- 50]. For example, in a sample of adolescents without psychiatric disorders, Spann et al. (2012) found a significant association between physical abuse/neglect and perseverative errors on the WCST. Also, a previous study conducted in our laboratory observed that adolescents with PTSDCSA show a lower capacity to maintain successful classification criteria on the WCST than their peers with no history of abuse [36]. Mothes et al. [51], meanwhile, found that adolescents who had suffered abuse showed deficiencies in cognitive flexibility when evaluated by the Trail-Making Task. PTSDG also showed difficulties in inhibiting both interference and preponderant responses when compared to CG, and problems with inhibitory control were mentioned by the girls’ caregivers on the BRIEF inhibit scale.
Turning to the Stroop test, PTSDG had more errors, fewer hits and higher execution times, three aspects that have been observed in impulsive people [52]. The Stroop task evaluates the ability to suppress distracter stimuli that can slow down the response to primary stimuli [53]. Similar results in female adolescents with PTSD secondary to CSA were reported in a study by Freeman and Beck [34], which used a version of the Stroop with emotional content. Those authors found that their participants generally showed a higher runtime to name the colors than a control group with no history of abuse.
Regarding the Stop-Signal task that assesses inhibitory motor control, we found that PTSDG presented a higher SSRT score, indicating that they needed more time to react to a stop stimulus. Similarly, earlier studies found that adolescents with a history of child abuse have lower performance on tasks that measure motor inhibition than their peers with no such antecedents [37,54].
Compared to controls, PTSDG also showed poorer performance on the Brief planning/organizing test with no significant tendency in the number of movements on the Tower of Hanoi task, which is used to evaluate planning [55]. To the best of our knowledge, this finding has not been reported in the literature to date. In addition, PTSDG presented a lower level of abstraction than CG, but this result has been reported in previous studies of adolescents who suffered child abuse [22].
We did not find between-group differences in verbal fluency or on the task used to evaluate decision-making. It is likely that the latter result is due to the low sensitivity of the BANFE-2 “card game”, since in this version, unlike the gambling task [56], the rewards and punishments do not have incentive or monetary value, so they were probably insufficiently sensitive to evaluate the level of risk taken by the girls. Another explanation of the lack of significant differences between the two groups is that children and adolescents with a history of early stress are less sensitive to a reward [57] of an economic type [58]. Dillon et al. [59] used fMRI to explore the effect of rewardprocessing on the activation of the basal ganglia in adults with a history of mixed childhood maltreatment and psychiatric disorders. Using a delayed monetary incentive task, they found that relative to non-abused controls those adults rated reward-predicting cues as less positive, and displayed a weaker response to reward cues in the left globus pallidus.
Finally, PTSDG showed higher scores on the BRIEF initiation and emotional control scales, which means that they encounter more problems when asked to begin a task or activity, and in generating ideas, responses, or problem-solving strategies independently. In addition, they presented difficulty in modulating their emotional responses.
The deficiencies observed in almost all the cognitive processes evaluated suggest a broad prefrontal dysfunction in adolescents with PTSD secondary to CSA that could be a consequence of anatomical and functional changes in certain brain regions. For example, previous studies have reported that victims of child maltreatment exhibit a smaller volume of the dorsolateral PFC [21,23,60] and right temporal and bilateral parietal lobes [60], as well as lower density of the arcuate and superior longitudinal fascicles that connect the PFC to the temporal and parietal areas [61,62]. These regions and their inter connections participate in working memory, flexibility, planning, abstraction and fluency [63,64].
The victims of child maltreatment also had a lower volume of the orbital prefrontal cortex [24,60] and the anterior cingulate [20,21], both of which are involved in decision-making, emotional regulation, motivation and inhibitory control [65]. The orbital prefrontal cortex has extensive connections with the amygdala and the insular and temporal pole cortices [66]. In this regard, it has been reported that women with PTSD secondary to CSA show lower blood flow in the medial prefrontal cortex, subcallosus gyrus (Brodman area 25) and anterior cingulate when recalling the sexual abuse they suffered [67], accompanied by hyperactivity in the amygdala [68].
The study of children with a background of early stress by Eluvathingal et al. (2006) found a decrease in the white matter density of the left uncinate fascicle, which connects the orbitofrontal cortex to the anterior area of the temporal lobe, including the amygdale. This fascicle plays a crucial role in emotional regulation, as will be discussed below.
At the outset, we hypothesized that the girls in PTSDG will have higher cortisol levels in all saliva samples compared to CG, as reported in previous studies [10,69]. But our results also show correlations between cortisol levels and all BANFE-2 and BRIEF indexes. This means that in the participants with higher cortisol levels, the performance of the cognitive functions related to the prefrontal cortex is lower and the executive impairment in daily life greater. This relation could indicate that stressful early life environments perturb the hypothalamic-pituitary-adrenal (HPA) axis which, in turn, may predispose such individuals to cognitive and behavioral disorders in adolescence.
Teicher, Tomoda and Andersen [70] proposed that early stress produces a cascade of events mediated, precisely, by stress that lead to the release of stress hormones (cortisol, adrenalin, vasopressin) and enhance the turnover of neurotransmitters (dopamine, serotonin, norepinephrine) in key brain regions. Stress-induced changes in neurohumors then generate an accelerated loss of neurons, delays in myelination, developmental abnormalities such as neuronal pruning, and inhibition of neurogenesis and gliogenesis or brain growth factors [15-18]. Hence, in this perspective, certain brain regions may be vulnerable to stress because they have a high density of glucocorticoid receptors and play an important role in postnatal development [19].
There are 2 types of cortisol receptors in the brain: type 1 (for mineralocorticoids), which is saturated first to improve the functions of different brain areas, including the hippocampus; and type 2 (for glucocorticoids), which rebounds causing a critical level of saturation that can have a cytotoxic effect on neurons [22]. This phenomenon commonly occurs during acute or chronic stress. The PFC is especially vulnerable to the effects of stress because it has mostly type 2 receptors and participates in late postnatal development. In addition, during stress cortisol blocks extracellular catecholamine transporters located in glial cells that contribute to removing excess dopamine and noradrenaline from synapses. This results in an extracellular increase of both types of catecholamines, which causes a deterioration of the processes in which the PFC is involved [71], including the executive functions, attention, fluency and behavioral and emotional regulation. It is important to note in this regard that studies with rats have shown that chronic stress provokes a loss of spines and dendrites in the PFC [72,73].
Prefrontal dysfunction could be implicated in the impairment of emotional regulation observed in our PTSDG group because the prefrontal control systems modulate emotion-generating systems, like the amygdala, which is responsible for detecting affectivelyarousing stimuli and shows hyperactivity in CSA survivors [68,74]. Early chronic stress could trigger this hyperactivity due to a permanent alteration in the composition of the GABAA complex of the amygdala, which produces a lower affinity for benzodiazepines, as well as a reduction of the density of both the GABAA receptors and the benzodiazepine binding sites in these receptors [75,76].
To explain how the PFC modulates emotional responses it is important to consider that prefrontal structures include dorsal regions of the lateral prefrontal cortex (PFC) involved in selective attention and working memory, ventral areas of the PFC implicated in response inhibition, the ACC, which participates in monitoring control processes, and the dorso-medial PFC, which is implicated in monitoring affective states [77]. When individuals deliberately regulate affective responses, activation increases in the PFC, but decreases in the amygdale [78,79], suggesting that PFC projections to the amygdala exert an inhibitory top-down influence [80,81]. For this reason, stress-related damage in the PFC and uncinate fasciculus could impair proper regulation of emotional responses elicited by the amygdala.
Finally, some limitations of the present study should be mentioned. The first is that the sample assessed is small due to the difficulty of finding participants who met the strict inclusion criteria. Although we screened girls from three foster homes in the state of Jalisco, Mexico, we were unable to obtain a larger sample because many potential participants had low IQs, were in a school grade below their age, had a maternal history of drug use, or had been institutionalized for over five years.
Second, it was impossible to separate and distinguish the impacts of PTSD and child sexual abuse because we could not gather a sample of participants with child sexual abuse who had not suffered PTSD as well. For this reason, the high incidence of PTSD in institutionalized child sexual abuse survivors is a topic that needs to be analyzed in future research.
Conclusion
Cortisol levels showed a significant correlation with prefrontal cognitive functioning in both the adolescents with PTSD secondary to sexual abuse and their healthy pairs with no traumatic background, but the PTSD group had deficiencies in many cognitive processes related to the PFC, which were evident in their daily behavior. Also, they had higher basal levels of salivary cortisol. We believe that the information provided by this study improves our understanding of the impact of early stressful events on the HPA system and their relation to cognitive and affective process in which the PFC plays a central role.
Grants
This research was supported by CONACYT grant CB/2012/180981.
References
- Spear LP (2013) Adolescent neurodevelopment. J Adolesc Health 52: 7-13.
- Lenroot RK, Giedd JN (2006) Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30: 718-129.
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, et al. (2014) The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage 95: 193-207.
- Scherf KS, Smyth JM, Delgado MR (2013) The amygdala: an agent of change in adolescent neural networks. Horm Behav 64: 298-313.
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ (2005) Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry 57: 624-632.
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, et al. (2008) Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry 63: 927-934.
- Bagley C (1992) Characteristics of 60 children and adolescents with a history of sexual assault against others: evidence from a comparative study. J Forens Psychiatry 3: 299-309.
- Paolucci EO, Genuis ML, Violato C (2001) A meta-analysis of the published research on the effects of child sexual abuse. J Psychol 135: 17-36.
- Teicher MH, Andersen SL, Polcari AM, Anderson CM, Navalta CP, et al. (2003) The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 27: 33-44.
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, et al. (2002) Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry 51: 575-582.
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, et al. (1999) Developmental traumatology. part ii: brain development. Biol Psychiatry 45: 1271-1284.
- Lupien SJ, Lepage M (2001) Stress, memory, and the hippocampus: can't live with it, can't live without it. Behav Brain Res 127: 137-158.
- Wolf OT (2003) Hpa axis and memory. Best Pract Res Clin Endocrinol Metab 17: 287-299.
- Sapolsky RM (1992) Stress, the aging brain, and the mechanisms of neuron death. MIT press, Cambridge, MA.
- De Bellis MD (2005) The psychobiology of neglect. Child Maltreat 10: 150-172.
- Lauder JM (1983) Hormonal and humoral influences on brain development. Psychoneuroendocrinology 8: 121-155.
- Bohn MC (1980) Granule cell genesis in the hippocampus of rats treated neonatally with hydrocortisone. Neuroscience 5: 2003-2012.
- McEwen BS (2008) Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583: 174-185.
- Teicher MH (2002) Scars that won't heal: the neurobiology of child abuse. Sci Am 286: 68-75.
- Kitayama N, Quinn S, Bremner JD (2006) Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord 90: 171-174.
- Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, et al. (2009) Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage 47: 66-71.
- Lee V, Hoaken PN (2007) cognition, emotion, and neurobiological development: mediating the relation between maltreatment and aggression. Child Maltreat 12: 281-298.
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, et al. (2008) Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 20: 292-301.
- De Brito S, Viding E, Sebastian C, Kelly P, Mechelli A, et al. (2013) Reduced orbitofrontal an temporal gray matter in a community sample of maltreatment children. J Child Psychol Psych 54: 105-112.
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC (2009) The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage 47: 864-871.
- Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8: 383-395.
- Roozendaal B, McEwen BS, Chattarji S (2009) Stress, memory and the amygdala. Nat Rev Neurosci 10: 423-433.
- Lezak MD (1982) The problem of assessing executive functions. Int J Psychol 17: 281-297.
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, et al (2000) The unity and diversity of executive functions and their contributions to complex "frontal lobe" tasks: a latent variable analysis. Cogn Psychol 41: 49-100.
- Verdejo-García A, Pérez-García M (2007) Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology 190: 517-530.
- Beers SR, De Bellis MD (2002) Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. Am J Psychiatry 159: 483-486.
- DePrince AP, Weinzierl KM, Combs MD (2009) Executive function performance and trauma exposure in a community sample of children. Child Abuse Negl 33: 353-361.
- Rivera-Vélez GM, González-Viruet M, Martínez-Taboas A, Pérez-Mojica D (2014) Post-traumatic stress disorder, dissociation, and neuropsychological performance in latina victims of childhood sexual abuse. J Child Sex Abus 23: 55-73.
- Freeman JB, Beck JG (2000) Cognitive interference for trauma cues in sexually abused adolescent girls with posttraumatic stress disorder. J Clin Child Psychol 29: 245-256.
- Mezzacappa E, Kindlon D, Earls F (2001) Child abuse and performance task assessments of executive functions in boys. J Child Psychol Psychiatry 42: 1041-1048.
- Rizo-Martínez LE, Sanz-Martin A, Guevara MA, Hernández-González M, Inozemtseva O, et al. (2015) Eeg correlations during wcst performance in female adolescents with sexual abuse-related post-traumatic stress disorder. Behav Brain Res 5: 239-250.
- Navalta CP, Polcari A, Webster DM, Boghossian A, Teicher MH (2006) Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J Neuropsychiatry Clin Neurosci 18: 45-53.
- Wechsler D (2007) Escala wechsler de inteligencia para niños-iv. Manual Moderno, México.
- American_Psychatric_Association (2014) Manual diagnóstico y estadístico de los trastornos mentales dsm-5. Editorial Médica Panamericana, USA.
- Bustos P, Rincón P, Aedo J (2009) Validación preliminar de la escala infantil de síntomas del trastorno de estrés postraumático en niños/as y adolescentes víctimas de violencia sexual. PSYKHE 18: 113-126.
- Zolotor AJ, Runyan DK, Dunne MP, Jain D, Peturs HR, et al. (2009) Ispcan child abuse screening tool children's version (icast-c): instrument development and multi-national pilot testing. Child Abuse Negl 33: 833-841.
- Kovacs M (2004) Inventario de depresión infantil, TEA ediciones, España.
- Flores-Lázaro J, Ostrosky-Shejet F, Lozano-Gutiérrez A (2014) Banfe-2 batería neuropsicológica de funciones ejecutivas y lóbulos frontales, 2a Edición, El Manual Moderno, España.
- Verbruggen F, Logan GD, Stevens MA (2008) Stop-it: windows executable software for the stop-signal paradigm. Behav Res Methods 40: 479-483.
- Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000) Brief: behavior rating inventory of executive function. Psychological Assessment Resources, Lutz, FL.
- Flores-Lázaro J (2006) Neuropsicología de los lóbulos frontales. universidad juárez autónoma de tabasco, Villa Hermosa.
- Samuelson KW, Krueger CE, Burnett C, Wilson CK (2010) Neuropsychological functioning in children with posttraumatic stress disorder. Child Neuropsychol 16: 119-133.
- Barcelo F (2001) Does the wisconsin card sorting test measure prefontral function? Span J Psychol 4: 79-100.
- Demakis GJ (2003) A meta-analytic review of the sensitivity of the wisconsin card sorting test to frontal and lateralized frontal brain damage. Neuropsychology 17: 255-264.
- Heaton SK, Chelune GJ, Talley JL, Kay GG, Curtiss G (1993) Wisconsin card sorting test manual: revised and expanded. In. Psychological Assessment Resources, Odessa, FL.
- Mothes L, Haag Kristensen C, Grassi-Oliveira R, Fonseca RP, I.I. dLA, et al. (2015) Childhood maltreatment and executive functions in adolescents. Child and Adolescent Mental Health 20: 56-62.
- Logan GD, Schachar RJ, Tannock R (1997) Impulsivity and inhibitory control. Psychol Sci 8: 60-64.
- Nigg JT (2000) On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull 126: 220-246.
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, et al. (2010) Early-life stress is associated with impairment in cognitivecontrol in adolescence: an fmri study. Neuropsychologia 48: 3037-3044.
- Shallice T (1982) Specifics impairments of planning. philosophical transactions of the royal society of london, B 298: 199-209.
- Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7-15.
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, et al. (2006) Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J Am Acad Child Adolesc Psychiatry 45: 1059-1067.
- Hevia-Orozco J, Sanz-Martin A, Guevara MA, Hernández-Gonzalez M (2017) Eeg correlation during social decision-making in institutionalized adolescents. J Abnorm Psychol 3: 2-8.
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, et al. (2009) Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry 66: 206-213.
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, et al. (2010) Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci 30: 7466-7472.
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH (2009) Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry 65: 227-234.
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT (2010) altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (Tbss). Cereb Cortex 20: 561-569.
- Stuss DT, Alexander MP (2000) Executive functions and the frontal lobes: a conceptual view. Psychol Res 63: 289-298.
- Gourtzelidis P, Tzagarakis C, Lewis SM, Crowe DA, Auerbach E, et al. (2005) Mental maze solving: directional fmri tuning and population coding in the superior parietal lobule. Exp Brain Res 165: 273-282.
- Cummings J (1995) Anatomic and behavioral aspects of frontal-subcortical circuits. in structure and functions of the human prefrontal cortex, Grafman J, Holyoak KJ, and Boller F. Annals New York Academy of Sciences: 1-13.
- Tucker DM, Luu P, Pribram KH (1995) Social and emotional self-regulation. in structure and functions of the human prefrontal cortex. In: Grafman J, Holyoak KJ, and Boller F (eds) Annals of the New York Academy of Sciences: 213-240.
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, et al (1999) Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 156: 1787-1795.
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, et al (2005) Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med 35: 791-806.
- Putnam F, Trickett P (1997) Psychobiological effects of sexual abuse. a longitudinal study. Ann N Y Acad Sci 821: 150.
- Teicher MH, Tomoda A, Andersen S (2006) Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci 1071: 313-232.
- Shansky RM, Lipps J (2013) Stress-Induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci 7: 123.
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, et al. (2005) Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol 196: 199-203.
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH (2009) Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex 19: 2479-2484.
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, et al. (2012) Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71: 286-293.
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ (2000) The effects of early rearing environment on the development of gabaa and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22: 219-229.
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, et al. (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95: 5335-5340.
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, et al. (2004) For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 23: 483-499.
- Beauregard M, Levesque J, Bourgouin P (2001) Neural correlates of conscious self-regulation of emotion. J Neurosci 21: 165.
- Harenski CL, Hamann S (2006) Neural correlates of regulating negative emotions related to moral violations. Neuroimage 30: 313-324.
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL (2007) Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2: 303-312.
- Davidson RJ, Jackson DC, Kalin NH (2000) Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull 126: 890-909.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi