Review Article, Clin Oncol Case Rep Vol: 1 Issue: 1
Phytotherapy of Prostate Cancer: How far are we?
Guy-Armel Bounda*
Department of Clinical Pharmacy, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, 24# Tong Jia Xiang, Jiangsu Nanjing 210009, P.R. China
*Corresponding Author : Guy-Armel Bounda
Department of Clinical Pharmacy, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, 24# Tong Jia Xiang, Jiangsu Nanjing 210009, P.R. China
E-mail: Armelb80@hotmail.com
Received: March 29, 2018 Accepted: May 30, 2018 Published: June 06, 2018
Citation: Bounda G (2018) Phytotherapy of Prostate Cancer: How far are we?. Clin Oncol Case Rep 1:1.
Abstract
Prostate cancer (PCa) is the second most diagnosed cancer in men, principally affecting men over 50 years old, and is the fifth leading cause of cancer-related deaths in men worldwide. In recent years, the use of complementary and alternative medicine (CAM) has increased, especially among oncology patients. Treatment options are limited in androgen-independent prostate cancer. To reduce this tremendous health burden, new approaches have been directed toward extremes of the disease spectrum centering on strategies for prostate cancer prevention and for treating advanced androgen-independent cancers. Medicinal herbs and their derivative phytocompounds are being increasingly recognized as useful complementary treatments for cancer. Great number of clinical studies have reported the beneficial effects of herbal medicines on the survival, immune modulation, and quality of life (QOL) of cancer patients, when these herbal medicines are used in combination with conventional therapeutics. Although, tremendous efforts have been done in phytomedicine and phytotherapy, we still have a long way to go.
Keywords: Clinical trial; Herbal medicine; Prostate cancer; Phyototherapy
Introduction
Cancer, a cohort of diseases in which abnormal cells divide without control and are able to invade other tissues (through the blood and lymph systems), brings devastating consequences for the patients’ life and indeed is a leading cause of death worldwide [1]. Being a multifaceted issue; it is a therapeutically challenging disease and rapidly emerging pre-clinical and clinical studies have started to shed light on the molecular mechanisms, which underlie cancer development and progression [2]. Prostate cancer (PCa) is the second most diagnosed cancer in men, principally affecting men over 50 years old [3]. It is the fifth leading cause of cancer-related deaths in men worldwide [4]. Current screening techniques are based on the measurement of serum prostate specific antigen (PSA) levels and digital rectal examination.
The incidence of prostate cancer is high. Autopsy studies have demonstrated that 60-70% of older men have some area showing cancer within the prostate [5,6]. It is estimated that a 50-year-old man has a lifetime risk of 42% of developing prostate cancer, but only a 9.5% risk of developing the disease clinically and being diagnosed and a 2.9% risk of dying from prostate cancer [7]. The clinical behavior can vastly differ in different men with prostate cancer of similar staging, PSA levels, and histological appearance. The existing clinical biomarkers for prostate cancer (PCa) are not ideal, since they cannot specifically differentiate between those patients who should be treated immediately and those who should avoid overtreatment [8].
Prostate Cancer Diagnostic and Treatment
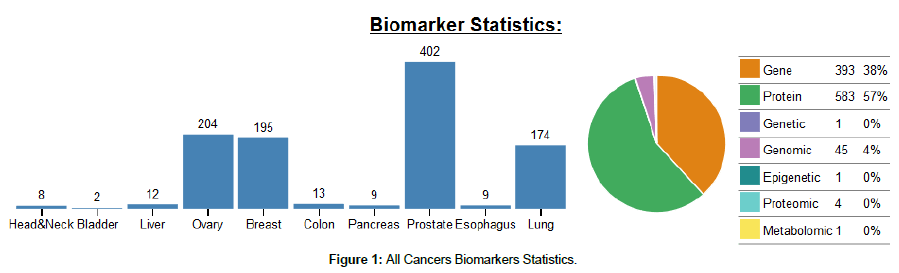
Over the past several decades, tremendous efforts have been made to screen and characterize useful cancer biomarkers for the use in clinical practice. According to the biomarkers statistics database from the Division of Cancer Prevention of the American National Cancer Institute, prostate cancer biomarkers represent the leading large group of investigated biomarkers by scientists, then followed by ovary, breast and lung cancers, respectively (Figure 1). This shows that among the various neoplasms that suffer men, prostate cancer remains the critical health issue. Several sources for the study of these potential novel biomarkers for PCa are being analyzed, each one with its advantages and disadvantages [9]. According to their clinical use, they currently fall into three major categories: (1) prognostic, (2) predictive, and (3) pharmacodynamics markers [10].
Conventional prostate cancer treatments include prostatectomy, radiotherapy, hormonotherapy, chemotherapy, and biological therapy. Side effects such as sexual function disorder, impotence, diabetes, and cardiovascular disease have been observed [11]. Currently used diagnostic standards consist of determination of prostate specific antigen (PSA), clinical stage, and total Gleason grade. Unfortunately, they do not give sufficient justification for choosing the optimal therapy for a particular patient [12]. Hence, it is necessary to search for new biomarkers to allow for the prediction of disease dynamics and personalization of therapy [13].
Prostate Cancer Phytotherapy
Statistically, in 25% of men worldwide with PCa that develop metastatic disease, the bones are the principal targets of PCa metastasis [14]. Given the fact that PCa is characterized by a long latency period, a strong dietary influence, and limited treatment strategies for the advanced disease; therefore, many patients turn to complementary and alternative medicine (CAM) with the belief that these medicines represent a viable therapeutic option that may be free of adverse side effects [15]. This folkloric belief is strongly upheld in many Asian cultures. Herbal remedies have been used for thousands of years with very minimal side effects and clearly merit extended research for their ability to selectively kill prostate cancer cells. Several herbal products have recently been incorporated into cancer research [16,17], among them is Nigella sativa whose seeds have been used medicinally for centuries in a variety of diseases [18].
There has long been a keen interest in herbal or alternative therapies for prostate cancer, particularly those that may not have the same side effects as androgen deprivation therapy. Numerous pharmacologic interventions have been developed in attempts to retard prostate tumor growth after the emergence of androgen-independent disease [19]. Several cytotoxic chemotherapeutics have shown substantial palliative benefits but little improvement delaying disease progression or mortality [20]. The inability of conventional approaches to reverse the progression of advanced disease coupled with a desire for therapies with fewer perceived toxicities has prompted patients and clinicians to consider unconventional or complementary alternatives [19]. Although there are often multiple cell-based and even animal models demonstrating efficacy of the CAM, the problematic remains the paucity of clinical trial data. The reliable source of the CAM, lot-to-lot variability, lack of reliable biomarkers, and a paucity of pharmacokinetics data to base decision regarding schedule or dose, are the various issues that make clinical trials more difficult to run until the end [21]. Complementary medicine has suffered for many years from lack of solid evidence in order to justify its use in clinical medicine. It appears that we have reached the phase that this claim is less and less valid, as more evidence-based products are allowed to be commercialized and used under the supervision of an authoritative professional (oncologist, palliative care specialist, pain specialist, and others) [1].
PC-SPES (FDA non-approved combination of eight different herbs), was commercially available in China since 1996. The complementary therapy garnered significant interest due to clinical studies reporting measurable responses in advanced prostate cancer [19]. A series of clinical studies were conducted to assess the effect and mechanism of PS-SPES activity [22,23]. Although the therapeutic application of PC-SPES seemed to be promising, unfortunately, PCSPES was recalled and withdrawn from the market because certain batches of testing PC-SPES samples were found to be contaminated with US Food and Drug Administration-(FDA) controlled prescription drugs [24].
The widespread use and possible success of PC-SPES led to a search for other ‘‘natural’’ or ‘‘herbal’’ remedies after it became unavailable [25]. Today, as many as one-third of prostate cancer patients use alternative medications [26]. In most cases, these therapies are not endorsed and often not even monitored by physicians, making it hard to discern their value. Therefore, it has been an urgent matter and a great interest to carry out clinical studies on phytotherapy of Prostate Cancer. Several clinical studies are currently in process and are shown in (Tables 1a-1c). While some of them have been completed, few have been withdrew or terminated due to various reasons. However, future more studies are in need to find the “Golden Phytotherapic Drug” for patient with prostate cancer.
| No | ClinicalTrials.gov Identifier | Phase | Patients | Allocation (Masking) |
Intervention/Treatment | Primary Purpose | Status | Country |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT01682941 | II | 40 | Randomized/Double | Soy Bread Soy-Almond Bread |
Treatment | C | USA |
| 2 | NCT03211104 | N/A | 107 | Randomized/Double | Curcumin Placebo |
Treatment | C | N/A |
| 3 | NCT00005828 | II | 54 | Single group/Open label | Green tea extract | Treatment | C | USA |
| 4 | NCT00731848 | II | 30 | Single group/Open label | Pomegranate liquid extract | Treatment | U | USA |
| 5 | NCT00719030 | N/A | 25 | Randomized/Open Label | Pomegranate pill Pomegranate pill placebo |
Prevention | C | USA |
| 6 | NCT00060086 | NA | 40 | Single group/Open label | Pomegranate juice | Treatment | AnR | USA |
| 7 | NCT00732043 | II | 200 | Randomized/Quadruple | Pomegranate extract Pomegranate juice Placebo |
Prevention | U | USA |
| 8 | NCT00685516 | NA | 113 | Randomized/Open Label | Green Tea Placebo Decaffeinated Black Tea |
Treatment | AnR | USA |
| 9 | NCT00765479 | III | 284 | Randomized (Open Label) | Soy protein isolate Placebo |
Treatment | C | USA |
| 10 | NCT00058266 | II | 36 | Randomized (Open Label) | Genistein Conventional Surgery |
Treatment | T | USA |
Table 1a: Clinical trials on Prostate Cancer Phytotherapy.
| No | ClinicalTrials.gov Identifier | Phase | Patients | Allocation (Masking) |
Intervention/Treatment | Primary Purpose | Status | Country |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT01912820 | I | 31 | Randomized/Double | Green tea extract Quercetin Conventional Surgery |
Prevention | AnR | USA |
| 2 | NCT00607932 | N/A | 66 | Randomized/Double | Brassica vegetable Indole-3-carbinol Questionnaire administration Adjuvant therapy |
Treatment | C | USA |
| 3 | NCT01340599 | II | 5 | Randomized/Double | Defined green tea catechin extract. Placebo. Questionnaire administration Conventional surgery. |
Treatment | T | USA |
| 4 | NCT00535977 | N/A | 22 | Non-randomized Open Label | Broccoli Peas |
Basic Science | C | UK |
| 5 | NCT01917890 | N/A | 40 | Randomized/Double | Curcumin Placebo |
Supportive Care | C | IRAN |
| 6 | NCT00617617 | II | 52 | Randomized/Triple | Prevastein HC® Placebo |
Prevention | C | USA |
| 7 | NCT02759380 | N/A | 240 | Randomized/Single group | Phytoestrogen-rich foods | Prevention | R | Sweden |
| 8 | NCT03087903 | N/A | 41 | Single group/Open label | Grape Seed Extract | Treatment | R | USA |
| 9 | NCT00200824 | II | NA | Randomized/Double | Soy Isoflavone Nutritional Supplements | Prevention | C | USA |
| 10 | NCT01083771 | N/A | 21 | Single group/Open label | Olive Oil | Prevention | C | USA |
| 11 | NCT03084913 | N/A | 30 | Randomized/Open Label | Plant- based, olive oil diet PCa Foundation diet |
Treatment | C | USA |
| 12 | NCT00669656 | II | 43 | Single group (Open label) | Prostate Health Cocktail | Treatment | C | USA |
| 13 | NCT02766478 | II | 24 | Randomized/Double | Genistein/Placebo | Prevention | R | USA |
| 14 | NCT01521949 | II | 21 | Single group (Open label) | Acai Juice Product | Treatment | C | USA |
| 15 | NCT01950143 | N/A | 78 | Randomized/Double | Standard broccoli soup Beneforte broccoli soup Beneforte extra broccoli soup |
Prevention | AnR | UK |
Table 1b: Clinical trials on Prostate Cancer Phytotherapy.
| No | ClinicalTrials.gov Identifier | Phase | Patients | Allocation/Masking | Intervention/Treatment | Primary Purpose | Status | Country |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT01912820 | I | 31 | Randomized/Double | Green tea extract Quecertin Placebo |
Prevention | AnR | USA |
| 2 | NCT01126879 | II | 36 | Randomized/Double | Genistein/Placebo | Treatment | AnR | USA |
| 3 | NCT01681823 | II | 60 | Single group/Open label | PectaSol-C Modified Citrus Pectin (MCP) | Treatment | R | ISRAEL |
| 4 | NCT00336934 | N/A | 183 | Randomized/Quadruple | Pomegranate juice Placebo |
Treatment | C | USA |
| 5 | NCT00651417 | II | 101 | Randomized/Quadruple | Organic Germanium Placebo |
Prevention | U | USA |
| 6 | NCT02144649 | N/A | 45 | Randomized (Open Label) | Tangerine tomato juice Red tomato juice Questionnaire administration |
Treatment | C | USA |
| 7 | NCT01126879 | II | 36 | Randomized/Double | Genistein Placebo Conventional Surgery |
Treatment | AnR | USA |
| 8 | NCT01009736 | II | 60 | Single group/Open label | Tomato-soy juice Conventional Surgery |
Treatment | C | USA |
| 9 | NCT01823549 | N/A | 120 | Cohort | Question administration | NA | AnR | USA |
| 10 | NCT01100866 | N/A | 1 | Randomized/Quadruple | POMELLA™ (pomegranate extract) Placebo |
Treatment | T | CANADA |
Table 1c: Clinical trials on Prostate Cancer Phytotherapy.
Conclusion
For centuries if not millennia, various plants have been used as medicines and disease therapeutics in most human cultures. Recently, scientists have showed great interest in herbal medicines as anti-tumor and chemoprevention agents. While continuous and systematic effort is needed, a number of notable “breakthroughs” have occurred in the field of medicinal plant research and botanical drugs in the last few years. In April 2008, a partially purified fraction of the water extract of green tea leaves from Camellia sinensis, named “Veregen”, was the very first FDA approved botanical drug, for topical treatment of external genital and perianal warts [27]. In January 2013, the FDA approved, for the first time, an oral botanical drug, “Crofelemer”, for treatment of diarrhea in HIV/AIDS patients [24]. Although these two pioneer FDA-approved botanical drugs are not therapies for cancer, they certainly pave way for such future developments [24]. “PHY906” (also known as KD018) is a four herbal-plant-composed TCM formulation, which has been conferred with good chemotherapy evidence [28]. Its ongoing FDA phase III clinical trial lead the way and will bring hope in the development of CAM for cancer patients. With the various other new clinical trials ongoing, CAM may start playing critical roles in future health care of aging populations [24].
Although considerable effort has been made in phytotherapy, there is a lot still to be done and we hope that several traditional remedies or multiple herb formulations will receive FDA approval as phytomedicines and botanical drugs for chemotherapy/ chemoprevention.
References
- Zaid H, Silbermann M, Amash A (2017) Medicinal Plants and Natural Active Compounds for Cancer Chemoprevention/Chemotherapy. Evid-Based Complementary Altern Med.
- World-Health-Organization (2015) Cancer: Fact Sheet No 297.
- Rigau M, Olivan M, Garcia M (2013) The present and future of prostate cancer urine biomarkers. Int J Mol Sci 14: 12620-12649.
- Ferlay J, Soerjomataram I, Dikshit R (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: 359-386.
- Rullis I, Shaeffer JA, Lilien OM (1975) Incidence of prostatic carcinoma in the elderly. Urology 6: 295–297.
- Sakr WA, Grignon DJ, Crissman JD (1994) High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: an autopsy study of 249 cases. In Vivo 8: 439–443.
- Scardino PT (1989) Early detection of prostate cancer. Urol Clin North Am 16: 635–655.
- Doll A, Clark J, Nelson C (2015) Biomarker and Translational Prostate Cancer Research. BioMed Res Int.
- Filella X, Foj L (2016) Prostate Cancer Detection and Prognosis: From Prostate Specific Antigen (PSA) to Exosomal Biomarkers. Int J Mol Sci 17: 1784.
- Mordente A, Meucci E, Martorana GE (2015) Cancer Biomarkers Discovery and Validation: State of the Art, Problems and Future Perspectives. Springer Science Business Media Dordrecht 9-26.
- Zhao YQ, Chen R, Wang Y (2017) In Vitro and In Vivo Efficacy Studies of Lavender angustifolia Essential Oil and Its Active Constituents on the Proliferation of Human Prostate Cancer. Integr Cancer Ther 16: 215–226.
- Gondek T, Szajewski M, Szefel J (2014) Evaluation of 12-Lipoxygenase (12-LOX) and Plasminogen Activator Inhibitor 1 (PAI-1) as Prognostic Markers in Prostate Cancer. BioMed Res Int
- Martin SK, Vaughan TB, Atkinson T (2012) Emerging biomarkers of prostate cancer (Review). Oncol Rep 28: 409–417.
- Lima AR, Bastos ML, Carvalho M (2016) Biomarker Discovery in Human Prostate Cancer: an Update in Metabolomics Studies. Translational Oncol 9: 357–370.
- Nelson PS, Montgomery B (2003) Unconventional therapy for prostate cancer: good, bad or questionable? Nat Rev Cancer 3: 845–858.
- Singh S, Khar A (2006) Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem 6: 259–270.
- Kuwajerwala N, Cifuentes E, Gautam S (2002) Resveratrol induces prostate cancer cell entry into s phase and inhibits DNA synthesis. Cancer Res 62: 2488–2492.
- Ali BH, Blunden G (2003) Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17: 299–305.
- Bonham M, Posakony J, Coleman I (2015) Characterization of Chemical Constituents in Scutellaria baicalensis with Antiandrogenic and Growth-Inhibitory Activities toward Prostate Carcinoma. Clin Cancer Res 11: 3905-3914.
- GilliganT, Kantoff PW (2002) Chemotherapy for prostate cancer. Urology 60: 94-100.
- Klempner SJ, Bubley G (2012) Complementary and Alternative Medicines in Prostate Cancer: From Bench to Bedside? Oncologist 17: 830–837.
- Pandha HS, Kirby RS (2002) PC-SPES: Phytotherapy for prostate cancer. Lancet 359: 2213–2215.
- Small EJ, Frohlich MW, Bok R (2000) Prospective Trial of the Herbal Supplement PC-SPES in Patients With Progressive Prostate Cancer. J Clin Oncol 18: 3595–3603
- Yin SY, Wei WC, Jian FY (2013) Therapeutic Applications of Herbal Medicines for Cancer Patients. Evid-Based Complementary Altern Med.
- Bhatt RS, Bubley GJ (2008) The Challenge of Herbal Therapies for Prostate Cancer. Clin Cancer Res 14: 7581-7582.
- Clement J, Bubley G (2008) Prostasol and venous thromboembolism. Urology 72: 664- 666.
- Wu KM, Ghantous H, Birnkrant DB (2008) Current regulatory toxicology perspectives on the development of herbal medicines to prescription drug products in the United States. Food Chem Toxicol 46: 2606–2610.
- Lam W, Bussom S, Guan F (2010) Chemotherapy: the four-herb Chinese medicine PHY906 reduces chemotherapy induced gastrointestinal toxicity. Science Translational Med 2.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi