Research Article, J Sleep Disor Treat Care Vol: 7 Issue: 2
Physiologic Remodeling of the Upper Airway: Pneumopedics
Dave Singh G*
President and Chief Medical Officer, Vivos BioTechnologies, USA
*Corresponding Author : Prof G. Dave Singh, DMD, PhD, DDSc
President and Chief Medical Officer, Vivos BioTechnologies, Inc. 12218 Hwy 14 N, Suite 1309, Cedar Crest, NM 87008, USA
Tel: +1 (971) 302-2234
Fax: +1 (866) 201-3869
E-mail: drsingh@vivoslife.com
Received: January 03, 2018 Accepted: May 24, 2018 Published: June 01, 2018
Citation: Singh GD (2018) Physiologic Remodeling of the Upper Airway: Pneumopedics. J Sleep Disor: Treat Care 7:2. doi: 10.4172/2325-9639.1000213
Keywords: Obstructive sleep apnea; Sleep disordered breathing; Pediatric sleep apnea; Rapid palatal expansion
Abbreviations
OSA: Obstructive Sleep apnea; AP: Antero- Posterior; SDB: Sleep Disordered Breathing; RPE: Rapid Palatal Expansion; CBCT: Cone-Beam Computed Tomography
Introduction
Adult and pediatric obstructive sleep apnea (OSA) are relatively common upper airway disorders, which consist of repetitive upper airway collapse during sleep. This perturbation of upper airway function is associated with oxygen desaturation and disruption of the normal sleep architecture. Although the patho-etiology of upper airway collapse during sleep is not fully understood, various predisposing factors including a smaller upper airway size [1], abnormal central respiratory control, and dysfunction of the upper airway/pharyngeal constrictors muscles have been postulated. In addition, other factors that may influence upper airway morphology include obesity, male gender [2], ages, and adenotonsillar hypertrophy, inter alia. Clinically, OSA is associated with excessive daytime sleepiness, systemic hypertension, myocardial infarction, congestive heart failure, stroke, diabetes mellitus, and road traffic accidents. Therefore, a thorough understanding of the upper airway’s structural and functional behavior is imperative in the successful treatment of both adult and pediatric OSA.
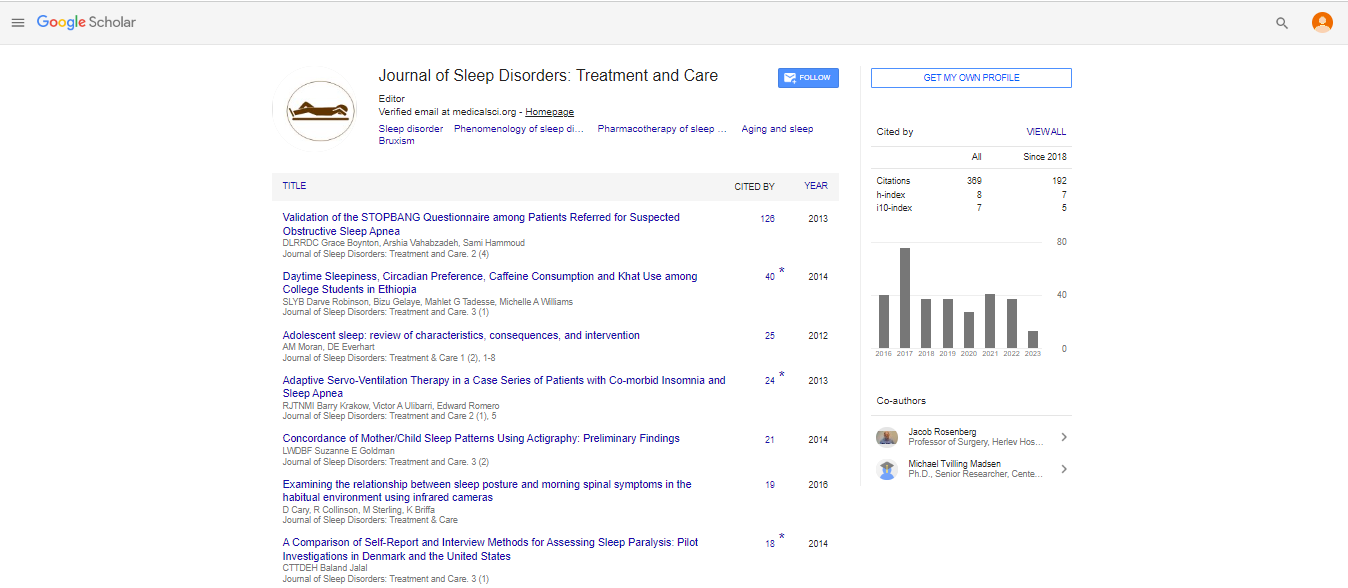
During normal growth and development, various changes in upper airway morphology have been described. For example, in a study investigating developmental changes of the pharyngeal airway structures, it was found that the upper pharyngeal depth increased with age, principally by vertical extension, and these developmental changes were greater in males than in females [4]. Gonçalves et al. [5] investigated the effects of age and gender on upper airway antero-posterior (AP) width during growth in Brazilian youths. They reported that while the airway width increased with age, the upper airway width was similar in both genders. As for the pattern of growth, the upper airway width exhibited: incremental growth between the ages of 6 to 18yrs; a growth plateau between the ages of 6 to 9yrs.; growth acceleration from 9 to 16 yrs., and then another plateau between the ages of 16 to 18yrs (Figure 1). Indeed, Zhang et al. [6] further investigated the relationship between AP upper airway morphology and craniofacial growth patterns in teenagers with normal occlusion. Although they also found no differences in the AP width of the upper airway between adolescent Chinese males and females, their analysis showed that upper airway morphology was associated with craniofacial growth patterns. Thus, the human upper airway appears to show some degree of heterogeneity according to phenotypic variation. In this regard, Tarkar et al. [7] evaluated the AP upper pharyngeal airway width in young Indian adults with different craniofacial growth patterns. Their results showed that subjects with a vertical skeletal pattern had narrower upper airways than those with a horizontal growth pattern. Consequently, in another study [8], the AP upper pharyngeal airway distance in young Indian adults with Class II malocclusion was compared with normal controls. It was reported that subjects with a Class II phenotype showed a narrower upper pharyngeal width when compared to the normo-divergent facial phenotype. This narrowing of the upper pharyngeal airway is considered to be a predisposing factor for mouth breathing, and likely increases the risk of sleep disordered breathing (SDB), since the retrognathic mandible in Class II cases might impinge on the functional space of the upper airway.
Figure 1: Upper airway growth between the ages of 6yrs to 60yrs. The plot shows incremental growth between 6yrs to 18yrs (7cm3-15cm3); a growth plateau between the ages of 6yrs to 9yrs. (7cm3-8cm3); growth acceleration from 9yrs to 16 yrs. (8cm3-15cm3); and then another plateau between the ages of 16yrs to 21yrs (15cm3) with a slow decline at around 30yrs ((13cm3). All values are approximate. After Schendel et al. [49] and Gonçalves et al. [5].
In another study, changes in the pediatric upper airway in the midsagittal plane were assessed during 1-11yrs of age [9]. It was found that the upper airway underwent developmental changes, measured along the clivus-menton axis, which demonstrated that the size of the nasopharyngeal airway increased with age. Indeed, this study suggested that the upper airway grows proportionally to the skeletal jaw structures, which ostensibly appears to support the concept of craniofacial pneumatization [10]. In terms of function, however, it appears that while children have smaller upper airways dimensionally during growth, functionally, children have a less collapsible upper airway during sleep compared to adults [11]. By contrasting the upper airway pressure-flow relationship during sleep in normal children and adults, it was found that the pediatric upper airway had a lesser tendency to collapse during sleep. These children exhibited a robust response to both a relative-negative (collapsing) airway pressure and hypercapnia, making the pediatric upper airway more resistant to nocturnal collapse. The corollary of this inference is that while pharyngeal airway responses present in normal children might compensate for a relatively smaller upper airway, children with a deficient or compromised upper airway may have an impaired ability to breathe efficiently while asleep, predisposing them to an increased risk of SDB. In this respect, it is worth noting the role of rapid palatal expansion (RPE). It is broadly accepted that widening of the nasal cavity occurs after RPE in children [12]. These widening permits a reduction in nasal airway resistance, presumably with a concomitant improvement of respiratory behavior; for example, Changes a mouth-breather into a nasal breather. In fact, there are some preliminary studies documenting nasal airway changes in adults also, using a novel protocol somewhat similar to RPE [13]. However, the effects of RPE on the upper airway further distally are less well documented. Therefore, other consequences of RPE apart from dentofacial characteristics need to be further addressed.
Antero-Posterior Changes of the Upper Airway
There are several variants of the generic protocol often referred to as RPE in the medical, dental and orthodontic literature. For example, Hiyama et al. [14] studied the effects of maxillary protraction, a modification of RPE, on craniofacial structures and upper airway dimensions in the AP plane. The sample consisted of Japanese children aged about 10yrs old with Class III malocclusions. The authors claimed to have increased maxillary growth in the AP plane, although this would be difficult to substantiate using lateral cephalometry alone [15], but consequent beneficial effects on the dimension of the superior upper airway were reported. In support of these findings, a similar study on Korean children of a comparable age with Class III malocclusions [16] also found improved nasopharyngeal airway measurements. The Korean study concluded that the nasopharyngeal airway could be enhanced, at least in the short term, with maxillary protraction in children. These results have been further substantiated recently in a study on slightly older Turkish children with Class III malocclusions [17]. Using variations of RPE (different, alternate rapid maxillary expansion protocols), an increase in maxillary growth allied with an increase in upper pharyngeal airway dimensions was found, even using different protocols. Therefore, it appears that a range of protocols that address midfacial development [18,19] appear to enhance the nasopharyngeal airway, at least in children, However, the efficacy of this approach in adults in not fully known.
In adults, an alternative option to positive airway pressure therapy is the use of mandibular advancement devices for the treatment of mild to moderate OSA. In fact, historically, various mandibular devices have also been used to treat children with Class II malocclusions exhibiting mandibular retrognathia [20]. More recently, the effects of several treatments of Class II malocclusion on the AP upper pharyngeal airway distance have also been investigated. In one study [21], three different Class II treatments (headgear, activator, and a bite-jumping appliance) were used to determine changes in AP upper airway width. All three modalities enabled small increases in pharyngeal width after treatment. Nevertheless, these modest enhancements in the pharyngeal airway might potentially decrease the risk of developing SDB in later life. Indeed, Ciavarella et al. [22] hypothesized that AP repositioning of the mandible might alter the position of the tongue and hyoid bone, leading to a remodeling of the upper airway space. The results of their study in children aged about 9yrs. showed an increase in the width of the superior pharyngeal airway space, and they concluded that an enhanced posterior airway space could consequently improve respiratory function. More specifically, Restrepo et al. [23] evaluated changes in airway dimensions in pre-pubertal children with a retrognathic mandible who received treatment with functional appliances (either a Klammt or a Bionator). After 1 yr., the only airway dimensions that increased were located in the adenoidal region of the nasopharynx. Bearing in mind the contribution of adenotonsillar hypertrophy to the increased risk of developing SDB [3], these protocols may be able to target the site or perhaps the severity of the upper airway obstruction. Consequently, in a similar study in Korean adolescents with Class II malocclusions [24], long-term changes in the pharyngeal airway dimensions after functional appliance treatment were assessed. Prior to treatment, it was found that the length of the upper pharyngeal region and the pharyngeal airway area were smaller in Class II adolescents when compared to matched controls. However, following treatment with a functional appliance, the Class II adolescents showed increased pharyngeal airway dimensions, which were similar to those of the control group. Thus, it appears that not only do functional appliances increase the pharyngeal airway dimensions in adolescents, but also that this effect appears to be maintained, at least until the completion of the pubertal growth spurt. Despite these advantages, one of the drawbacks of functional appliance therapy is its heavy reliance on excellent patient compliance. In the absence of ideal compliance, fixed appliances are often favored by the orthodontic profession. In one study [25], the pharyngeal airway effects of a skeletally-anchored appliance (using bilateral miniplates inserted onto the mandibular symphysis) were compared to a control group treated using a dentally-fixed (Herbst) appliance. The sample consisted of teenage patients with skeletal Class II malocclusion and mandibular retrusion. Post-treatment, there were significant changes in the maxillo-mandibular relationship, and the pharyngeal airway dimensions were increased in both groups. In addition, however, the oropharyngeal area also increased in both groups. Thus, skeletal changes produced by these fixed appliances were associated with both linear changes in the sagittal plane as well as an increased cross-sectional area of the pharyngeal airway. Therefore, it appears that a range of protocols that address mandibular development appear to enhance the pharyngeal airway in both children and adolescents. That these changes might be beneficial in the treatment and prevention of SDB in pediatric patients remains promising.
Medio-Lateral Changes of the Upper Airway
In terms of patho-etiology, an anatomically-narrow airway in the AP plane is considered to be a predisposing factor for obstruction of the upper respiratory tract. This deficiency appears to be amenable to maxillary protraction in children, which often results in increased AP width and area of the pharyngeal airway [26]. In addition, there appears to be a correlation between the upper airway and craniofacial morphology [27]. There is, however, a dearth of studies of the upper airway in the transverse dimension. One study [28] noted that the nasopharynx is transversely larger in prognathic subjects compared to retrognathic counterparts. Moreover, the interaction appears to be of a linear nature, as alluded to below. While it is not disputed that midfacial development appears to improve nasal volume, with current developments in 3D imaging technology and mathematical modeling [29], the deficiencies of lateral cephalometric analysis have become more apparent [30]. In another recent study [31], conebeam computed tomography (CBCT) was used assess changes in the upper airway after RPE in children aged about 10.5 yrs. old. As with previous 2D studies, an increase in width of the nasal floor was found. In addition, however, similar to an adult CBCT study [13] the nasal airway volumes were also increased. These are pertinent findings since a prior study that assessed the relationship between nasal cross-sectional area and nasal airway volume [32] determined that a linear relationship might exist between nasal airway area and volume, and that upper airway resistance is likely present in adults with a nasal airway surface area < 0.4 cm2. In other words, the nasal cross-sectional area might alter upper airway volume. Therefore, 3D changes in the upper airway further distally need to be considered.
Volumetric Changes of the Upper Airway
The notion that RPE in children can enhance nasal cavity volume and decrease upper airway resistance is becoming more widely accepted. For example, when acoustic rhinometry was used to evaluate nasal cavity volume, it was found that the palatal area and volume increased [33]. In this respect, a study using CBCT scans [34] evaluated skeletal, oropharyngeal airway, and nasal airway volume changes after RPE in teenagers. As might be expected in growing adolescents, transverse skeletal parameters (medial orbital width, lateral nasal width, maxillary width, and mandibular width) were enhanced. However, while there was an increase in nasopharyngeal airway volume for the RPE group, there were no significant changes in the oropharyngeal airway volume. Therefore, specific sites within the craniofacial complex might exhibit heterogeneity during allometric changes associated with various treatment modalities. For example, in another CBCT study in 11 yr. olds [35], favorable changes in the retroglossal region and in the oropharyngeal airway volume were reported in the correction of Class II malocclusion, using a mandibular device. But, since the nasal cross-sectional area also often increases, newer morphometric and functional analyses are needed to elucidate the effects of upper airway growth and remodeling, and their impact on the quality of sleep and breathing. One such study [36] correlated morphologic (CBCT) changes of the upper airway with functional sleep data obtained by polysomnography after RPE in 8yr. old children. Not surprisingly perhaps, RPE was associated with both an increase in total airway volume as well as respiratory performance in pediatric cases. Therefore, it appears that in actively growing patients, various maxillomandibular protocols are advantageous in the treatment of SDB.
On the other hand, the adult upper airway is less well understood in terms of its developmental and structural dynamics. Sutherland et al. [37] investigated changes in adult upper airway size and regional fat with weight loss in men with OSA. It was reported that, in addition to a reduction in weight, the AHI also fell. In contrast, the velopharyngeal airway volume increased while craniofacial and parapharyngeal fat volumes decreased. The authors concluded that while weight loss increases velopharyngeal airway volume in men, changes in upper airway length appear to have a greater influence on the reduction in the AHI. Therefore, changes in upper airway architecture might be a possible mechanism for addressing sleep issues in adults [38] (Figure 2). Indeed, anatomic components predisposing to an increased risk of OSA may be the result of interaction between craniofacial skeletal tissues [39], soft tissues, dental tissues [40] and functional spaces [2]. Thus, the pathogenesis of OSA is complex and may vary, in fact, according to ethnicity [41] inter alia. In a study of Japanese-Brazilians and Caucasians, it was found that the Japanese-Brazilians had smaller craniofacial skeletal dimensions (cranial base, maxillary, and mandibular lengths), whereas the Caucasians had larger craniofacial soft tissues (tongue length and volume). These recent findings corroborate the work of Singh et al. [42-44] who reported on the craniofacial heterogeneity of Asian and Caucasian children, and the implication of cranial base morphology on upper airway behavior in terms of SDB [45]. Therefore, a deeper understanding of development dynamics of upper airway remodeling is warranted.
Figure 2: Size- and shape-changes in the 3D morphology of the upper airway. The 3D models show size- and shape-changes in the morphology of the upper airway of a 60yr. old patient who underwent biomimetic oral appliance therapy for the treatment of mild obstructive sleep apnea. The red model on the left is the pre-treatment upper airway; the blue model on the right shows an increased volume upper airway the post-treatment.
Mechanisms of Physiologic Airway Remodeling
The medical literature reveals numerous studies on upper airway remodeling in pathologic conditions such as asthma, chronic obstructive pulmonary disease, emphysema, etc. In contrast, there is a dearth of studies that have documented physiologic airway remodeling. However, in one study [46], it was suggested that after RPE, improvements in nasal breathing and spontaneous forward repositioning of the mandible might occur. These maxillo- mandibular effects might enhance the upper airway volume with a concomitant repositioning of the tongue after treatment. Surprisingly however, in that study on children age about 7.5 yrs. no differences in oropharyngeal airway and mandibular displacement after RPE were detected. The authors suggested that improvement in upper airway and breathing after RPE might instead be related to nasopharyngeal and nasal airway changes, as noted above. In contrast, a recent case study in an adult diagnosed with OSA found that spontaneous forward repositioning of the mandible occurred after midfacial redevelopment, and that the AHI returned to within normal limits even with no device in the mouth while sleeping [47]. Therefore, various maxillo-mandibular protocols in both pediatric and adult cases of SDB might need to more precisely selected to target the suspected site and severity of the upper airway obstruction, once the craniofacial phenotype has been evaluated.
While it has been argued that the adult craniofacial is not amenable to (orthodontic) growth modification techniques, it is wellknown that the facial skeleton under goes changes with age. Indeed, in an early study, Harris et al. [48] reported that dentofacial dimensions continue to change throughout adulthood. Thus, evidence is also emerging that the adult upper airway may also be subject to the ageing process. For example, Schendel et al. [49] assessed changes in the upper airway during growth and development using 3D CBCT data. They reported that the upper airway size and length increase until age 20yrs. then plateau similar to findings reported previously [5] . With further ageing, however, the upper airway appears to decrease in size slowly, and then more rapidly after age 40yrs. These data suggest that there may be an inherent, physiologic mechanism for upper airway remodeling. In fact, a cephalometric study investigated morphological changes in the pharynx in adults with age [50]. It was found that while the AP depth of the nasopharynx increased, the depth of the oropharyngeal airway decreased, and the soft palate became longer and thicker with age. These findings suggest that pharyngeal morphology is not immutable, and although the structure of the upper airway develops during childhood and adolescence, its form changes throughout adult life. It can, therefore, be suggested that these age-related upper airway changes might represent a form of physiologic upper airway remodeling, and it might be further ventured that the development mechanisms underlying these changes could be harnessed for clinical purposes in the context of SDB (Figure 2). For example, in a CBCT study in Japanese adults, the influence of aging on the oropharynx was investigated [51]. In men, the airway lengthened with age and, more specifically, the lower oropharynx soft tissue volume increased with age. These changes might lead to a more collapsible upper airway in males, which could subsequently contribute to the development of OSA. On the other hand, retardation of the ageing process might prevent the early onset of OSA or provide an alternative option in terms of treatment.
While some literature suggests that a potential relationship exists between RPE and upper nasal airway changes [52,53], other studies do not provide precise corroboration of the nature of this relationship [54]. Physiologic upper airway remodeling, in this context, refers to the development of specific but potentially reversible structural changes in the tissues of the upper airway, which lead to clinically-identifiable, morphologic changes [38,55,56] (Figure 2). In chronic rhinosinusitis, however, a recent study showed that remodeling appears to occur in parallel rather than subsequent to inflammation. Furthermore, several factors are thought to modulate phases of pathologic upper airway remodeling, including coagulation factors, cytokines, growth factors, and proteases [57]. In fact, both inflammatory and remodeling factors have been found in chronic rhinosinusitis, including TGF-β1 and its receptors, as well as TNF-α and IL-1 β inter alia. These findings suggest that these cytokines play a central role in the remodeling processes associated with inflammatory rhino-sinusitis [58]. Thus, research on human nasal epithelial stem cells might shed further light on the patho-etiology of upper airway diseases, since this remodeling includes migration, proliferation, and differentiation of epithelial cells [59]. However, the interactions between epithelial and mesenchymal cells is not yet fully understood, and the contribution of epithelial remodeling in physiologic upper airway remodeling remains to be elucidated. For example, Polotsky et al. [60] studied the effects of leptin and obesity on upper airway function in mice. Obese, leptin-deficient mice showed dulled ventilatory control, which could worsen neuromechanical upper airway function, and predispose to increased airway collapsibility during sleep. However, this mechanism might not explain structural changes that might be perceived as upper airway remodeling. On the other hand, since age, obesity and male sex are risk factors for the development of SDB, Skelly et al. [61] investigated both structure and function of the sternohyoid muscle in male rats. They reached the conclusion that, during ageing, the sternohyoid muscle shows indefinite growth, and is protected from oxidative stress in rats. Presumably, this type of growth potential, in conjunction with epithelial remodeling, could conceivably account for clinically-identifiable, morphologic changes of the human upper airway also. Indeed, it is thought by some [62,63] that SDB is a generalized abnormality, that may involve the upper airway from the nasopharynx to the larynx. Thus, techniques that address both abnormal pharyngeal structure and function, perhaps deploying physiologic airway remodeling, may be preferential in the treatment of SDB [64].
References
- Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T (2002) Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med 165: 260-265.
- Singh GD, Olmos S (2007) Use of a sibilant phoneme registration protocol to prevent upper airway collapse in patients with TMD. Sleep Breath 11: 209-216.
- Jordan AS, McSharry DG, Malhotra A (2014) Adult obstructive sleep apnoea. Lancet 383: 736-747.
- Tsai HH (2007) Developmental changes of pharyngeal airway structures from young to adult persons. J Clin Pediatr Dent 31: 219-221.
- Gonçalves Rde C, Raveli DB, Pinto Ados S (2011) Effects of age and gender on upper airway, lower airway and upper lip growth. Braz Oral Res 25: 241-247.
- Zhang QF, Jin L, Yao H, Li ZY, Huang X, et al. (2016) Relationship between upper airway and cranial-facial growth patterns in adolescents with normal occlusion. Shanghai Kou Qiang Yi Xue 25: 702-706.
- Tarkar JS, Parashar S, Gupta G, Bhardwaj P, Maurya RK, et al. (2016) An evaluation of upper and lower pharyngeal airway width, tongue posture and hyoid bone position in subjects with different growth patterns. J Clin Diagn Res 10: 79-83.
- Mani P, Muthukumar K, Krishnan P, Senthil Kumar KP (2015) Upper and lower pharyngeal airway space in West-Tamil Nadu population. J Pharm Bioallied Sci 7: S539-542.
- Arens R, McDonough JM, Corbin AM, Hernandez ME, Maislin G, et al. (2002) Linear dimensions of the upper airway structure during development: assessment by magnetic resonance imaging. Am J Respir Crit Care Med 165: 117-122.
- Belforte G, Sacco M (1970) Mutual relationships between the development of dental alveolar arches and the pneumatisation of the paranasal sinuses. Minerva Stomatol 19: 442-446.
- Marcus CL, Fernandes Do Prado LB, Lutz J, Katz ES, Black CA, et al. (2004) Developmental changes in upper airway dynamics. J Appl Physiol 97: 98-108.
- McNamara JA Jr, Lione R, Franchi L, Angelieri F, Cevidanes LH, et al. (2015) The role of rapid maxillary expansion in the promotion of oral and general health. Prog Orthod 16: 33.
- Singh GD, Heit T, Preble D, Chandrashekhar R (2016) Changes in 3D nasal cavity volume after biomimetic oral appliance therapy in adults. Cranio 34: 6-12.
- Hiyama S, Suda N, Ishii-Suzuki M, Tsuiki S, Ogawa M, et al. (2002) Effects of maxillary protraction on craniofacial structures and upper-airway dimension. Angle Orthod 72: 43-47.
- Moyers RE, Bookstein FL (1979) The inappropriateness of conventional cephalometrics. Am J Orthod 75: 599-617.
- Lee JW, Park KH, Kim SH, Park YG, Kim SJ (2011) Correlation between skeletal changes by maxillary protraction and upper airway dimensions. Angle Orthod 81: 426-432.
- Celikoglu M, Buyukcavus MH (2017) Changes in pharyngeal airway dimensions and hyoid bone position after maxillary protraction with different alternate rapid maxillary expansion and construction protocols: A prospective clinical study. Angle Orthod.
- Singh GD, Rozihan MH, Nidzam MTM, Shamim AK, Samsudin AR, et al. (2007) 3-D reconstruction of nasopharyngeal airways in Malaysian subjects. Springer, Berlin, Heidelberg, Germany.
- Kuroedova VD, Cikor TA, Makarova AN, Kim AA (2016) Orthodontic treatment effect of on the condition of the upper airways. Wiad Lek 69: 734-736.
- Singh GD, Clark WJ (2001) Localization of mandibular changes in patients with Class II division 1 malocclusions treated using Twin Block appliances: finite-element modeling. Am J Orthod Dentofacial Orthop 119: 419-425.
- Godt A, Koos B, Hagen H, Göz G (2011) Changes in upper airway width associated with Class II treatments (headgear vs activator) and different growth patterns. Angle Orthod 81: 440-446.
- Ciavarella D, Lo Russo L, Mastrovincenzo M, Padalino S, Montaruli G, et al. (2014) Cephalometric evaluation of tongue position and airway remodelling in children treated with swallowing occlusal contact intercept appliance (S.O.C.I.A.). Int J Pediatr Otorhinolaryngol 78: 1857-1860.
- Restrepo C, Santamaría A, Peláez S, Tapias A (2011) Oropharyngeal airway dimensions after treatment with functional appliances in class II retrognathic children. J Oral Rehabil 38: 588-594.
- Han S, Choi YJ, Chung CJ, Kim JY, Kim KH (2014) Long-term pharyngeal airway changes after bionator treatment in adolescents with skeletal Class II malocclusions. Korean J Orthod 44:13-19.
- Celikoglu M, Buyuk SK, Ekizer A, Unal T (2016) Pharyngeal airway effects of Herbst and skeletal anchored Forsus FRD EZ appliances. Int J Pediatr Otorhinolaryngol 90: 23-28.
- Oktay H, Ulukaya E (2008) Maxillary protraction appliance effect on the size of the upper airway passage. Angle Orthod 78: 209-214.
- Guilleminault C, Stoohs R (1990) Chronic snoring and obstructive sleep apnea syndrome in children. Lung 168 Suppl: 912-919.
- Stellzig-Eisenhauer A, Meyer-Marcotty P (2010) Interaction between otorhinolaryngology and orthodontics: correlation between the nasopharyngeal airway and the craniofacial complex. GMS Curr Top Otorhinolaryngol Head Neck Surg 9: Doc04.
- Singh GD (2008) Digital diagnostics: Three-dimensional modelling. Br J Oral Maxillofac Surg., 46: 22-26.
- Bookstein FL (2016) Reconsidering "The inappropriateness of conventional cephalometrics". Am J Orthod Dentofacial Orthop 149: 784-797.
- Izuka EN, Feres MF, Pignatari SS (2015) Immediate impact of rapid maxillary expansion on upper airway dimensions and on the quality of life of mouth breathers. Dental Press J Orthod 20: 43-49.
- Hinton VA, Warren DW, Hairfield WM, Seaton D (1987) The relationship between nasal cross-sectional area and nasal air volume in normal and nasally impaired adults. Am J Orthod Dentofacial Orthop 92: 294-298.
- De Felippe NL, Bhushan N, Da Silveira AC, Viana G, Smith B (2009) Long-term effects of orthodontic therapy on the maxillary dental arch and nasal cavity. Am J Orthod Dentofacial Orthop 136: 490.
- El H, Palomo JM (2014) Three-dimensional evaluation of upper airway following rapid maxillary expansion: a CBCT study. Angle Orthod 84: 265-273.
- Erbas B, Kocadereli I (2014) Upper airway changes after Xbow appliance therapy evaluated with cone beam computed tomography. Angle Orthod 84: 693-700.
- Fastuca R, Meneghel M, Zecca PA, Mangano F, Antonello M, et al. (2015) Multimodal airway evaluation in growing patients after rapid maxillary expansion. Eur J Paediatr Dent 16: 129-134.
- Sutherland K, Lee RW, Phillips CL, Dungan G, Yee BJ, et al. (2011) Effect of weight loss on upper airway size and facial fat in men with obstructive sleep apnoea. Thorax 66: 797-803.
- Singh GD, Wendling S, Chandrashekhar R (2011) Midfacial development in adult obstructive sleep apnea. Dent Today 30: 124-127.
- Caprioglio A, Zucconi M, Calori G, Troiani V (1999) Habitual snoring, OSA and craniofacial modification. Orthodontic clinical and diagnostic aspects in a case control study. Minerva Stomatol 48:125-137.
- Guilleminault C, Abad VC, Chiu HY, Peters B, Quo S (2016) Missing teeth and pediatric obstructive sleep apnea. Sleep Breath 20: 561-568.
- Schorr F, Kayamori F, Hirata RP, Danzi-Soares NJ, Gebrim EM, et al. (2016) Different Craniofacial Characteristics Predict Upper Airway Collapsibility in Japanese-Brazilian and White Men. Chest 149: 737-746.
- Singh GD, McNamara JA Jr, Lozanoff S (1998) Craniofacial heterogeneity of prepubertal Korean and European-American subjects with Class III malocclusions: Procrustes, EDMA and Cephalometric Analyses. Int J Adult Orthod Orthog Surg 13: 227-240.
- Singh GD, McNamara JA Jr, Lozanoff S (1999) Allometry of the cranial base in prepubertal Korean subjects with Class III Malocclusions. Finite-Element Morphometry. Angle Orthod 69: 507-514.
- Singh GD, McNamara JA Jr, Lozanoff S (1999) Finite element morphometry of soft tissues in prepubertal Korean and European-Americans with Class III malocclusions. Arch Oral Biol 44: 429-436.
- Banabilh SM, Suzina AH, Dinsuhaimi S, Singh GD (2007) Cranial base and airway morphology in adult Malays with obstructive sleep apnoea. Aust Orthod J 23: 89-95.
- Fastuca R, Zecca PA, Caprioglio A (2014) Role of mandibular displacement and airway size in improving breathing after rapid maxillary expansion. Prog Orthod 15: 40.
- Heit T, Sebastian J, Singh GD (2016) A novel combined protocol for the resolution of severe obstructive sleep apnea. J Sleep Disord Ther 5: 251-254.
- Harris JE, Kowalski CJ, LeVasseur FA, Nasjleti CE, Walker GF (1977) Age and race as factors in craniofacial growth and development. J Dent Res 56: 266-274.
- Schendel SA, Jacobson R, Khalessi S (2012) Airway growth and development: a computerized 3-dimensional analysis. J Oral Maxillofac Surg 70: 2174-2183.
- Johnston CD, Richardson A (1999) Cephalometric changes in adult pharyngeal morphology. Eur J Orthod 21: 357-362.
- Shigeta Y, Ogawa T, Venturin J, Nguyen M, Clark GT, et al. (2008) Gender- and age-based differences in computerized tomographic measurements of the orophaynx. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106: 563-570.
- Gray LP (1975) Results of 310 cases of rapid maxillary expansion selected for medical reasons. J Laryngol Otol 89: 601-614.
- Ortu E, Giannoni M, Ortu M, Gatto R, Monaco A (2014) Oropharyngeal airway changes after rapid maxillary expansion: the state of the art. Int J Clin Exp Med 7: 1632-1638.
- Warren DW, Hershey HG, Turvey TA, Hinton VA, Hairfield WM (1987) The nasal airway following maxillary expansion. Am J Orthod Dentofacial Orthop 91: 111-116.
- Alsufyani NA, Al-Saleh MA, Major PW (2013) CBCT assessment of upper airway changes and treatment outcomes of obstructive sleep apnoea: a systematic review. Sleep Breath 17: 911-923.
- Morgan TD (2016) Novel approaches to the management of sleep-disordered breathing. Sleep Med Clin 11: 173-187.
- Watelet JB, Dogne JM, Mullier F (2015) Remodeling and Repair in Rhinosinusitis. Curr Allergy Asthma Rep 15: 34.
- Van Bruaene N, Van Crombruggen K, De Ruyck N, Holtappels G, Van Cauwenberge P, et al. (2012) Inflammation and remodelling patterns in early stage chronic rhinosinusitis. Clin Exp Allergy 42: 883-890.
- Yu F, Zhao X, Li C, Li Y, Yan Y, et al. (2012) Airway stem cells: review of potential impact on understanding of upper airway diseases. Laryngoscope 122: 1463-1469.
- Polotsky M, Elsayed-Ahmed AS, Pichard L, Richardson RA, Smith PL, et al. (2012) Effects of leptin and obesity on the upper airway function. J Appl Physiol 112: 1637-1643.
- Skelly JR, O'Connell RA, Jones JF, O'Halloran KD (2011) Structural and functional properties of an upper airway dilator muscle in aged obese male rats. Respiration 82: 539-549.
- Hoffstein V (1996) How and why should we stabilize the upper airway? Sleep 19: S57-60.
- Marcus CL, Fernandes Do Prado LB, Lutz J, Katz ES, Black CA, et al. (2004) Developmental changes in upper airway dynamics. J Appl Physiol 97: 98-108.
- Singh GD, Kraver M, Chernyshev O (2018) Restoration of sleep using a novel biomimetic protocol for adult OSA: Clinical case report. Cranio (in press).
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi