Review Article, J Pulm Med Vol: 5 Issue: 7
Pattern Recognition, Toll-Like Receptors: Mechanisms and Implications in Lung Injury and Pulmonary Medicine
Shakir Ali1*, Syed Mansoor Ali2
1Department of Biochemistry, School of Chemical and Life Sciences, Jamia Hamdard, Delhi, India
2Department of Biotechnology, Jamia Millia Islamia, New Delhi, India
- *Corresponding Author:
- Shakir Ali
Department of Biochemistry,
School of Chemical and Life Sciences,
Jamia Hamdard,
Delhi;
Tel: 7011183625;
E-mail: sali@jamiahamdard.ac.in
Received date: August, 23, 2021; Accepted date: November 17, 2021; Published date: November 26, 2021
Citation: Ali S, Ali SM (2021) Pattern Recognition, Toll-Like Receptors: Mechanisms and Implications in Lung Injury and Pulmonary Medicine. J Pulm Med 5: 7.
Abstract
Toll-like receptor (TLR) is a clan of receptor proteins that belong to a group of receptors called pattern recognition receptors or PRRs. These receptors are present in a variety of immune and non-immune cells and help the cell recognize unique Pathogen-associated molecular patterns (PAMPs), such as the bacterial lipopolysaccharide, viral nucleic acid etc., and Damage-associated molecular patterns (DAMPS) from dying or injured host cells. TLRs respond to these pathogenic stimuli (PAMPS/DAMPs) and relay signal into the interior of the cell with the help of adaptor proteins (MyD88, TRIF etc.) and generate an initial immune response against the invading pathogen or a stimulus.
Keywords: Pulmonary injury; tissue repair; receptors; adaptorproteins; interstitial diseases; chemokine’s; cytokines
Introduction
Lungs, being at the interface of the interior surface of the human body and atmosphere, are directly and continuously exposed to a plethora of substances and stimuli which include dust, chemicals, pollens and other allergens including a number of microorganisms and their spores, viruses etc. and are equipped with a sophisticated protective mechanism. The pulmonary host defense against various environmental intimidations include soluble mediators (lysozyme, secretory IgA, antimicrobial peptides and surfactant proteins), besides its unique anatomy, the alveolar macrophages and dendritic cells for a continuous observance of pathogenic machineries, as well as inhibit T cell response against non-pathogenic antigens [1]. A number of components which include the complement, a system of plasma proteins [2], lactoferrin [3], defensing, a family of cationic peptides [4], and soluble proteins collections (Collagen-containing C-type lectins) can recognize an invading pathogen or substance with the help of specific groups or molecules such as the oligosaccharides or lipids on the microorganism surface, triggering a collection of host defense responses against injury. Collectins, for example, provoke the host innate immune response against microorganism aggregation, complement activation, opsonization, activation of phagocytosis, or inhibition of microbial growth, as well as modulate the inflammatory and allergic response, adaptive immunity and clearance of apoptotic cells [5]. Briefly, these molecules or receptor proteins upon activation lead to the synthesis and secretion of specific substances or proinflammatory molecules which coordinate to provide a protective response against pathogen; even though, in doing so, sometime an aberrant activation of the system may lead to adverse consequences including immunodeficiency, septic shock, or induction of autoimmunity. The recent SARS-Cov2 infection (COVID-19) is an example of such an exaggerated response [6]. In this article, we shall be reviewing the recent literature on the role of Toll-like receptors (TLR) in pulmonary injury and their potential as candidate proteins for designing and development of pulmonary medicine.
Toll-like receptors
Cells express a variety of unique conserved receptor proteins called the Pathogen or pattern recognition receptors (PRRs) which sense and detect conserved products of microbial, as well as nonmicrobial, origin and elicit host immune response [7, 8]. These receptors are expressed mainly by the cells of the innate immune system, as well as other cell types, and can be found on the cell surface, the subcellular compartments (endosomal membrane), in cytosol, and also extracellularly in blood stream and interstitial fluid [9]. In the lung, the lung epithelial, myeloid and lymphoid cells, for example, carry these receptors.
The PRR family includes four distinct types of receptors, namely, the carbohydrate binding proteins called the C-type lectin receptors (CLRs), RNA helicase retinoic acid-inducible gene I (RIG-I) like receptors (RLRs), NOD (nucleotide binding oligomerization domain)- like receptors (NLRs), and Toll-like receptors (TLRs) [10, 11]. Their expression and activation ultimately culminate in the expression of a variety of pro-inflammatory substances providing an early response to pathogen attack and shaping the adaptive immunity. In this system, TLRs are particularly important for their ability to recognize unique microbial patterns, commonly called as Pathogen-associated molecular patterns (PAMPs) [12, 13]. LPS, lipoteichoic acid, peptidoglycan and flagellin are prominent PAMPs in bacteria. In viruses, dsRNA and ssRNA act as PAMP [7, 14, 15]. The receptors which identify PAMPs called PRRs are present on both immune cells (monocytes, dendritic cells, macrophages, neutrophils etc) and nonimmune cells (endothelial cells, epithelial cells etc), as well as in intracellular compartments (lysosomes). The distribution of PRR ensure detection of pathogen or an antigen at both extracellular and intracellular level.
Toll like receptors or TLRs constitute a crucial component of the innate immune system. Toll is a gene, initially identified in fruit fly[16]. Toll-like receptors have been reported in human to recognize not only the PAMPs, but also Damage-associated molecular patterns (DAMPS) from dying or injured cells[16]. TLR is a Type 1 integral membrane protein, having an N-terminal (ligand recognition) ectodomain of a tandem of a Leucine-rich repeat (LRR) consensus sequence, typically 22-29 amino acids containing hydrophobic residues spaced at distinctive intervals [17] and a C-terminal signalling domain called the Toll/Interleukin-1 receptor or TIR domain, named for its homology with the IL-1R signalling domain containing three highly homologous regions or boxes called Box 1, Box 2 and Box 3. IL-1R and TLR have a similar cytoplasmic TIR domain and similar downstream proteins[18, 19].
TLRs are glycosylated conserved proteins. More than 10 functional TLRs have been reported in human. TLR1-6, and TLR10 are expressed as cell surface receptors, but TLR3, 7, 8 and 9 are present on the intracellular vesicles[21]. TLR11, 12, 13, like TLR3, 7, 8, 9, are intracellular TLRs, but not found in human. TLR10 is non-functional in mouse [14]. The endolysosomal or intracellular TLRs (TLR3, 7, 8, 9, 11, 12 and 13), which are expressed on ER, endosome and lysosome, recognize either nucleic acids (TLR3, 7, 8, 9, 13) or microbial components (TLR11, 12) [22]. Localisation of the nucleic acid sensor TLRs (TLR3, 7, 8 and 9) is important to prevent an inappropriate immune response as “Self-DNA” is rarely present in endolysosomal compartments, where the microorganisms are degraded. DAMPs, on the other hand, are endogenous substances (danger molecules) released from damaged and dying cells and which bind to TLR. DAMPs include nuclear protein HMGB1 (High-mobility group box 1), S100 and heat and cold shock proteins, purines and peroxiredoxins[23, 24]. Besides this, urate crystals, hyaluronan and fragments of extracellular matrix functions in a manner to microbial PAMPs and act as DAMPs [25, 28].
TLR Signaling
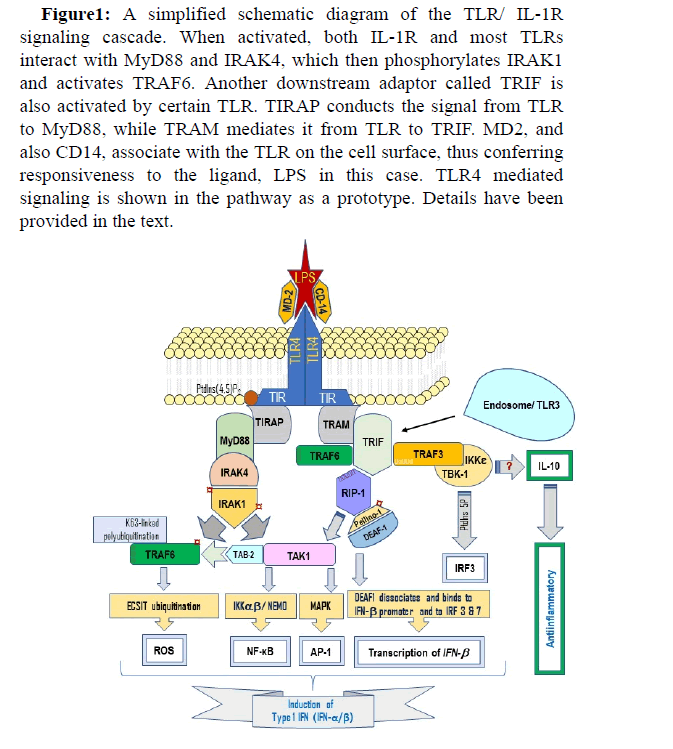
A controlled and short-term TLR cascade in response to external (PAMPs) or internal (DAMPs) stimuli provides an immediate defence, an innate immune response that guides the adaptive immune system to initiate antigen-specific response against the stimuli, and to repair tissue damage. Downstream TLR signalling upon activation promotes the expression of chemokines, cytokines and other chemical mediators and recruit other immune cells. It uses a set of adaptor proteins called TIR domain-containing adaptors, which include Myeloid differentiation primary response 88 (MyD88), MyD88 adaptor-like (MAL)/TIRAP (TIR domain-containing adaptor protein), TRIF (TIR domain- [37]containing adaptor inducing IFN-β, also called TICAM-1), TRIF-related adaptor molecule (TRAM, also called TICAM-2), SARM (Sterile- and Armadillo motif protein), BCAP (PIK3AP1) and SCIMP, a transmembrane non-TIR adaptor [19, 29-36]. Of these, MyD88 is utilized by almost all TLRs, perhaps except TLR3 [37], inducing the expression of proinflammatory chemokines and cytokines via NF-κB and MAPKs pathway, as well as the generation of the reactive oxygen species by the mitochondria and the cell, and leukocyte migration to the site of infection or injury [38]. TIRAP act as a sorting adaptor which recruits MyD88 to cell surface TLRs, as well as participates in signalling through endosomal TLRs [32]. TIRAP is characterized by a lipid-biding domain which binds to phosphatidylinositol 4,5-bisphosphate (PtdIns (4,5)P2) at the plasma membrane, or to PI(3)P on endosomes, and facilitates the delivery of MyD88 to activated TLR4 [39]. On the other hand, the lipid-binding domain of TIRAP binds to phosphatidylinositol 3P on endosome and mediates the formation of TLR9 signaling complex [40]. Phosphatidylinositol 3P 5-kinase inhibition preferentially blocks TLR9 [40]. However, higher concentrations of TLR9 agonists have been reported to activate cells independent of TIRAP [41]. TRAM is precisely involved in TLR4-mediated MyD88-independent pathway as demonstrated in TRAM-deficient mice, which had a defective cytokine production in the presence of TLR4 ligand [33]. On the other hand, BCAP (B cell adaptor for PI3K) is a exclusive TIR domaincontaining adapter which negatively regulates/inhibits TLR response. BCAP is crucial for linking TLRs to PI3K and its absence leads to an exaggerated inflammation following infection [42]. Negative regulators of TLR pathway are crucial and while some are induced by the TLR signaling, others are constitutively expressed. Negative regulators intersect the TLR pathway at almost every step. SCIMP (SLP adaptor and CSK interacting membrane protein) is a universal trans membrane non-TIR TLR adaptor in macrophage [35, 43].
TLRs respond to a stimulus (PAMP/DAMP) by dimerization of cytoplasmic domain in response to (pathogen-induced) dimerization of the extracellular domain, or work as monomer [44, 45]. A TLR dimer can be homo- or heterodimer. TLR4, for example, is a homodimer, and TLR1/TLR2 a heterodimer. The stimulation (activation upon binding) of TLR by a pathogenic ligand, such as lipopolysaccharide (LPS), which binds to TLR4, is assisted by a co-receptor protein MD-2 (also known as LY96) which binds to the extracellular domain of TLR, serving as a ‘link’ between TLR and its ligand, and another coreceptor CD14 (Fig. 1). C3H/HeJ and C57BL/10ScCr mice, which are low responders to LPS, have a mutated TLR4. In such mutations, transfection of TLR4 alone does not confer LPS receptiveness on cells, demonstrating a prerequisite for an additional molecule, MD-2 [46]. CD14 and LPS-binding protein, LBP help deliver and load the LPS to the TLR4-bound MD-2 [45]. Once bound to the ligand, the Cterminal signaling domain TIR interacts with intracellular adaptor molecules (MyD88 etc. including the negative regulators). TRAM is definitely involved in TLR4-mediated MyD88-independent signaling cascade [33] (Fig. 1).
Figure 1: A simplified schematic diagram of the TLR/ IL-1R signaling cascade. When activated, both IL-1R and most TLRs interact with MyD88 and IRAK4, which then phosphorylates IRAK1 and activates TRAF6. Another downstream adaptor called TRIF is also activated by certain TLR. TIRAP conducts the signal from TLR to MyD88, while TRAM mediates it from TLR to TRIF. MD2, and also CD14, associate with the TLR on the cell surface, thus conferring responsiveness to the ligand, LPS in this case. TLR4 mediated signaling is shown in the pathway as a prototype. Details have been provided in the text.
MYD88-Dependent TLR Signaling
Signaling through TLR orients the immune response by inducing the synthesis and secretion of proinflammatory chemokines/cytokines and Type 1 interferons (Type 1 IFNs, important against viral infections) [47-49]. The process has been reported to be both MyD88- dependent and MyD88 independent [50]. In the signaling process engaging MyD88, MyD88 interacts with TLR (or IL-1 receptors) and soon after the assembly culminates in an oligomeric assembly of IL-1R-associated kinases (IRAKs), leading to the formation of a multi protein complex called Myddosome [51]. In the process of myddosome formation, IRAK4 activates (phosphorylates) IRAK1, which then undergoes sequential autophosphorylations to regulate its own availability as an adaptor. A hyperphosphorylated IRAK1 induces the activations of Tumour necrosis factor receptor-associated factor 6 (TRAF6) which induces K63-linked polyubiquitination on TRAF6 and MAP3K7, also known as Transforming growth factor β (TGF-β)- activated kinase 1 (TAK1), a member of the MAP kinase family [29]. IRAK1 auto phosphorylation dissociates it from MyD88, but does not affect its association with TRAF6, which, along with the ubiquitinconjugating enzyme UBC13 and UEV1A, promotes K63-linked polyubiquitination. TRAF6 is linked to TAK1 through a regulatory protein called TAK1-binding protein 2 (TAB2) [52, 53]. It (TRAF6) promotes ubiquitination of the evolutionarily conserved signaling intermediate in Toll pathways (ECSIT), resulting in an increased production of the reactive oxygen species by the mitochondria and the cell. TAK1, on the other hand, leads to the activation of the inhibitor of NF-κB (IκB) kinase (IKK) and MAPKs, which regulate the NF-κB and AP1 (MAPK pathway), respectively. TAK1 is a key regulator TLR pathway. Ribosomal S6 kinase 1 (S6K1) has been reported to negatively regulate the TLR pathway by inhibiting TAK1 [54].
TAK1 activation is crucial as it activates two different complexes/ pathways—NF-κB pathway via IKK, and the MAP kinase pathway. IKK is composed of two subunits (IKKα and IKKβ) and a regulatory IKKγ subunit (also known as NEMO). TAK1 binding to IKK complex, via ubiquitin chains, permits it to phosphorylate and trigger IKKβ. The (IKK) complex then phosphorylates IκBα, an NF-κB inhibitory protein which undergoes proteasomal degradation, thus allowing NF-κB to translocate into the nucleus and induce the expression of proinflammatory genes [55]. TAK1 also activates the MAPK proteins (ERK1/2, p38 and JNK) which mediate the activation of AP-1 [56]. ECSIT, as indicated above, also interacts with TRAF6. In bacterial infections (and after TLR activation), TRAF6 interacts with and ubiquitinates ECSIT, which is a mitochondrial respiratory chain assembly factor. TRAF6-promoted ECSIT ubiquitination leads to an increased generation of the reactive oxygen species, thus killing the bacteria inside the cell and also regulate NF-κB in TLR4 signaling [57, 58]. The endogenous ECSIT can form a complex with p65/p50 NF-κB (ECSIT-silenced THP-1 cells failed to activate NF-κB DNAbinding activities of p65 and p50, indicating a role of NF-κB in TLR4 signaling [57]. TRAF6 is also reported to be trans located to mitochondria in infections caused by the bacteria [58].
The MyD88 is essential for TLR2, 4, 5, 7, 8, and 9 signaling cascades. In fact, all TLRs except TLR3 use MyD88. TLR3 binds to TRIF, which acts independently of MyD88 in a TRIF-dependent pathway. TRIF binds to TLR3 directly, but interacts with TLR4 indirectly through TRAM, a TRIF related adaptor [59]. TRAM is important as it mediates the TLR4 signaling independent of MyD88, that is, in a TRIF-dependent way.
TRIF dependent pathway
TRIF mediated pathway, which, for example, is initiated by TLR3 and TLR4, activates TRIF directly or indirectly, resulting in the production of IFN- (Fig. 1), indicating the essentiality of TRIF in TLR3 and TLR4 signaling, facilitating the antiviral defense. Mice deficient in the gene encoding TIR domain-containing adaptor (TRIF) had a defective TLR3 and TLR4 mediated IFN- production. The cytokine production in response to TLR4 activation is diminished in TRIF-knock out macrophages, but not with other TLR ligands [60].
Signaling diversity in TLR pathways is an interesting phenomenon, albeit less understood. For example, in TLR4 cascade mediated by the TRIF–TRAM (MyD88 independent) pathway, TLR4 specifically induces the expression of Type I IFN via TRAF-family-memberassociated NF-κB activator (TANK)-binding kinase-1 (TBK-1) and IFN regulatory factor 3 (IRF3). On the other hand, Type I IFN genes are also expressed in MyD88 pathway upon stimulation of TLR7 and TLR9, suggesting the role of some other controlling components in the diversification of TLR response. In TRIF–TRAM mediated signaling of TLR4, TRAF3 has a unique role in signal diversification. It recruits TBK-1 and IKK and phosphorylate (activate) IRF3 in a process facilitated by the PtdIns5P (PtdIns5P forms a complex between TBK1 and IRF3). The (TRAF3) pathway is essential for the expression of IFN-responsive genes and IL-10, an anti-inflammatory cytokine [61]. The activation cascade involving TRAF3 (which activates IRF7 or IRF3 and stimulates the production Type I IFN, as well as IL-10) could be a potential pharmacotherapeutic target in various diseases, including the pulmonary disease. TRAF6 in TRIF pathway, on the other hand, recruits RIP-1, which activates TAK1 complex and MAPK. Upon activation, RIP-1 regulates ubiquitination by Pellino-1 is an E3 ubiquitin ligase which, when phosphorylated by IRAK1, TBK1, and IKK [62], induces the transcription of IFN- via a transcription factor called Deformed Epidermal Autoregulatory Factor 1 (DEAF1). The Pellino-1—DEAF-1 interaction is independent of the E3 ligase activity of Pellino, but it is weakened when Pellino-1 is phosphor ylated. DEAF1 then binds to the IFN- promoter and to IRF3 and IRF7 (which are required for the transcription of IFN-). DEAF1 is also crucial for TLR3 mediated production of IFN-, indicating its role in dsRNA-mediated production of IFN [63].
Regulation of Myd88-Dependent and Independent Signaling
Several negative regulators have been reported in literature that can modulate the TLR signaling, by inhibiting either signaling complex formation or ubiquitination. For example, MyD88 is suppressed by ST2825, NRDP-1, SOCS1, and Cbl-b, while TRIF is suppressed by SARM and TAG. SARM is a negative regulator of TRIF and therefore regulates TLR3 and 4 signaling. TRAF3 is suppressed by SOCS3 and DUBA. TRAF6 is reported to be suppressed by A20, USP4, CYLD, TANK, TRIM38, and SHP. NF-κB is suppressed by Bcl-3, IκBNS, Nurr1, ATF3, and PDLIM2, while IRF3 activation is negatively regulated by Pin1 and RAUL [29].
TLRs in pulmonary tissue
TLRs are vital not only for host defence against infections and have implications in lung infections, but also play a role in the pathogenesis of non-infectious lung injury, including the airways disease, acute pulmonary injury, and interstitial lung disease. While a controlled TLR response initiates pulmonary innate immune response, a more exaggerated response elicits an intense chemokine/ cytokine response characteristic of the pro-inflammatory M1 macrophage phenotype in cells and tissues, including the lungs [64]. In the lung, TLR proteins are widely expressed on both resident and infiltrating macrophages and lymphoid cells [21]. The alveolar macrophages in the terminal airways and airspaces, which characterize major macrophage population of the lung, express almost all mammalian TLRs, most prominently TLR2, 3, 4, 5 and 6 [65]. The human alveolar macrophage expresses lower levels of TLR3, 5 and 9, and higher levels of TLR1, 2, 4, 7 and 8 [66, 67]. TLR1 to TLR10, which subsequently release CXCL8 or IL-8 upon activation, are expressed on primary bronchial epithelial cells [68]. The plasmacytoid and myeloid dendritic cells located within the epithelium and interstitium also express a multitude of TLR receptors, with the plasmacytoid dendritic cells preferentially expressing TLR7/8 and TLR9, and the myeloid dendritic cells producing TLR2, 3, 4 and 9 [69]. Lung dendritic cells are particularly important as they bridge innate and adaptive immunity and, depending on the context, may induce Th1, Th2 or Th17 response to infections. These dendritic cells can also prevent harmful immune responses to harmless inhaled antigens via Treg cells or via neutralizing mucosal IgA antibodies [70].
The pulmonary stromal (structural) cells including the pulmonary endothelium, airway and alveolar epithelium, and fibroblasts also engage in TLR-mediated signaling [68]. TLR4 on lung endothelial cells is particularly important for capillary permeability and neutrophil recruitment in response to LPS [71]. Recruited neutrophils also express TLR1, 2, 4, 5 and 9 [72]. In the respiratory system, the lower respiratory tract (trachea, bronchi, alveoli) cells express TLR3 on the luminal and basal side, whereas TLR1, 2 and 6 on basolateral side. Briefly, pulmonary TLR signaling has been implicated in the synthesis and secretion of a variety of chemokine’s and cytokines including the TNF-α, MIP-1α, RANTES, MIP-1β, GRO-α, -β and -γ, IL-8, IL-6, IL-5 and TGF-β to promote the influx of professional phagocytes (neutrophils, monocytes, macrophages) and dendritic cells, and the production of anti-microbial substances such as defensing, lysozyme, nitric oxide and IL-37 in the respiratory tract [73-75]. However, the TLR levels in a cell cannot always be correlated with the functional response [66, 67]. To illustrate this, the heightened secretion of TNF-α by the alveolar macrophage cells, and IL-6 and IL-10 by interstitial macrophages have been observed even when the levels of TLR mRNA were comparable [66].
TLRs in Lung Infections
Viral Infection
Toll-like receptors are like a double-edged sword, contributing both to eliminate the pathogenic substance, including the viruses, and, at the same time, have potential to harm the host due to persistent immune activation, inflammation and tissue destruction. In virus infection, such as the SARS-Cov-2 (COVID-19), TLR activation leads to the inflammation activation and IL-1β production, inducing IL-6 [76]. The activation of JAK/STAT by TLR may further lead to the macrophage activation syndrome. Ordinarily, in virus infections, the virus nucleotides or proteins can be sensed by various PAMP receptors/TLR. Viral RNA is recognized by TLR8, 7 and 3. Specifically its dsRNA is sensed by TLR3 receptors, and ssRNA is by TLR8-7 [7]. TLR3 induces the secretion of IFN type-1 and proinflammatory cytokines, but the reports are conflicting concerning the protection in vivo. Cytomegalovirus (MCMV) infected TLR3- deficient animals showed reduction in secretion of IFN type-1, IFN-γ and IL-12 subunit p40 (IL-12p40, or IL-12B) and a drop in activation of NK cells, making these (deficient) mice more vulnerable to virus infection [77]. However, according to some other studies of infections with MCMV, Reoviruses and Lymphocytic choriomeningitis virus (LCMV), TLR3 deficiency did not influence CD8+ and CD4+ cells, denouncing its role in defence against virus infection [78]. The virus deoxynucleic acid (DNA) is sensed by TLR9 [22, 79-81]. TLRbinding of viral nucleotides stimulates the expression and secretion of cytokines and IFN type-1 [82]. In virus infections, the virus structural proteins are sensed by the cell surface TLRs, TLR4 and TLR2. TLR4 can also sense the envelop protein of mouse mammary tumour virus (MMTV). TLR4-deficient mice exhibited a reduced IL-12 secretion and reduced levels of inflammatory cells infiltration leading to a decrease in viral clearance. [83]. The glycoproteins of Herpes simplex virus 1 (HSV1), Human cytomegalovirus (CMV) and Measles virus hemagglutinin (MV-H) can be sensed by the TLR2 receptors and induce inflammatory cytokines secretions [2]. TLR2 is also reported to initiate IFN type-1 production in cell type-specific manner. The dendritic cells and macrophages, when infected with inactivated Vaccine virus, induce inflammatory cytokines production, but not IFN type-1 via TLR2, although inflammatory monocytes induced IFN type-1 through TLR2 [84]. TLR2-mediated stimulation of IFN type-1 does not need nucleotides [85].
Bacterial Infection
Briefly, the bacterial lipoproteins can be detected by TLR6, 2 and 1. Lipopolysaccharide is detected by TLR4, whereas the bacterial flagellin can be detected by TLR5, and bacterial RNA and DNA, respectively, by TLR7 and 9. The lipoteichoic acid, and also lipoprotein, is an important group of PAMP that can be sensed by TLR6-TLR2 heterodimer. In TLR2-deficient mice, a decrease in survival has been reported when these mice were infected with Staphylococcus aureus [86]. However, in another study, in bacterial brain abscess, the Control and TLR2-deficient mice showed indistinguishable responses. TLR2-deficient mice were less vulnerable to infection than MyD88-deficient mice, indicating a role of some other TLR and IL-1R family members in immune response [86, 87]. In a study on to Salmonella typhimurium infection in TLR4-deficient mice, the knockout mice were more vulnerable to infection. Interestingly, TLR4 and TLR2-deficient group was more vulnerable to the infection than TLR4 deficient mice, suggesting an efficient response of TLR2 in absence of a functional TLR4 [88]. In the infection caused by Mycobacterium (tuberculosis, TB), TLR9, 4 and 2 on alveolar macrophages recognized the infection and initiated transcription of IL-6, TNF-α and IL-12 [89, 90]. MyD88-deficient mice which showed a reduced secretion of TNF-α and IL-12 were vulnerable to TB [90].
Fungal Infection
In fungal infection, TLR4 and 2 recognize mannan, which is expressed by Candida albicans and Saccharomyces cerevisiae. TLR4 stimulation is reported to lead to the secretion of TNF-α [91]. TLR4- deficient C3H/HeJ mice infected with C. albicans which exhibited reduced cytokine secretion by the macrophage were vulnerable to infection as compared with the control group. However, the reports with regard to TLR4 response in candida infection are conflicting [91]. According to some studies, TLR2 was redundant against fungal infection. TLR2-knockout mice were more susceptible to Candida infection and secreted low level of chemokines and cytokines. Other reports suggested that the TLR2-mediated detection of Candida can be damaging for the host species (as TLR2-knockout mice demonstrated a reduced production of IL-10 and increased secretion of IFN-γ and IL-12; hence were more resistance against infection than control group [92, 93]. TLR6, and 2 as well, also recognize β-glucan and zymosan. The fungal RNA can be detected by TLR7 and its DNA by TLR9 [91, 94]. As reported in invasive candidiasis, dendritic cells have been reported to recognize the Candida infection and initiate cytokine secretion and upregulate the chemical mediators that cause naïve T cell differentiation in Th17, Th1, Th2, and Treg cells. In candidiasis, Th17 and Th1 responses are crucial for protection against Candida, while the Treg and Th2 responses are detrimental for the host as they repress the innate immunity.
Toll-Like Receptors in Pulmonary Injury (Non- Infectious)
Airway Diseases, Acute Lung Injury and Pulmonary Fibrosis
TLRs play a role in non-infectious pulmonary diseases ranging from asthma, bronchitis and chronic obstructive pulmonary disease (COPD) to acute lung injury and pulmonary fibrosis. Many TLRs have been implicated in airway diseases (COPD, asthma, bronchitis, bronchiectasis and emphysema). Low, but not large, doses of LPS, which acts through TLR4, potentiates asthma—a response blunted in TLR4-deficient mice [95]. However, TLRs such as TLR7 and 9 have been reported to suppress asthmatic inflammation, indicating their potential as a therapeutic target. TLR7 acts as a bronchodilator in allergic asthma in guinea pigs [96], a response primarily attributed to the suppression of Th2 response. Similarly, TLR9 promotes DC polarization towards DC1 in response to allergens [97], a response which can partly be attributed to the CD4+CD25+ Treg cells known to inhibit allergic inflammation [98]. CpG oligodeoxynucleotides, a TLR9 agonist, can significantly decrease airway inflammation and bronchial hyperactivity, and IgE [99]. Role of TLR4 is somewhat paradoxical in COPD, promoting injury [100, 101], and preventing pulmonary emphysema (TLR4-knockout mice develop emphysema with age) [102]. TLR is also engaged in cystic fibrosis, a genetic ailment characterized by chronic airway inflammation and persistent/ recurrent infection, primarily due to bacteria such as P. aeruginosa. Incidentally, flagellin, a TLR5 agonist, is abundantly expressed in P. aeruginosa. Innate immunity mediated by TLR5 has been suggested as a potential target in cystic fibrosis pulmonary disease [103].
TLRs also contribute to the alveolar capillary injury resulting in pulmonary edema [104]. The injury, commonly known as acute lung injury can be produced by lung infections (pneumonia, sepsis), as well as exposure to non-infectious substances such as bleomycin and ozone. TLR4 promotes lung injury in response to ozone [105]; however, TLR4 either alone or in combination with TLR2, can also reduce injury in other models [105]. TLR4-deficient mice are characterized by an increased susceptibility to hyperoxia-induced pulmonary injury—an effect attributed to decreased expression of heme oxygenase-1, a cytoprotective gene reported to inhibit apoptosis [101, 106]. In general, usually the TLR2 and TLR4 are more protective in lung injury models, as compared with TLR3, which seems to promote lethal lung injury in hyperoxia-induced acute lung injury model [107]. Moreover, the endogenous factors uniquely present in the lung also affect the lung function. For example, the lung surfactant protein A (SP-A) dampen injurious TLR-mediated signaling [108]. The protein blocks the interaction of TLRs with agonists.
Interstitial Lung Diseases
Interstitial lung diseases result in variable amounts of lung parenchymal inflammation and fibrosis, an exaggerated wound healing and repair response mediated by the fibroblasts and myofibroblasts. The fibroproliferative response of the lung may be initiated or modified by the TLR. TLR4, for instance, in conjunction with TLR2, suppresses fibrogenesis [109]. An enhanced radiationinduced lung fibrosis was reported in TLR2/TLR4 double-knockout mice in this study, but not in TLR2 or TLR4 alone single-knockout animals, indicating a protective effect in combination. However, TLRs such as TLR9 promote a dysregulated fibrotic response [110]. In another study, TLR3 agonist Poly (I: C) helped in matrix production and differentiation of fibroblast-to-myofibroblast [111].
Lung Cancer and TLR
TLRs are crucial in the development and progression of lung cancer [112], as well as may enhance anticancer efficiency and tumor rejection [113]. Specific TLR ligand can be used in cancer monotherapy, or as an adjuvant. Bacillus Calmette-Guerin (BCG) vaccine which is also used for the treatment of superficial bladder tumor is a TLR2/4 ligand and an approved FDA-approved TLR ligand [114, 115].
A number of endogenous damage-associated molecular patterns/ DAMPs such as the heat-shock proteins, high-mobility group protein B1, calprotectin (a complex of two Ca2+-binding proteins of S100 family, s100A8 and S100A9), heparanase, and the lung collectin, SPA are can be recognized by the TLR and may contribute to the lung cancer in a positive, or negative way. HSP70 and HMGB1, for example, bind to TLR2/4 and TLR4, respectively, and produce an anticancer effect by inducing the Th cell cytotoxicity (HSP70), or inhibit signals that control growth of cancer cells (HMGB1) [116, 117]. HSP27 and HSP60 contribute to cancer by promoting cancer growth and progression. Extracellular HSP27 binds to TLR3 and mediates angiogenesis [118]. Soluble HSP60, on the other hand, stimulates TLR2 on human CD45RO+ memory and CD45RA+ naive T cells, regulating the T cell behavior. TLR2 mediated signaling stimulated by HSP60 leads to the activation of T cell adhesion to fibronectin, and also inhibition of T cell chemotaxis, and down-regulated chemokine receptor CXCR4 and CCR7 expression [119]. Calprotectin and SP-A also contribute to cancer development and its spread. Calprotectin is an endogenous TLR4 agonist that has been reported to amplify cancer [120]. SP-A regulates the expression of TLR2 and 4 and reduces the TLR4 and pro-inflammatory response [120, 121]. The protein has been reported to express in about 49% primary non-small cell lung carcinoma (NSCLC) [122]. Heparanase is another protein (enzyme) that plays a role in lung cancer. Heparanase cleaves heparan sulphate (HS) into HS fragments (Mol mass, 5 to 7). The enzyme is overexpressed in about 75% of lung cancer patients, and had an inverse correlation with survival. Heparanase is one of the many DAMP whose role has been confirmed in lung carcinoma [112]. HS fragments can stimulate the release of pro-inflammatory mediators such as the TNF and interleukins (IL) 1β, 6, 8 and 10 by the peripheral blood mononuclear cells and activate dendritic cells, both through TLR4, mediating an inflammatory response [123, 124].
Expression of TLRs on dendritic cells is similarly significant in lung cancer. The myeloid dendritic cells (mDCs), acting as guards, look for antigens (to be presented and processed by the T cells to generate adaptive immune responses), bridging the gap between innate and adaptive immunity [125]. TLR 1-9 are expressed by the mDCs, but their role in lung cancer is not fully understood. On the other hand, the plasmacytoid dendritic cells (pDCs), also known as natural IFN producing cells, specializes in sensing the viral nucleic acid (RNA/ DNA) via TLR7 and 9, and massive quantity of type 1 IFN [126]. Once it has produced IFN (type 1), pDC differentiates into professional antigen-presenting cells capable of stimulating T cells of adaptive immune system. TLR2, 4. 7/8 and 9 are among highly expressed TLRs in lung cancer and stimulate IFN type-1 and IFN-γ, promoting the production of IL-10 (an anti-inflammatory cytokine, also known as cytokine synthesis inhibitory factor) and the immunosuppressive TGF-β, as well as tolerogenic enzyme indoleamine-2,3-dioxygenase [127, 128]. These cytokines and indoleamine-2,3-dioxygenase induce the production of adaptive and natural Tregs [129]. B cells express TLR7 and 9. TLR9 binds to CpG and stimulate the B cell polarization, acquiring B-1 phenotype, reported to promote tumor regression [128, 130]. The macrophage cells in lung express high levels of TLR2, 3, 4 and 6, when compared with TLR7 and 9, and are polarized to M2 phenotype in lung cancer. These ‘Tumor-associated macrophage’ (TAM) cells express high levels of TLR2, which, upon stimulation, promotes lung metastasis [131]. TLR4 signaling in TAMs also increases cancerous lesions [132]. TLR9 recruits large number of macrophages in lung cancer [133]. TLR pathway in macrophage and myeloid-derived suppressor cells (MDSCs) repress immune surveillance of the cancer cells [134]. In a recent study on MDSCs, TLR2 (activation) promoted immune evasion and enhanced tumor growth in lung adenocarcinoma, lymphoma and colon carcinoma [135]. In another study, TLR9, upon stimulation by its agonist CpG, promoted the growth of tumor epithelial lung cells in human [136], but along with cetuximab, regressed tumor growth [137]. Cetuximab is an inhibitor of EGFR signaling. TLR9 is also reported to induce the release of VEGF and promote cancer [138]. Recently, TLR3 has been reported as a new marker to detect high risk early stage NSCLS [139]. TLR7 and TLR8, stimulated by the ssRNA, cause an increase in NF-B and upregulation of Bcl-2, thereby favoring tumor cell survival and chemoresistance [140].
TLRs: a potential therapeutic target in lung cancer
A TLR can be targeted in two ways – (1) Using agonists that specifically bind to TLR to augment the response, and which can be used as adjuvant in vaccine therapies, and (2) Inhibiting TLR activation (by way of antibodies, small molecule inhibitors, oligopeptides, and endogenous anti-TLR substances). The negative regulation of TLR signaling is another important approach that can be used a novel therapeutic strategy. The modulation of TLR signalling pathway has demonstrated its usefulness in infectious, as well as noninfectious, and inflammatory diseases, allergy and autoimmunity [141]. A brief description of TLR agonists and antagonists is provided in the following section.
TLR agonists
TLR2, 3, 4, 5, 7 and 9 has been linked with numerous diseases and could be stimulated by agonists making them fascinating immunomodulators and adjuvants. The monophosphoryl lipid A (MPL), derived from Salmonella Minnesota, is used as a vaccine adjuvant in Europe for Hepatitis B and HPV virus [142]. It is also used in allergy vaccine and for the treatment of asthma. The lipid, MPL, specifically triggers TRAM/TRIF pathway and controls CD80/86 complex [143]. Another TLR4 agonist, OM-174, purified form of lipid A of E. coli, has demonstrated anticancer activity in mice. It acts by increasing IFN-γ [144, 145]. E6020, on the other hand, is a chemically synthesized TLR4 agonist [146, 147]. Aminoalkyl glucosaminide phosphates (AGPs) are also lipid A derivatives which have been synthesized and are safe and effective as adjuvants for influenza virus vaccine [148, 149]. Poly-γ-glutamic acid (γ-PGA), known to down regulation Th2 cytokines, airway inflammation and eosinophilia while increasing the manifestation of co-stimulatory molecules, CD80, CD86 and CD40 on dendritic cells, has been found effective in allergic type lung inflammation [150]. Another synthetic TLR4 agonist (ER803022) has been found effective in mouse model of asthma. ER803022 causes TLR-dependent MyD88 activation and IL-12/IFN-γ production [151].
Pam3CSK4 is a synthetic TLR2 agonist. TLR2 binds to LPS and recognizes several ligands more specifically of the Gram-positive bacteria. It can reduce the Th2 cytokine release, airway inflammation etc in asthma. Pam3CSK4 binds to a TLR2 pocket and a small channel of TLR1, thus bridging the two [152]. SMP-105 is also a TLR2 agonist, from Mycobacterium bovis. It is an approved drug for bladder cancer [153]. SMP-105 upregulates NF-κβ via TLR2, in a TLR4- independent manner. It causes a decrease in TNF-α and IL-6, and has been found useful as an adjuvant in inflammation [153]. Similarly, lipopeptide CGP 40774 (LP40), the most potent analogue of synthetic lipopeptides, stimulated TLR2 (but not TLR4) and potentiated the production of IFN-γ and IL-10, but not IL-4 and IL-5 by human T cells and inhibited allergen-induced IgE production [154]. There are other TLR agonists such as the Macrophage activating lipopeptide-2 (MALP-2). MALP-2 is obtained from Mycoplasma fermentans and binds to TLR2/TLR6 heterodimer, inducing IFN-γ, CD80, CD86, MHC I and II in response to allergies [155]. It decreases the airway hyperresponsiveness, eosinophilia and Th2 cytokines and stimulates the accumulation of T and B cells and NK cell in lung in murine model of asthma [156]. TLR7 agonists also suppress Th2-mediated airway inflammation, as well as IgE production, and inhibit IL-17 and IL-13 production through IL-10 pathway [157]. Imiquimod is a TLR7 agonist that works against asthma, virus-induced respiratory infection, as well as basal cell carcinoma and external genital wart [158]. It causes an increase in IFN-α via TLR7–MYD88 pathway. SA-2, a synthetic TLR7 agonist, regulates T-cell production via IFN-α, IL-27 and IL-10 with great therapeutic potential [159]. ANA773, a small molecule TLR7 agonist, has antiviral potential [160]. R-848 (Resiquimod) is a TLR7 and TLR8 agonist which can reduce lung eosinophilia and airway inflammation [161, 162]. It induces IFN-α, IL-12 and TNF-α production.
CpG-ODN is a TLR9 agonist reported to stimulate IL-6, IFN-γ, IL-12 and CD4 T cells, in turn eliciting a Th1 type of inflammatory response along with enhanced IL-12 and IL-10 production [163]. CpG-ODN suppresses Th2 cytokine, airway eosinophilia, IgE levels, and bronchial hyperreactivity and has been found effective in asthma and allergic rhinitis [164-168]. Rintatolimod, a TLR3 agonist, which affects the RNase L, has been found effective in severe acute respiratory syndrome, influenza, hepatitis infections and cancer [169]. In a recent study, the TLR1-10 is associated with improved survival outcomes in NSCLC.
Small molecule such as N-methyl-4-nitro-2-(4-(4- (trifluoromethyl)phenyl)-1H-imidazol-1-yl)aniline (CU-T12-9) have also been found to facilitate TLR (TLR1/2 heterodimeric) complex formation and induce inflammatory response by inducing NF-κB and downstream effectors TNF-α, IL-10 and iNOS [170]. TLR5-mediated recognition of flagellin has been reported to involve in activating pulmonary defense against P. aeruginosa [171]. In another study, TLR5 agonist entolimod stimulated NK-dendritic-CD8+ T-cell axis and suppressed metastasis [172]. TLR10, which has no (known) ligand specificity and biological function and which acts as a modulatory receptor (mainly inhibitory effect) has been suggested as a potential target in therapeutics, owing to its unique anti-inflammatory properties [173]. These and other PRRs and their agonists need to be explored further in lung and airways diseases and allergies.
TLR antagonists
Antagonist treatment reduces the unwarranted effects of TLR activation. TLR2 and 4 antagonists show great potential as potential molecules. The diphosphoryl lipid A from the nontoxic LPS of Rhodobacter sphaeroides (RsDPLA) is a reported TLR4 antagonist which blocks the binding and internalization of LPS in RAW macrophages [174]. When inhaled, it benefits asthma by preventing eosinophilia and lymphocytosis, by falling Th2 cytokines and lower airway hyperresponsiveness [175]. NI-0101, a synthesized antibody, blocks TLR4 dimerization and can reduce cytokine secretion, averting flu and its symptoms [176]. 1A6, another anti-TLR4 monoclonal antibody, has also been reported to reduce inflammation, showing positive signs in lung injury [177]. Eritoran TM (E5564) is another TLR4 antagonist. It affects TLR4/MD-2/LPS complex formation [178]. OPN-305, a fully humanized IgG4 monoclonal TLR2-specific antibody, blocks TLR2-mediated pro-inflammatory cytokines [179]. R837, a TLR7 antagonist, engages in the NO production and relaxes airway passage [180]. Capsazepine and its analogues inhibit TLR3 and repress pro-inflammatory TNF-α and IL-8 in asthma [181]. Resveratrol in grapes and peanuts also downregulate TLR3, and one of its adapters TRIF, providing protection in asthma [182]. However, despite the benefits, it is important to exercise caution in antagonist therapy as its, and also the agonist therapy, use can damage to the local immune defence and result in damage and opportunistic disorders. A summary of TLR agonists and antagonists with therapeutic potential and possible use and contraindications in pulmonary diseases are shown in Table 1.
| TLR | Cell surface TLRs | Intracellular TLRs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Types | TLR1 (CD281) |
TLR2 (CD282) |
TLR4* (CD284) |
TLR5 | TLR6 (CD286) |
TLR10 (CD290) |
TLR3 (CD283) |
TLR7 | TLR8 (CD288) |
TLR9 (CD289) |
| Agonist | CU-T12-9 | BCG [114] | AGPs [148, 149] | Flagellin [141] Entolimod [172] |
MALP-2 [155] | No known ligand specificity [173] | Poly(I:C), a dsRNA mimetic [185] Rintatolimod [169] |
ANA773 [160] Imiquimod [186], R-848 [161, 162] SA-2 [159] |
CL075 [187] R-848 [161, 162] |
CpG-ODN [167] |
| Antagonist | LP40 [154] | BCG [114] | TH1020 [189] | Capsazepine [#] Resveratrol [182] |
R837 [180] | CU-CPT9a [190] | IRS-869 [191] | |||
Table 1: TLR agonists and antagonists: implications in lung diseases and contraindications *TLR4 can be active on cell surface, as well as intracellularly in specific cells [192].
Conclusions
Lungs have a huge surface area, roughly the size of a tennis court, and a total airway length roughly about 1,500 miles, breathing 12-15 times a minute. With such a huge surface exposed to atmosphere, the respiratory system is always facing danger of exposure to a plethora of substances and injury. The pattern recognition receptors called TLR has drawn particular attention in host defense and a key mechanism in providing protective mechanism against pulmonary injury, both infectious and non-infectious. TLR signaling is not only imperative for strengthening the host defense, but also play a role in tissue remodeling and repair. In this article, we have attempted to highlight the key research on TLR in pulmonary injury, providing a mechanistic approach and insights on therapeutic implications of TLR agonists and antagonist in lung diseases. TLRs appear to have immense potential as therapeutic targets in various lung injuries, including virus and microbial infections, allergies, and even cancer, but need to be dealt with extra caution as an aggravated or inhibited response can damage to the local immune defense and result in damage and opportunistic disorders. A detailed understanding of the TLR-associated mechanisms is required to fully exploit the potential of TLRs in lung injury caused by various agents and substances.
References
- Wilmott RW, Khurana-Hershey G, Stark JM (2000) Current concepts on pulmonary host defense mechanisms in children. Curr opin pediatr 12(3):187-93.
- Watford WT, Ghio AJ, Wright JR (2000) Complement-mediated host defense in the lung. American J Phys Lung Cell Mol Phys 279(5):L790-8
- Campione E, Cosio T, Rosa L, Lanna C, Di Girolamo S, Gaziano R, et al. (2020) Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int J Mol Sci 21(14):4903.
- van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS (1999) Defensins: key players or bystanders in infection, injury, and repair in the lung? J Allergy Clin Immunol. 104(6):1131-8.
- Casals C, Campanero-Rhodes MA, García-Fojeda B, Solís D (2018) the Role of Collectins and Galectins in Lung Innate Immune Defense. Front Immunol 9:1998
- Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. (2021) Evolution of antibody immunity to SARS-CoV-2. Nature. 2021; 591 (7851):639-44.
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140(6):805-20.
- Janeway CA, Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54 Pt 1:1-13.
- Medzhitov R, Janeway CA, Jr (1997) Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 9(1):4-9.
- Kawai T, Akira S (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. International immunology 21(4):317-37.
- Hoving JC, Wilson GJ, Brown GD (2014) Signalling C-type lectin receptors, microbial recognition and immunity. Cellular microbiology 16(2):185-94.
- Lafferty EI, Qureshi ST, Schnare M (2010) The role of toll-like receptors in acute and chronic lung inflammation. J Inflamm (Lond) 7:57.
- Arora S, Dev K, Agarwal B, Das P, Syed MA (2018) Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 223(4):383-96.
- Yamamoto M, Takeda K (2010) Current views of toll-like receptor signaling pathways. Gastroenterology research and practice
- Yang L, Seki E (2012) Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol 3:138.
- Lindsay SA, Wasserman SA (2014) Conventional and non-conventional Drosophila Toll signaling. Developmental and comparative immunology 42(1):16-24.
- Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM (2003) Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends in immunology 24(10):528-33.
- O'Neill LAJ, Bowie AG (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature Reviews Immunology 7(5):353-64.
- Hughes MM, Lavrencic P, Coll RC, Ve T, Ryan DG, Williams NC, et al. (2017) Solution structure of the TLR adaptor MAL/TIRAP reveals an intact BB loop and supports MAL Cys91 glutathionylation for signaling. Proc Natl Acad Sci U S A 114(32):E6480-e9.
- Jezierska A, Kolosova IA, Verin AD (2011) Toll Like Receptors Signaling Pathways as a Target for Therapeutic Interventions. Current signal transduction therapy 6(3):428-40.
- Arora S, Ahmad S, Irshad R, Goyal Y, Rafat S, Siddiqui N, et al. (2019) TLRs in pulmonary diseases. Life sciences 233:116671.
- Akira S, Uematsu S, Takeuchi O (2006) Pathogen Recognition and Innate Immunity. Cell 124(4):783-801.
- Roh JS, Sohn DH (2018) Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune network 18(4):e27.
- Gülke E, Gelderblom M, Magnus T (2018) Danger signals in stroke and their role on microglia activation after ischemia. Therapeutic advances in neurological disorders 11:1756286418774254.
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature medicine 11(11):1173-9.
- Gasse P, Riteau N, Charron S, Girre S, Fick L, Pétrilli V, et al. (2009) Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. American journal of respiratory and critical care medicine 179(10):903-13.
- Campo GM, Avenoso A, Nastasi G, Micali A, Prestipino V, Vaccaro M, et al. (2011) Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochimica et biophysica acta 1812(9):1170-81.
- Morwood SR, Nicholson LB (2006) Modulation of the immune response by extracellular matrix proteins. Archivum immunologiae et therapiae experimentalis 54(6):367-74.
- Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Frontiers in immunology 5:461.
- Belinda LW, Wei WX, Hanh BT, Lei LX, Bow H, Ling DJ. (2008) SARM: a novel Toll-like receptor adaptor, is functionally conserved from arthropod to human. Mol Immunol 45(6):1732-42.
- Deguine J, Barton GM. (2014) MyD88: a central player in innate immune signaling. F1000Prime Rep 6:97.
- Sampaio NG, Kocan M, Schofield L, Pfleger KDG, Eriksson EM. (2018) Investigation of interactions between TLR2, MyD88 and TIRAP by bioluminescence resonance energy transfer is hampered by artefacts of protein overexpression. PloS one. 13(8):e0202408.
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, et al. (2003) TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nature immunology. 4(11):1144-50.
- Luo L, Lucas RM, Liu L, Stow JL.( 2019) Signalling, sorting and scaffolding adaptors for Toll-like receptors. J Cell Sci. 133(5).
- Luo L, Bokil NJ, Wall AA, Kapetanovic R, Lansdaal NM, Marceline F, et al. (2017)SCIMP is a transmembrane non-TIR TLR adaptor that promotes proinflammatory cytokine production from macrophages. Nat Commun. 8:14133.
- O'Neill LA, Fitzgerald KA, Bowie AG. (2003) The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 24(6):286-90
- Teixeira HS, Zhao J, Kazmierski E, Kinane DF, Benakanakere MR. (2020) TLR3-Dependent Activation of TLR2 Endogenous Ligands via the MyD88 Signaling Pathway Augments the Innate Immune Response. Cells 9(8).
- Hamerman JA, Pottle J, Ni M, He Y, Zhang ZY, Buckner JH. (2016) Negative regulation of TLR signaling in myeloid cells--implications for autoimmune diseases. Immunol Rev. 269(1):212-27.
- Kagan JC, Medzhitov R. (2006) Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125(5):943-55.
- Hayashi K, Sasai M, Iwasaki A. (2015) Toll-like receptor 9 trafficking and signaling for type I interferons requires PIKfyve activity. International immunology. 27(9):435-45.
- Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W, et al.( 2014) A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell. 156(4):705-16.
- Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, et al. (2012)Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci U S A. 109(1):273-8.
- Luo L, Curson JEB, Liu L, Wall AA, Tuladhar N, Lucas RM, et al. (2020)SCIMP is a universal Toll-like receptor adaptor in macrophages. J Leukoc Biol. 107(2):251-62.
- Zhang H, Tay PN, Cao W, Li W, Lu J. (2002) Integrin-nucleated Toll-like receptor (TLR) dimerization reveals subcellular targeting of TLRs and distinct mechanisms of TLR4 activation and signaling. FEBS letters. 532(1-2):171-6.
- Botos I, Segal DM, Davies DR. (2011)The structural biology of Toll-like receptors. Structure (London, England : 1993). 19(4):447-59.
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. The Journal of experimental medicine. 189(11):1777-82.
- McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. (2015) Type I interferons in infectious disease. Nature Reviews Immunology. 15(2):87-103.
- Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. (2004) Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 112(3):428-36.
- Routray I, Ali S. (2016) Boron Induces Lymphocyte Proliferation and Modulates the Priming Effects of Lipopolysaccharide on Macrophages. PloS one. 11(3):e0150607.
- Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, et al. (2007) MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 178(2):1164-71.
- Gay NJ, Gangloff M, O'Neill LA. (2011) What the Myddosome structure tells us about the initiation of innate immunity. Trends Immunol. 32(3):104-9.
- Kollewe C, Mackensen AC, Neumann D, Knop J, Cao P, Li S, et al. (2004) Sequential autophosphorylation steps in the interleukin-1 receptor-associated kinase-1 regulate its availability as an adapter in interleukin-1 signaling. The Journal of biological chemistry. 279(7):5227-36.
- Kishida S, Sanjo H, Akira S, Matsumoto K, Ninomiya-Tsuji J (2005) TAK1-binding protein 2 facilitates ubiquitination of TRAF6 and assembly of TRAF6 with IKK in the IL-1 signaling pathway. Genes to cells: devoted to molecular & cellular mechanisms. 10(5):447-54.
- Kim SY, Baik KH, Baek KH, Chah KH, Kim KA, Moon G, et al. (2014) S6K1 negatively regulates TAK1 activity in the toll-like receptor signaling pathway. Mol Cell Biol. 34(3):510-21. Epub 20131125.
- Ali S, Mann DA. (2004) Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 22(2):67-79.
- Dey N, Liu T, Garofalo RP, Casola A. (2011) TAK1 regulates NF-ΚB and AP-1 activation in airway epithelial cells following RSV infection. Virology. 418(2):93-101.
- Mi Wi S, Park J, Shim JH, Chun E, Lee KY. (2015)Ubiquitination of ECSIT is crucial for the activation of p65/p50 NF-κBs in Toll-like receptor 4 signaling. Mol Biol Cell. 26(1):151-60.
- Tiku V, Tan MW, Dikic I. (2020) Mitochondrial Functions in Infection and Immunity. Trends Cell Biol. 30(4):263-75.
- Takeuchi O, Kawai T, Mühlradt PF, Morr M, Radolf JD, Zychlinsky A, et al. (2001) Discrimination of bacterial lipoproteins by Toll-like receptor 6. International immunology. 13(7):933-40.
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301(5633):640-3
- Hoebe K, Beutler B. (2006)TRAF3: a new component of the TLR-signaling apparatus. Trends Mol Med 12(5):187-9.
- Smith H, Liu XY, Dai L, Goh ET, Chan AT, Xi J, et al. (2011) The role of TBK1 and IKKε in the expression and activation of Pellino 1. Biochem J. 434(3):537-48.
- Ordureau A, Enesa K, Nanda S, Le Francois B, Peggie M, Prescott A, et al. (2013) DEAF1 is a Pellino1-interacting protein required for interferon production by Sendai virus and double-stranded RNA. J Biol Chem. 288(34):24569-80.
- Grassin-Delyle S, Abrial C, Salvator H, Brollo M, Naline E, Devillier P (2020) The Role of Toll-Like Receptors in the Production of Cytokines by Human Lung Macrophages. Journal of innate immunity 12(1):63-73.
- Maris NA, Dessing MC, de Vos AF, Bresser P, van der Zee JS, Jansen HM, et al. (2006)Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. Eur Respir J. 28(3):622-6.
- Hoppstädter J, Diesel B, Zarbock R, Breinig T, Monz D, Koch M, et al. (2010) Differential cell reaction upon Toll-like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respiratory Research. 11(1):124.
- Maris N, Dessing M, De Vos A, Bresser P, Van der Zee J, Jansen H, et al. (2006) Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. European Respiratory Journal. 28(3):622-6.
- Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP (2004) Activation of airway epithelial cells by toll-like receptor agonists. American journal of respiratory cell and molecular biology. 31(3):358-64.
- Kovach MA, Standiford TJ (2011) Toll like receptors in diseases of the lung. Int Immunopharmacol. 11(10):1399-406.
- Plantinga M, Hammad H, Lambrecht BN (2010) Origin and functional specializations of DC subsets in the lung. Eur J Immunol. 40(8):2112-8.
- Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, et al. (2003) Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. The Journal of clinical investigation. 111(7):1011-20.
- Koller B, Kappler M, Latzin P, Gaggar A, Schreiner M, Takyar S, et al. (2008) TLR Expression on Neutrophils at the Pulmonary Site of Infection: TLR1/TLR2-Mediated Up-Regulation of TLR5 Expression in Cystic Fibrosis Lung Disease. The Journal of Immunology. 181(4):2753-63.
- McClure R, Massari P (2014)TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front Immunol. 5:386.
- Ohkuni T, Kojima T, Ogasawara N, Masaki T, Fuchimoto J, Kamekura R, et al. (2011) Poly (I: C) reduces expression of JAM-A and induces secretion of IL-8 and TNF-α via distinct NF-κB pathways in human nasal epithelial cells. Toxicology and applied pharmacology. 250(1):29-38.
- Wu CA, Peluso JJ, Zhu L, Lingenheld EG, Walker ST, Puddington L (2010) Bronchial epithelial cells produce IL-5: implications for local immune responses in the airways. Cellular immunology. 264(1):32-41.
- de Rivero Vaccari JC, Dietrich WD, Keane RW, de Rivero Vaccari JP (2020). The Inflammasome in Times of COVID-19. Front Immunol. 11:583373.
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, et al. (2004) Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. PNAS. 101(10):3516-21.
- Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB (2004) Does Toll-like receptor 3 play a biological role in virus infections? Virology. 322(2):231-8.
- Blasius AL, Beutler B (2010) Intracellular toll-like receptors. Immunity. 32(3):305-15.
- Gilliet M, Cao W, Liu Y-J (2008) Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nature Reviews Immunology. 8(8):594-606.
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V (2011) Plasmacytoid dendritic cells: recent progress and open questions. Annual review of immunology. 29:163-83.
- Kawai T, Akira S (2011) Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 34(5):637-50.
- Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, et al.( 2008) Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 133(2):235-49.
- Barbalat R, Lau L, Locksley RM, Barton GM (2009) Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nature immunology. 10(11):1200.
- Shi Z, Cai Z, Sanchez A, Zhang T, Wen S, Wang J, et al. (2011) A Novel Toll-like Receptor That Recognizes Vesicular Stomatitis Virus. Journal of Biological Chemistry. 286(6):4517-24.
- Takeuchi O, Hoshino K, Akira S. (2000) Cutting Edge: TLR2-Deficient and MyD88-Deficient Mice Are Highly Susceptible to Staphylococcus aureus Infection. The Journal of Immunology. 165(10):5392-6.
- Miller LS, O'connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, et al. (2006) MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 24(1):79-91.
- Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A (2004) Toll-like receptors are temporally involved in host defense. The Journal of Immunology. 172(7):4463-9.
- Gerold G, Zychlinsky A, Juana L, editors. (2007) what is the role of Toll-like receptors in bacterial infections? Seminars in immunology, Elsevier.
- Saiga H, Shimada Y, Takeda K. (2011) Innate immune effectors in mycobacterial infection. Clinical and Developmental Immunology. 2011.
- Netea MG, Maródi L. (2010) Innate immune mechanisms for recognition and uptake of Candida species. Trends in immunology. 31(9):346-53.
- Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, Van Krieken JH, et al. (2004) Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. The Journal of Immunology. 172(6):3712-8.
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg B-J, et al. (2006) Toll-like receptor 2 controls expansion and function of regulatory T cells. The Journal of clinical investigation. 116(2):485-94.
- Romani L (2004) Immunity to fungal infections. Nature Reviews Immunology. 4(1):11.
- Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. (2002) Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 196(12):1645-51.
- Kaufman EH, Fryer AD, Jacoby DB. (2011)Toll-like receptor 7 agonists are potent and rapid bronchodilators in guinea pigs. J Allergy Clin Immunol. 127(2):462-9.
- Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, et al. (2003)
- Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directi ng T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 197(1):101-9.
- Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. (2004) Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 173(7):4433-42.
- Givens BE, Geary SM, Salem AK (2018) Nanoparticle-based CpG-oligonucleotide therapy for treating allergic asthma. Immunotherapy. 10(7):595-604.
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E (2004) Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 172(4):2522-9.
- Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, et al. (2005) Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 175(8):4834-8.
- Zhang X, Shan P, Jiang G, Cohn L, Lee PJ (2006) Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 116(11):3050-9.
- Blohmke CJ, Victor RE, Hirschfeld AF, Elias IM, Hancock DG, Lane CR, et al. (2008) Innate immunity mediated by TLR5 as a novel antiinflammatory target for cystic fibrosis lung disease. J Immunol. 180(11):7764-73.
- Qureshi ST, Zhang X, Aberg E, Bousette N, Giaid A, Shan P, et al. (2006) Inducible activation of TLR4 confers resistance to hyperoxia-induced pulmonary apoptosis. J Immunol 176(8):4950-8
- Kleeberger SR, Reddy SP, Zhang LY, Cho HY, Jedlicka AE. (2001) Toll-like receptor 4 mediates ozone-induced murine lung hyperpermeability via inducible nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol 280(2):L326-33
- Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM (2000) Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol. 278(2):L312-9
- Murray LA, Knight DA, McAlonan L, Argentieri R, Joshi A, Shaheen F, et al. (2008) Deleterious role of TLR3 during hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 178(12):1227-37.
- Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS (2008) Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 180(12):7847-58.
- Paun A, Fox J, Balloy V, Chignard M, Qureshi ST, Haston CK (2010) Combined Tlr2 and Tlr4 deficiency increases radiation-induced pulmonary fibrosis in mice. Int J Radiat Oncol Biol Phys. 77(4):1198-205.
- Meneghin A, Choi ES, Evanoff HL, Kunkel SL, Martinez FJ, Flaherty KR, et al. (2008) TLR9 is expressed in idiopathic interstitial pneumonia and its activation promotes in vitro myofibroblast differentiation. Histochem Cell Biol. 130(5):979-92.
- Sugiura H, Ichikawa T, Koarai A, Yanagisawa S, Minakata Y, Matsunaga K, et al. (2009) Activation of Toll-like receptor 3 augments myofibroblast differentiation. Am J Respir Cell Mol Biol. 40(6):654-62.
- Łagiedo M, Sikora J, Kaczmarek M (2015) Damage-Associated Molecular Patterns in the Course of Lung Cancer--A Review. Scand J Immunol. 82(2):95-101.
- Urban-Wojciuk Z, Khan MM, Oyler BL, Fåhraeus R, Marek-Trzonkowska N, Nita-Lazar A, et al. (2019) The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Frontiers in immunology. 10:2388.
- Morales A, Eidinger D, Bruce AW (1976) Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. The Journal of urology. 116(2):180-3.
- Holmes EC. BCG immunotherapy of lung cancer (1980) The Japanese journal of surgery. 10(1):1-6
- Figueiredo C, Wittmann M, Wang D, Dressel R, Seltsam A, Blasczyk R, et al. (2009) Heat shock protein 70 (HSP70) induces cytotoxicity of T-helper cells. Blood. 113(13):3008-16.
- Smolarczyk R, Cichoń T, Jarosz M, Szala S (2012) [HMGB1--its role in tumor progression and anticancer therapy]. Postepy Hig Med Dosw (Online). 66:913-20.
- Thuringer D, Jego G, Wettstein G, Terrier O, Cronier L, Yousfi N, et al. (2013) Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. Faseb j. 27(10):4169-83.
- Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O (2003) T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. Faseb j. 17(11):1567-9.
- Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 86(3):557-66.
- Liu L, Aron CZ, Grable CM, Robles A, Liu X, Liu Y, et al. (2021) Surfactant protein A reduces TLR4 and inflammatory cytokine mRNA levels in neonatal mouse ileum. Sci Rep. 11(1):2593.
- Bejarano PA, Baughman RP, Biddinger PW, Miller MA, Fenoglio-Preiser C, al-Kafaji B, et al. (1996) Surfactant proteins and thyroid transcription factor-1 in pulmonary and breast carcinomas. Mod Pathol. 9(4):445-52.
- Goodall KJ, Poon IK, Phipps S, Hulett MD (2014) Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One. 9(10):e109596.
- Johnson GB, Brunn GJ, Kodaira Y, Platt JL. (2002) Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 168(10):5233-9.
- Lambrecht BN, Hammad H (2010) the role of dendritic and epithelial cells as master regulators of allergic airway inflammation. The Lancet. 376(9743):835-43.
- Li S, Wu J, Zhu S, Liu YJ, Chen J (2017) Disease-Associated Plasmacytoid Dendritic Cells. Front Immunol. 8:1268.
- Smit JJ, Lindell DM, Boon L, Kool M, Lambrecht BN, Lukacs NW (2008) The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One. 3(3):e1720.
- Xu H, Zhang G-X, Ciric B, Rostami A (2008) IDO: a double-edged sword for TH1/TH2 regulation. Immunology letters. 121(1):1-6.
- Gregori S, Bacchetta R, Passerini L, Levings MK, Roncarolo MG (2007) Isolation, expansion, and characterization of human natural and adaptive regulatory T cells. Immunological Tolerance: Springer, p. 83-105.
- Inoue S, Leitner WW, Golding B, Scott D. (2006) Inhibitory effects of B cells on antitumor immunity. Cancer research. 66(15):7741-7.
- Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Current opinion in immunology. 22(2):231-7.
- Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nature Reviews Cancer. 6(5):392.
- Sorrentino R, Morello S, Luciano A, Crother TR, Maiolino P, Bonavita E, et al. (2010) Plasmacytoid dendritic cells alter the antitumor activity of CpG-oligodeoxynucleotides in a mouse model of lung carcinoma. The Journal of Immunology. 1000881.
- Luo J-L, Maeda S, Hsu L-C, Yagita H, Karin M. (2004) Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer cell. 6(3):297-305.
- Li X, Jiang S, Tapping RI. (2010)Toll-like receptor signaling in cell proliferation and survival. Cytokine. 49(1):1-9.
- Leung DW, Cachianes G, Kuang W-J, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 246(4935):1306-9.
- Johnson B, Osada T, Clay T, Lyerly H, Morse M (2009) Physiology and therapeutics of vascular endothelial growth factor in tumor immunosuppression. Current molecular medicine 9(6):702-7.
- Sorrentino R, Morello S, Giordano MG, Arra C, Maiolino P, Adcock IM, et al. (2011) CpG‐ODN increases the release of VEGF in a mouse model of lung carcinoma. International journal of cancer. 128(12):2815-22.
- Bianchi F, Milione M, Casalini P, Centonze G, Le Noci VM, Storti C, et al. (2019) Toll-like receptor 3 as a new marker to detect high risk early stage Non-Small-Cell Lung Cancer patients. Sci Rep. 9(1):14288.
- Cherfils-Vicini J, Platonova S, Gillard M, Laurans L, Validire P, Caliandro R, et al. (2010)Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J Clin Invest. 120(4):1285-97.
- Hennessy EJ, Parker AE, O'Neill LA (2010) Targeting Toll-like receptors: emerging therapeutics? Nature reviews Drug discovery. 9(4):293-307.
- Qureshi N, Takayama K, Ribi E (1982) Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. The Journal of biological chemistry. 257(19):11808-15.
- Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. (2007) the vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science (New York, NY). 316(5831):1628-32.
- De Ridder M, Verovski VN, Chiavaroli C, Van den Berge DL, Monsaert C, Law K, et al. (2006) The radiosensitizing effect of immunoadjuvant OM-174 requires cooperation between immune and tumor cells through interferon-gamma and inducible nitric oxide synthase. International journal of radiation oncology, biology, physics. 66(5):1473-80.
- D'Agostini C, Pica F, Febbraro G, Grelli S, Chiavaroli C, Garaci E (2005) Antitumour effect of OM-174 and cyclophosphamide on murine B16 melanoma in different experimental conditions. International immunopharmacology. 5(7-8):1205-12.
- Przetak M, Chow J, Cheng H, Rose J, Hawkins LD, Ishizaka ST (2003) Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine. 21(9-10):961-70.
- Ishizaka ST, Hawkins LD (2007) E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert review of vaccines. 6(5):773-84.
- G Stöver A, Da Silva Correia J, T Evans J, W Cluff C, W Elliott M, W Jeffery E, et al. (2004) Structure-Activity Relationship of Synthetic Toll-like Receptor 4 Agonists. 4440-9 p.
- Cluff CW, Baldridge JR, Stöver AG, Evans JT, Johnson DA, Lacy MJ, et al. (2005) Synthetic Toll-Like Receptor 4 Agonists Stimulate Innate Resistance to Infectious Challenge. Infection and Immunity. 73(5):3044-52.
- Lee K, Kim SH, Yoon HJ, Paik DJ, Kim JM, Youn J (2011)Bacillus-derived poly-gamma-glutamic acid attenuates allergic airway inflammation through a Toll-like receptor-4-dependent pathway in a murine model of asthma. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 41(8):1143-56.
- Bortolatto J, Borducchi E, Rodriguez D, Keller AC, Faquim-Mauro E, Bortoluci KR, et al. (2008) Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-gamma axis. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 38(10):1668-79.
- Zhou C, Kang XD, Chen Z (2008) A synthetic Toll-like receptor 2 ligand decreases allergic immune responses in a mouse rhinitis model sensitized to mite allergen. Journal of Zhejiang University Science B. 9(4):279-85
- Murata M, Sato T, Miyauchi M, Koga E, Aoki M, Kimura T, et al. (2007) SMP-105, cell-wall skeleton purified from <em>Mycobacterium bovis</em> BCG Tokyo 172, activates innate immunity through TLR2/MyD88 pathway and prevents tumor metastasis into draining lymph nodes. Cancer Research. 67(9 Supplement):3554.
- Akdis CA, Kussebi F, Pulendran B, Akdis M, Lauener RP, Schmidt-Weber CB, et al. (2003) Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 33(10):2717-26.
- Weigt H, Muhlradt PF, Larbig M, Krug N, Braun A (2004) The Toll-like receptor-2/6 agonist macrophage-activating lipopeptide-2 cooperates with IFN-gamma to reverse the Th2 skew in an in vitro allergy model. Journal of immunology 172(10):6080-6.
- Weigt H, Nassenstein C, Tschernig T, Muhlradt PF, Krug N, Braun A (2005) Efficacy of macrophage-activating lipopeptide-2 combined with interferon-gamma in a murine asthma model. American journal of respiratory and critical care medicine. 172(5):566-72.
- Drake MG, Kaufman EH, Fryer AD, Jacoby DB (2012) The therapeutic potential of Toll-like receptor 7 stimulation in asthma. Inflamm Allergy Drug Targets. 11(6):484-91.
- Gkoulioni V, Eleftheriadou A, Yiotakis I, Ferekidou E, Chrisovergis A, Lazaris A, et al. (2010) The efficacy of imiquimod on dysplastic lesions of the oral mucosa: an experimental model. Anticancer research. 30(7):2891-6.
- Isobe Y, Tobe M, Ogita H, Kurimoto A, Ogino T, Kawakami H, et al. (2003) Synthesis and structure-activity relationships of 2-substituted-8-hydroxyadenine derivatives as orally available interferon inducers without emetic side effects. Bioorg Med Chem. 11(17):3641-7.
- Bergmann JF, de Bruijne J, Hotho DM, de Knegt RJ, Boonstra A, Weegink CJ, et al. (2011) Randomised clinical trial: anti-viral activity of ANA773, an oral inducer of endogenous interferons acting via TLR7, in chronic HCV. Alimentary pharmacology & therapeutics. 34(4):443-53.
- Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, et al. (2005) Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. Journal of immunology (Baltimore, Md: 1950). 174(3):1259-68.
- Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. (2005) Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science (New York, NY). 309(5739):1380-4.
- Gupta GK, Agrawal DK (2010) CpG oligodeoxynucleotides as TLR9 agonists: therapeutic application in allergy and asthma. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 24(4):225-35.
- Serebrisky D, Teper AA, Huang CK, Lee SY, Zhang TF, Schofield BH, et al. (2000) CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. Journal of immunology (Baltimore, Md: 1950). 165(10):5906-12.
- Banerjee B, Kelly KJ, Fink JN, Henderson JD, Jr., Bansal NK, Kurup VP (2004) Modulation of airway inflammation by immunostimulatory CpG oligodeoxynucleotides in a murine model of allergic aspergillosis. Infect Immun. 72(10):6087-94.
- Chiang DJ, Ye YL, Chen WL, Lee YL, Hsu NY, Chiang BL (2003) Ribavirin or CpG DNA sequence-modulated dendritic cells decrease the IgE level and airway inflammation. American journal of respiratory and critical care medicine. 168(5):575-80.
- Vollmer J, Krieg AM (2009) Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Advanced drug delivery reviews. 61(3):195-204.
- Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Muller P, et al. (2009) Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 39(4):562-70.
- Overton ET, Goepfert PA, Cunningham P, Carter WA, Horvath J, Young D, et al. (2014) Intranasal seasonal influenza vaccine and a TLR-3 agonist, rintatolimod, induced cross-reactive IgA antibody formation against avian H5N1 and H7N9 influenza HA in humans. Vaccine. 32(42):5490-5.
- Cheng K, Gao M, Godfroy JI, Brown PN, Kastelowitz N, Yin H (2015) Specific activation of the TLR1-TLR2 heterodimer by small-molecule agonists. Sci Adv. 1(3).
- Morris AE, Liggitt HD, Hawn TR, Skerrett SJ (2009) Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol. 297(6):L1112-9.
- Brackett CM, Kojouharov B, Veith J, Greene KF, Burdelya LG, Gollnick SO, et al. (2016) Toll-like receptor-5 agonist, entolimod, suppresses metastasis and induces immunity by stimulating an NK-dendritic-CD8+ T-cell axis. Proc Natl Acad Sci U S A. 113(7):E874-83.
- Fore F, Indriputri C, Mamutse J, Nugraha J (2020) TLR10 and Its Unique Anti-Inflammatory Properties and Potential Use as a Target in Therapeutics. Immune Netw. 20(3):e21.
- Kutuzova GD, Albrecht RM, Erickson CM, Qureshi N (2001) Diphosphoryl lipid A from Rhodobacter sphaeroides blocks the binding and internalization of lipopolysaccharide in RAW 264.7 cells. J Immunol. 167(1):482-9.
- Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN ( 2009) House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nature Medicine. 15(4):410-6.
- Monnet E, Lapeyre G, Poelgeest EV, Jacqmin P, Graaf K, Reijers J, et al.( 2017) Evidence of NI-0101 pharmacological activity, an anti-TLR4 antibody, in a randomized phase I dose escalation study in healthy volunteers receiving LPS. Clinical pharmacology and therapeutics. 101(2):200-8.
- Ungaro R, Fukata M, Hsu D, Hernandez Y, Breglio K, Chen A, et al. (2009) A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. American journal of physiology Gastrointestinal and liver physiology. 296(6):G1167-G79.
- Savov JD, Brass DM, Lawson BL, McElvania-Tekippe E, Walker JK, Schwartz DA (2005) Toll-like receptor 4 antagonist (E5564) prevents the chronic airway response to inhaled lipopolysaccharide. American journal of physiology Lung cellular and molecular physiology. 289(2):L329-37.
- Hennessy E, E Parker A, O'Neill L (2010) Targeting Toll-like receptors: Emerging therapeutics? 293-307 p.
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. (2002) Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 3(2):196-200.
- Mahmutovic-Persson I, Johansson M, Brandelius A, Calven J, Bjermer L, Yudina Y, et al. (2012) Capacity of capsazepinoids to relax human small airways and inhibit TLR3-induced TSLP and IFNbeta production in diseased bronchial epithelial cells. International immunopharmacology. 13(3):292-300.
- Zang N, Xie X, Deng Y, Wu S, Wang L, Peng C, et al. (2011) Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J Virol. 85(24):13061-8.
- Isambert N, Fumoleau P, Paul C, Ferrand C, Zanetta S, Bauer J, et al. (2013) Phase I study of OM-174, a lipid A analogue, with assessment of immunological response, in patients with refractory solid tumors. BMC Cancer. 13:172.
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 458(7242):1191-5.
- Stowell NC, Seideman J, Raymond HA, Smalley KA, Lamb RJ, Egenolf DD, et al. (2009) Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir Res. 10(1):43.
- Novak N, Yu CF, Bieber T, Allam JP. (2008) Toll-like receptor 7 agonists and skin. Drug News Perspect. 21(3):158-65. PubMed PMID: 18560614.
- Tanji H, Ohto U, Shibata T, Taoka M, Yamauchi Y, Isobe T, et al. (2015) Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat Struct Mol Biol. 22(2):109-15.
- Durai P, Shin HJ, Achek A, Kwon HK, Govindaraj RG, Panneerselvam S, et al. (2017) Toll-like receptor 2 antagonists identified through virtual screening and experimental validation. Febs j. 284(14):2264-83.
- Yan L, Liang J, Yao C, Wu P, Zeng X, Cheng K, et al. (2016) Pyrimidine Triazole Thioether Derivatives as Toll-Like Receptor 5 (TLR5)/Flagellin Complex Inhibitors. ChemMedChem. 11(8):822-6.
- Moen SH, Ehrnström B, Kojen JF, Yurchenko M, Beckwith KS, Afset JE, et al. (2019) Human Toll-like Receptor 8 (TLR8) Is an Important Sensor of Pyogenic Bacteria, and Is Attenuated by Cell Surface TLR Signaling. Front Immunol. 10:1209.
- Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, et al. (2005) Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 202(8):1131-9.
- Aryan Z, Holgate ST, Radzioch D, Rezaei N (2014) A New Era of Targeting the Ancient Gatekeepers of the Immune System: Toll-Like Agonists in the Treatment of Allergic Rhinitis and Asthma. International Archives of Allergy and Immunology. 164(1):46-63.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi