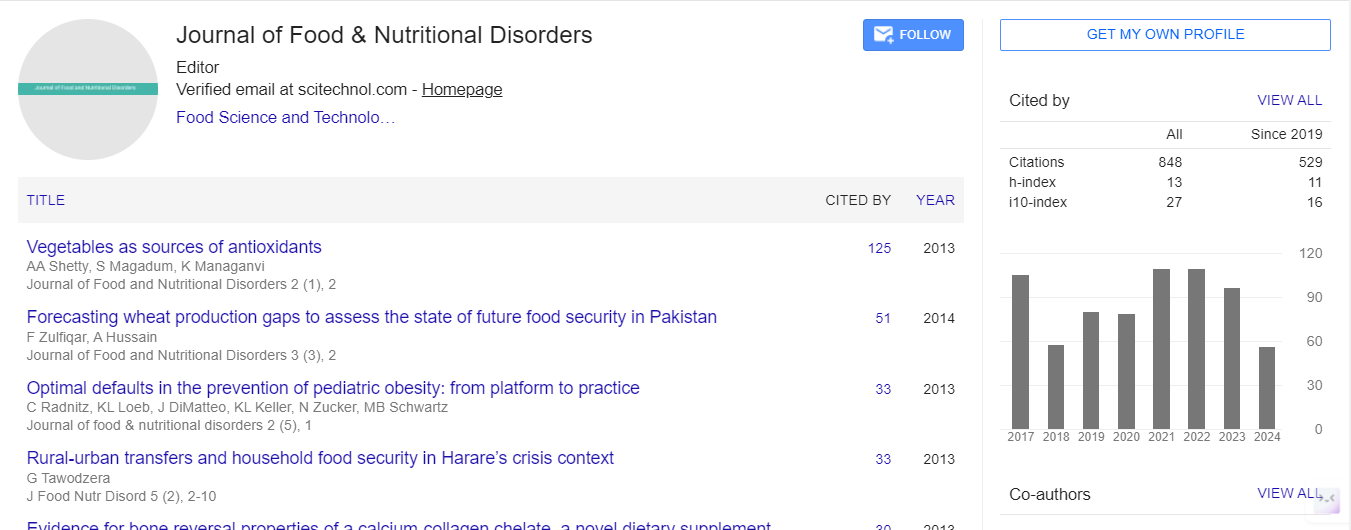
Editorial, J Food Nutr Disor Vol: 1 Issue: 1
Molecular Nutrition, Nutrigenomics and Health Promotion: A Long Road Ahead
| Moul Dey* |
| Molecular Nutrition and Nutrigenomics research program, Department of Health & Nutritional Sciences, South Dakota State University, Box 2203, Brookings, SD 57007, USA |
| Corresponding author : Moul Dey Molecular Nutrition and Nutrigenomics research program, Department of Health & Nutritional Sciences, South Dakota State University, Box 2203, Brookings, SD 57007, USA E-mail: moul.dey@sdstate.edu |
| Received: June 08, 2012 Accepted: June 11, 2012 Published: June 13, 2012 doi: 10.4172/2324-9323.1000e101 |
| Citation: Dey M (2012) Molecular Nutrition, Nutrigenomics and Health Promotion: A Long Road Ahead. J Food Nutr Disor 1:1 doi:10.4172/2325-9701.1000e101 |
Abstract
Molecular Nutrition, Nutrigenomics and Health Promotion: A Long Road Ahead
Advances in “omics”-based fields have produced an explosion of new information, fueling high expectations for improved public and individualized health. Unfortunately, there exists a widening gap between basic biochemistry and omics-based population research, with both disciplines failing to translate their full potential impact to human health applications. In an attempt to address this current situation, the National Institutes of Health (NIH) has made “translational” or “bench-to-bedside” research a priority, forming specialized centers and launching the Clinical and Translational Science Award (CTSA) program in 2006.
Introduction |
|
| Advances in “omics”-based fields have produced an explosion of new information, fueling high expectations for improved public and individualized health. Unfortunately, there exists a widening gap between basic biochemistry and omics-based population research, with both disciplines failing to translate their full potential impact to human health applications. In an attempt to address this current situation, the National Institutes of Health (NIH) has made “translational” or “bench-to-bedside” research a priority, forming specialized centers and launching the Clinical and Translational Science Award (CTSA) program in 2006 [1]. More recently, President Obama signed a spending bill that includes a provision to establish the National Center for Advancing Translational Sciences (NCATS) within the NIH. As a result, basic biomedical research and genomewide association studies (GWAS), the two ends of the translational paradigm, are booming. But their impacts, in terms of new therapies and intervention strategies, are growing at a far more modest pace [2]. Prevention, in particular, has suffered in the United States because of the use of a traditional “medical” model rather than a “health” model. Financing, medical education, and research support have favored disease treatment over prevention. This is ironic since, historically; the greatest gains in physical well-being have come from preventive rather than curative activities [3]. | |
| Nutrigenomics has the potential to lead to evidence-based dietary intervention strategies for restoring health and fitness and preventing disease. It may be viewed as an off-shoot of chemical biology, where nutrients are seen as signals or chemicals that trigger a sequence of reactions leading to changes in gene expression and other biological effects (together termed the “phenotype”) within a specific cell in the body. Recent advances in nutrigenomics are due to the completion of the human genome project and the new biomics technologies that provide the means for simultaneous determination of the expression of many thousands of genes at the 2 mRNA (transcriptomics), metabolite (metabolomics), and protein (proteomics) levels. Genomic and transcriptomic studies are mostly conducted by DNA/RNA microarray technologies, but proteomics and metabolomics do not yet have standardized large-scale procedures. Proteome analysis is usually performed by two- dimensional gel electrophoresis and liquid chromatography-mass spectrometry, while metabolome analysis can be carried out by gas/liquid chromatography-mass spectrometry and liquid chromatography-nuclear magnetic resonance spectroscopy. | |
| These technologies are usually applied in a “differential display” mode, that is, by comparing phenotypes in diseased versus healthy subjects to help achieve better association of clinical phenotypes with the corresponding genotypes. Nutrigenomics data are typically generated on a massive scale and require computational analyses to derive mechanistic understanding of the disease under study [4]. | |
| However, nutrigenomics requires a deep understanding of nutrition, genetics, bioinformatics, human physiology and pathology and biochemistry as well as an expanding array of omics technologies. Therefore, it is often difficult, even for professionals, to connect all the transdisciplinary information together and take it to the next step of translating into preventive approaches for optimizing health, delaying onset of disease, and diminishing disease severity. For example, identification of major as well as subtle genetic differences is the first step in better understanding human variation in response to diet and environment. Subsequently, an intervention discovery platform may identify potential cellular targets. While conventional in vitro and in vivo disease model-based research may be crucial, human specimen acquisition for patient-relevant in vitro models also becomes a critical step. Patient-relevant in vitro disease models can be targeted or personalized to the unique genetic mutations that define a patient’s disease type, progression, and consequently their inherent or acquired drug sensitivity and resistance profiles. However, since protocols for obtaining and processing human specimens are limited, implementation of human tissue research services will be necessary to ensure reproducible and reliable results. Furthermore, examination of a broad range of human specimens of diverse origin with sufficient numbers for the necessary statistical power is critical. The information obtained from studies with human specimens will greatly facilitate the design of clinical trials, assisting in the identification of the smaller patient population that would most likely benefit from the targeted experimental dietary intervention. This could potentially scale down human intervention studies to save millions of research dollars and take us a step closer to deriving the ultimate benefits of nutrigenomics research. | |
Acknowledgements |
|
| The author is supported by the National Institutes of Health grant R00AT4245, USDA/SD-AES grant 328100/318000, as well as by MGP Ingredients, Inc., Atchison, KS. | |
References |
|
|
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi