Review Article, Int J Ment Health Psychiatry Vol: 9 Issue: 1
Melatonin and Schizophrenia: Physiological, Pharmacological and Pathological Approach
Ali Rastqar1*, Niraj Patel1 and Zahra Ghasemzadeh2
1Department of Psychiatry and Neuroscience, Laval University, Quebec, Canada
2Department of Animal Biology, University of Tehran, Tehran, Iran
*Corresponding Author: Ali Rastqar
Department of Psychiatry and Neuroscience, Laval University, Quebec, Canada
Tel: +1-4189988540
E-mail: ali.rastqarfarajzadeh.1@ulaval.cac
Received date: 09 December, 2021, Manuscript No. IJMHP-21-49360;
Editor assigned date: 15 December, 2021, PreQC No. IJMHP-21-49360 (PQ);
Reviewed date: 29 December, 2021, QC No. IJMHP-21-49360;
Revised date: 13 March, 2023, Manuscript No. IJMHP-21-49360 (R);
Published date: 22 March, 2023, DOI: 10.4172/2471-4372.1000244
Citation: Rastqar A, Patel N, Ghasemzadeh Z (2023) Melatonin and Schizophrenia: Physiological, Pharmacological and Pathological Approach. Int J Ment Health Psychiatry 9:1.
Abstract
Schizophrenia includes a wide range of mental disorders that cause social and occupational disabilities, can leads to improper behaviours. Melatonin has not been widely focused on schizophrenic patients. However, the researchers have tried to design the drug to treat, prevent progression of the illness, or at least to minimize its physical and mental subsequent. Efforts for the finding of new approaches or new medications as well as endogen substances, which can improve the disease or even influence to its etiology, will be an outstanding success. Recently, it has proved that melatonin (an endogen neurohormone) can play an important role in various forms from preventing illness onset or can reduce the severity of medications side effects. This review aims to highlight the potential effects of melatonin on multiple aspects of schizophrenia.
Keywords: Melatonin, Schizophrenia, Psychiatric disease, Mental health
Introduction
Schizophrenia (SZ) is a neuropsychiatric disorder affecting over 1% of the world's population, which is characterized by distortions in thinking, perception, emotion, language, sense of self, and behaviour. A complex interaction between genetic susceptibility and environmental insults are likely to alter brain development. Typically, SZ accompanied by other mental problems such as anxiety, depression, or addiction. SZ symptoms are gradually increasing from early adulthood and will be a long-lasting mental disorder. Antipsychotics are one of the most widely used drugs for treatment of SZ. However, recent developments in treatment of schizophrenic patients have heightened the need for improved treatments because many patients continue to have symptoms despite their antipsychotics use. Melatonin has been thought of as a key biomarker in schizophrenia but this role has been challenged, as the melatonin levels in plasma of schizophrenic patient were higher, lower, or similar to healthy controls [1].
Researchers of psychiatric disorders suggest that melatonin has beneficial effects in the developmental etiology, course, and treatment of SZ. Various studies have revealed the role of melatonin in the treatment of SZ on bases of specific nature of some specific schizophrenic symptoms including circadian dysregulation, sleep disturbance, and metabolic disturbances by improving the neuroimmune and oxidative stress factors. Antipsychotic drugs have many adverse effects such as tardive dyskinesia, weight gain, and metabolic dysregulation, which emphasize the adjunctive use of melatonin with routine antipsychotics. Schizophrenic people have low levels of tryptophan in their cerebrospinal fluid. Melatonin interacts with the tryptophan catabolic pathway and its catabolites including Quinolinic (QA), 3-OH-Kynurenine (3HK), Picolinic (PA), Xanthurenic (XA) and Kynurenic Acid (KA) and Anthranilic Acid (AA) and is known to altered in SZ. Elevation of activation of the tryptophan catabolite pathway in SZ causes a depletion of normal tryptophan pool for production of serotonin and melatonin. This alters cognition abilities and motivational processing in the result of the disturbances in the cortex, amygdala and striatum, respectively [2].
Life expectancy downturn is recognized as a significant result of the induction of metabolic syndrome by antipsychotics. This downturn in life expectancy and its term health problems pose a moral dilemma for the clinical psychiatrist. On the other hands, antipsychotic medication is the only treatment available. As SZ is being recognized as an immuno-inflammatory disorder, obesity due to the medicine can interact with the course and management of SZ.
Literature Review
Biosynthesis of melatonin
Melatonin or N-acetyl-5-methoxytryptamine is a neurohormone that is discovered by Lerner and his colleagues. This neurohormone is mainly secreted by the pineal gland in mammals especially humans, but the bone marrow, retina, brain, lens, skin and lymphocytes are another source for melatonin. Secretion of melatonin is under circadian control so that, its level is high during nights and is low during days. Although pineal gland is the principal source of secreted melatonin in human, it is secreted by other organs like retina, skin, bone marrow and other organs. Tryptophan is the main precursor of melatonin. In pineal cells, this amino acid is converted to serotonin and then, via two enzymatic processes, melatonin is produced. In the first step, Arylalkylaminen-Acetyltransferase (AA-NAT) or serotonin N-acetyltransferase that is the rate-limiting enzyme, converts tryptophan to N-acetyl-5-Hydroxytryptamine (N-acetyl-5HT). Then in next step, a methyl group is transferred on obtained N-acetylserotonin by Hydroxyindole-O-Methyltransferase (HIOMT) to produce melatonin hormone. These steps are summarized in Figure 1. Under visible light, melatonin production as a result of NAT inhibition is shut down [3]. Melatonin has a small peak of production at around 4 pm and a much larger peak between approximately 10 pm and 3 am under normal light-dark conditions.
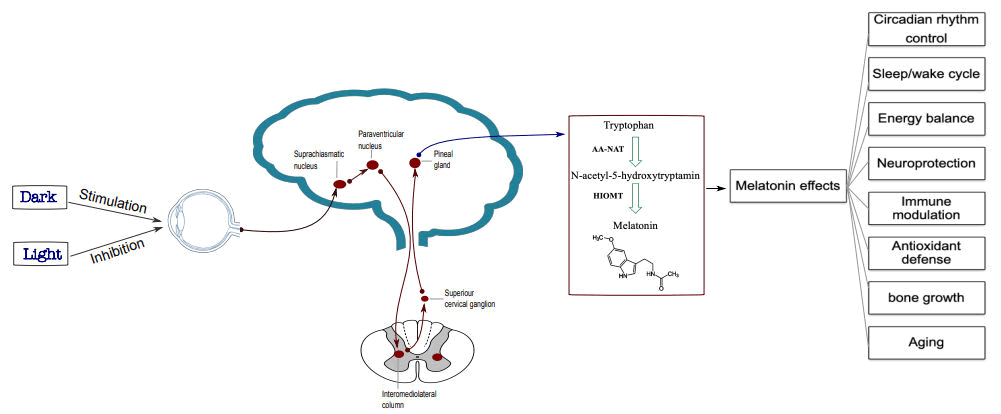
Figure 1: Melatonin synthesis and function.
Melatonin: Receptors, pharmacology and therapeutic perspective
The effects of melatonin (an indoleamine) exert through four ways including interaction with membrane or nuclear receptors, direct binding to cytoplasmic proteins and also as an antioxidant. There is evidence that melatonin receptors widely distributed within the central nervous system and periphery, which suggests its role in various functions of the body. Both MT1 and MT2 melatonin receptors are GProtein Coupled Receptors (GPCRs). There is also MT3 receptor, which belongs to the family of quinone reductases (has not been identified in mammals). In general, melatonin receptors signaling pathways are accompanied by a pertussis-toxin sensitive Gi that leads to inactivation of adenylate cyclase and decreases cAMP levels in target tissues [4].
But recent studies showed that, in different organs, melatonin receptors build mono and heterodimers that are linked to other kinds of GPCRs and activate different signaling pathways that result in various effects instead of diminishing PKA activity. Indoleamine is also known to be a ligand for retinoic acid receptor-Related Orphan Receptor/Retinoid Z Receptor (ROR/RZR). They have a role in the regulation of circadian rhythm, development of CNS and the immune system and also cell differentiation and proliferation. Figure 2 is summarized the signaling pathways of melatonin receptors. Interestingly, it is now well established that diverse functions of melatonin are associated with melatonin receptor oligomerization feature [5].
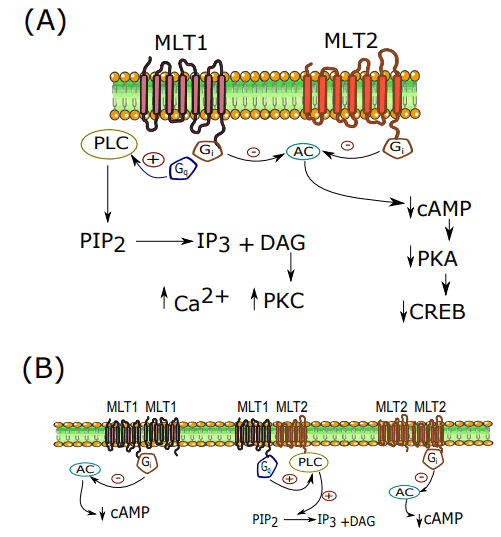
Figure 2: A) Melatonin receptors signaling; B) Melatonin monomeric and heteromeric signaling.
There are some melatonin receptors agonists such as agomelatine and ramelteon that are clinically used or some others such as tasimelteon and TIK-301 that are under assessment to enter into the markets. These are non-selective agonists for melatonin receptors but there are also other detailed lists of selective and non-selective MT1 and MT2 melatonin receptors antagonists that are not the purpose of this paper. In addition, agomelatine (valdoxan) is clinically used as a melatonin receptor agonist and 5-HT2C antagonist in some depressive disorders. However, research on melatonin and its agonists and antagonists in various diseases especially in psychiatric disorders are one of new study field on melatonin hormone [6].
Pathophysiology of schizophrenia
After a century of schizophrenia discovery, its main pathophysiological bases are not fully understood yet. The symptoms of schizophrenia are generally classified into two types: Positive symptoms, including delusions and hallucinations, and Negative symptoms including social isolation, affective dullness, cognitive impairments (working memory deficit, disorganization and inattention) and anhedonia, thought scarcity. Various possible causes for schizophrenia has been proposed such as changes in some neurotransmitter levels (such as dopamine, glutamate and GABA), functional impairment of frontal lobe, loss of prefrontal cortex volume, agenesis of corpus callosum, microcephaly and neurodegeneration, and also heterotopia (ectopic nodules of neurons) [30]. Moreover, abnormal bioenergetics, compromising of the neural development and connectivity, neuroinflammation and also genetic and environmental factors are involved in the pathogenesis of schizophrenia. According to genetic studies, alterations of gene encodeing the L-type voltage-dependent calcium channel subunit are correlated with schizophrenia. In recent years, the neuroinflammation base of schizophrenia etiology governed more attention. Increased peripheral inflammatory markers or increased microglial activity in schizophrenic patients is proven [7].
Melatonin deficit in schizophrenia
Data suggest that melatonin plays a role in the etiology of schizophrenia. In 1994, Rao and co-workers reported significant phase advance of serum tryptophan and melatonin of 1.2 h in drug free SZ patients. In schizophrenic individual, rhythm period of melatonin is longer than 24 h. Moreover, in medicated SZ patients it was shown that melatonin patterns were disrupted. Decreased nocturnal and lack of the typical diurnal variation in secretion pattern of melatonin in schizophrenic patients as well as pineal gland calcification evidence associated with cortical atrophy in CT scan analysis demonstrate the potential role of melatonin in etiology of schizophrenia [ ]. A large body of studies are available that suggest correlation between melatonin secretion and the size and degree of calcification of the pineal gland. Moreover, using Magnetic Resonance Imaging (MRI), Takahashi et al. showed that SZ patients regardless of their illness stage have smaller pineal volumes (both in first episodes and chronic stages). However, in affected individual’s gland volume does not change over time and also with dose or duration of antipsychotic medication. Thus, it seems that decreased pineal volume is not cause of the SZ and may be a risk factor for development of the disease [9].
It has conclusively been shown that melatonin deficiency cause to alteration in the structural and functional properties of neurons in schizophrenic patients. Additionally, decreases in nocturnal plasma melatonin levels have been reported in patients with schizophrenia, which suggests impairment of biosynthetic activity of the pineal gland in this patient. Low concentration of circulating melatonin in schizophrenic patients has also reported. It has been demonstrated that melanogenesis had increased in the schizophrenic patients showing defective melatonin synthesis in these subjects. Low melatonin secretion which seen in SZ patient might be directly or indirectly come from abnormality in the activities of the enzymes involved in its production namely the AA-NAT and the HIOMT. In the brain autopsy of SZ patient (11 people), it has been shown that HIOMT activity increased by about 25% as compared with non-schizophrenic subjects (67 people). They proposed that the observed low concentration of melatonin is associated with substrate lack or low enzyme activity prior to HIOMT. Furthermore, the relation between melatonin deficiency and etiology of schizophrenia has reported by Galvan- Arrieta and co-workers. These researchers showed that during gestation, melatonin (as a neuro-differentiation factor) stimulates neuron maturation. In schizophrenic patients, volume of pineal gland is significantly smaller than normal ones [10]. Axogenesis impairment observed in schizophrenia is related to melatonin deficiency. This evidence together with phase advance in circadian pattern of melatonin secretion and impaired activity of pineal gland in schizophrenic patient indicate this hormone may involve at least in part of schizophrenia’s pathology.
Effects of exogenous melatonin in SZ patients have been studied extensively. In the study conducted by Onaolapo and co-workers, the potential of oral melatonin to reverse ketamine- induced behavioral and brain oxidative stress changes were investigated. The results show the improving effects of exogenous melatonin on social interaction deficits, hyperlocomotion, memory deterioration, and brain antioxidant activity in ketamine- induce schizophrenia-like behavior. Moreover, it was shown that intraperitoneal administration of exogenous melatonin in mice treated with MK-801 (a NMDA receptor antagonist) induces schizophrenic like symptoms and attenuates the histological changes in the prefrontal cortex.
Discussion
Melatonin and the antioxidant activity in schizophrenia
In 2013, Morera-Fumero and Abreu-Gonzalez have reviewed the evidence for the involvement of melatonin in SZ and reported that injections of pineal extracts to schizophrenic patients led to increase in levels of glutathione. Indeed, melatonin cause to elevation in the rate limiting enzyme of the glutathione synthesis, namely γ- glutamylcysteine synthase [11]. The protective effect of melatonin has been shown that is related to increase in Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPx) activity (antioxidant enzymes) and gene expression. Additionally, the specific association between melatonin and the antioxidative effects was further demonstrated via western blot analysis. Receptor-mediated antioxidative effects of melatonin have also reported previously. Wu and his colleagues have found that in ventilator-induced lung injury, melatonin and Ramelteon (a melatonin receptor agonist) exert the antioxidative and anti-inflammatory effects, which the effect decreased by luzindole treatment (a melatonin receptor antagonist). This is further support for the important role of melatonin receptors in the antioxidative and anti-inflammatory effects of melatonin. Melatonin led to Antioxidant Response Elements (ARE) system activation that caused an increase Nrf2 levels and suppressing NF-κB activity. Transcription of many antioxidant proteins and enzymes occurs as a result of ARE activation. Under oxidative stress, melatonin increases Nrf2 levels in cells. Moreover, interactions of Nrf2 with ARE in the nucleus as a consequence of melatonin treatment is also reported. On the other hand, ERK–MAPK/JNK signaling pathway, which has important role in the oxidative stress, modulated by melatonin. Therefore, it seems that many physiological functions of melatonin, in part, mediated via receptor dependent signaling pathways. Melatonin receptor independent antioxidant effects related to its potent direct scavenger of free radicals (singlet oxygen, hydroxyl, peroxyl radicals, hydrogen peroxide, nitric oxide and peroxynitrite) properties. Antioxidative and anti-inflammatory effects of melatonin suppress the influence of oxidative stress regulation on the schizophrenia susceptibility genes. Preeclampsia induced oxidative stress during gestation elevate the risk of schizophrenia at the same time, melatonin levels, melatonin receptors, and the conversion of serotonin to N-Acetylserotonin (NAS) and melatonin by AANAT are noticeably diminished [12].
In the brain cortices, it has been demonstrated that treatment with melatonin results in amelioration of the AChE levels that causes the antischizophrenic effects. Moreover, in 2019, Andrabi and co-workers reported that melatonin treatment causes restoration of cholinergic system results in cognitive deficits improvement. In previous studies on melatonin, neuroprotective effects have been found to be related to elevation of D1 and D2 dopamine receptors activity, modulatory role on GABA transmission and also reduces norepinephrine receptors activity [13].
Melatonin and the immune pathophysiology in schizophrenia
More than a century ago, a probable link between schizophrenia and the immune system was proposed, and epidemiological studies imply associations with infection and systemic inflammation. In schizophrenia patients, several investigators have discovered immune system abnormalities (cellular and/or humoral) as well as infective or autoimmune processes. High levels of circulating immune complexes have been shown to be associated with the acute condition (relapse) of schizophrenia. Furthermore, antipsychotic medication has been shown to have a number of impacts on the immunological profile of schizophrenic patients, and changes in the cellular immune system are linked to treatment response. These changes could be possibilities for disease-related biological indicators. The imbalance between T helper Type 1 (Th1) and T helper Type 2 (Th2) cytokines was confirmed. Increased Th2-like and proinflammatory cytokines, changed in T cell activation, and also a Th1-like pattern occur as results of monocytic activation in SZ, which indicates a mixed immune response. Moreover, neuroinflammation have been reported to increase in SZ patients. The strong anti-inflammatory properties of melatonin were shown as melatonin cause to decreases proinflammatory cytokines and other inflammatory mediators. Variations in endogenous melatonin levels are likely to modulate such factors relevant to the course of schizophrenia because it regulates the immune and circadian systems, as well as being a powerful antioxidant, anti-inflammatory and inducer of mitochondrial oxidative phosphorylation. Melatonin (in addition to being an antioxidant) via the phosphorylation and inhibition of Glycogen Synthase Kinase-3b (GSK-3b) increases the production of Nuclear Factor Erythroid-derived-2 (NF-E2)-related factor (Nrf-2) induced endogenous antioxidants. It also raises levels of the lifespan protein sirtuin-1 in the neurons, implying a role in schizophrenia's significant loss in longevity. Combination of melatonin with antipsychotic drugs through targeting of neuro-inflammation and oxidative stress potentiates efficacy of those drugs [14].
Melatonin and sleep problems in schizophrenia
Sleep disturbance occurs in over 80% of people with schizophrenia, which is strongly linked with severe circadian and melatonin misalignment and it can occur despite stability in mental state and mood.
Slow wave sleep and also REM latency is decreased in SZ patients. Additionally, elevated sleep latency and attenuated total sleep time along with sleep efficiency are reported in SZ patients. It has reported that, in patients with schizophrenia, there is disrupted normal melatonin rhythmicity, reduction of nocturnal secretion of melatonin, and changed melatonin circadian rhythms. However, cortical atrophy and pineal calcification are reported in patient with schizophrenia. The quality of sleep is affected by schizophrenia, like many other psychiatric disorders. Additionally, there are some similarities between delusions, hallucinations, bizarre thinking and perceptual distortions in schizophrenia and dreaming that may link them together through a dopaminergic pathway. Furthermore, melatonin regulates dopamine's circadian cycle. Any directly and indirectly changes in phase of the melatonin circadian rhythm can causes both sleep disruption and circadian rhythm disturbance and may lead to an increase in dopamine activity in the brain.
The function of the prefrontal cortex is tightly related to the quality of sleep and any disruption on it makes worsen schizophrenia, especially in its positive phase. Preclinical investigations have also shown that antagonists of melatonin receptor can inhibit glutamate release in the prefrontal cortex, which has been linked to the pathophysiology of schizophrenia, particularly cognitive symptoms. Furthermore, it has been proven in an animal model of schizophrenia to improve behavioural impairments by decreasing brain oxidative stress. Melatonin has stimulatory effect on production of endogenous antioxidants phosphorylated Glycogen Synthase Kinase-3 (GSK-3) and induces an anti-inflammatory effect. Lower levels of phosphorylated GSK-3 subtype β are associated with a reduction in axogenesis and less expression of melatonergic receptors in schizophrenic patients. Human and animal experimental works suggest that schizophrenia symptoms such as somatic complaints, anxiety, depression, and paranoia are in close relationship with melatonin phase. Then treating circadian disruption might be a good focus for improving the lives and symptoms of schizophrenia patients.
It is found that the decrease in melatonin production at night in schizophrenia. Melatonin agonists have been proven to be effective treatments for schizophrenia-related sleep problems and drug-induced tardive dyskinesia. Participants with schizophrenia displayed a wide variety of circadian sleep wake cycle patterns, from normal to significantly disrupted, as measured by relative amplitude of day/night activity. Also, it is reported that melatonin (2 mg, controlled release) improved sleep efficiency as measured by wrist actimetry in a randomized, blinded, cross over study measuring urinary melatonin output in patients with chronic schizophrenia and assessing the effects of melatonin replacement on their sleep quality. According to a second research, melatonin or a placebo was administered to 19 individuals with DSM-IV schizophrenia throughout the course of two treatment periods of three weeks each, separated by one week. Urine was taken from each subject every three hours between 9:00 p.m. and 9:00 a.m. to measure endogenous melatonin synthesis. Melatonin production was low in all of the patients. When compared to placebo, melatonin replacement enhanced rest derived sleep efficiency and increased REM sleep latency, with the effect being considerably larger among low-efficiency sleepers. Melatonin increases sleep efficiency in schizophrenia patients with poor sleep quality, according to the authors. In a randomized, double-blinded, placebo controlled experiment including 40 stable DSM-IV schizophrenia outpatients with initial insomnia of at least 2 weeks, Kumar and his colleagues found that melatonin enhanced subjective sleep quality. Participants with a low relative amplitude, on the other hand, showed substantially greater activity and alertness during sleep, as well as lower activity day-to-day stability (interdaily stability index) than those with a normal relative amplitude. Another research in schizophrenia patients found that melatonin phases and sleep-wake cycles were significantly delayed and/or free-running, indicating that the circadian rhythms were poorly entrained to the light-dark cycle. The most frequent observed abnormities were reduced sleep efficiency, longer sleep latencies, and increased number of nighttime awakenings in those with schizophrenia. Moreover, polymorphism in the promoter of the gene coding the MT1 melatonin receptor was associated with insomnia symptoms in SZ patients.
Any abnormality in the genes that are involved control of circadian rhythm such as clock, per and cry genes can resulted in sleep disturbance, psychiatric disorders, and many other systemic disease like different forms of cancers. Few studies on circadian clock gene polymorphisms such as Clock 311C/T polymorphism, which is linked with higher dopaminergic neurotransmission in the SCN, have been shown in patients with schizophrenia. When compared to healthy controls, iPeriod-1 mRNA expression in the temporal lobe of postmortem schizophrenia patients was shown to be down regulated. It has been found lower amounts of the CSNK1 protein in the prefrontal cortex of schizophrenia patients. Consequently, the usage of melatonin in combination with psychotic drugs, can improve mood and social behavior of patients. However, the link between schizophrenia and circadian genes has to be addressed through largerscale research.
Melatonin as adjunctive into antipsychotic drugs
First generation of antipsychotics like dopamine agonists is effective at reducing the positive symptoms of schizophrenia, such as hallucinations, disordered thinking, and delusions by blocking the D2, D3, and D4 receptors. Second-generation antipsychotics operate by inhibiting the receptors of dopamine and serotonin. Melatonin, used in conjunction with antipsychotics, may improve medication efficacy by addressing immuno-inflammation and oxidative stress. In vivo and in vitro studies showed that melatonin concentrations increased following antipsychotic treatment. In drug-free SZ patients, an atypical antipsychotic treatment (olanzapine) had no effect on the melatonin circadian rhythm. Moreover, in healthy individuals’ treatment with an atypical antipsychotic quetiapine could not influence melatonin secretion. Antipsychotic drugs cause high blood pressure and metabolic syndrome in psychotic patients while melatonin as a vasodilator can lower the blood pressure in those patients. In addition, melatonin increases mitochondrial oxidative phosphorylation so it counteracts the weight gaining due to psychotic drugs. Melatonin by inhibition of antipsychotic-induced leptin resistance is able to control the disturbance of immune-anti-inflammatory that cause schizophrenia. In one study, ramelteon (agonist of melatonin) could improve the metabolic syndrome effects. Also, agomelatine acts as an anxiolytic and antidepressant and can be used in treatment of depression associated with schizophrenia; these drugs do not have the same potency, efficiency, and efficacy as melatonin has. Maybe in part, melatonin induces its effect via other physiological mechanisms. Adding of melatonin to antipsychotic drugs will enhance the efficacy of those drugs via its anti-inflammatory and anti-oxidative and will significantly diminish the recurrence of disease and drug side effects.
Conclusion
On the base of clinical, experimental, and animal studies, it is clear that melatonin and its agonists (as marketed drugs or pre-clinical and experimental substances) and other drugs that affect on melatonin receptors or melatonergic pathways. It seems that the beneficial effects of melatonin in schizophrenic patient rise from its modulatory effects on dopaminergic and serotoninergic pathways, and also antioxidant actions of melatonin. Melatonin acts through melatonin receptors, nuclear receptors, directly with cytoplasmic proteins and activates the ERK-MAPK/JNK and Nrf2-ARE signaling pathway, and also affects on NF-κB. They can reduce side effects of antipsychotic medications as well as sleep disturbance (at the same time, sleep abnormality induced disorders), antipsychotic-induced obesity and metabolic disturbances, and leptin resistance and inhibit the progression of the illness. As no significant side effects of melatonin or melatonergic complements have been reported, utilisation of them as adjunctive in antipsychotic drugs or using them in prescription along with antipsychotics will cease or at least attenuate adverse effects of those drugs and improve quality of life.
Funding
No funding required.
Conflict of Interest
None.
Authors Contribution
All authors wrote the manuscript. All authors contributed to the editing and reviewing of the manuscript.
References
- Morera-Fumero AL, Abreu-Gonzalez P (2013) Role of melatonin in schizophrenia. Int J Mole Sci 14: 9037-9050.
[Crossref] [Google Scholar] [PubMed]
- Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, et al. (2014) Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res 53: 133-140.
[Crossref] [Google Scholar] [PubMed]
- Mahmood D, Muhammad BY, Alghani M, Anwar J, el-Lebban N, et al. (2016) Advancing role of melatonin in the treatment of neuropsychiatric disorders. Egypt J Basic Appl Sci 3: 203-218.
- Wang AK, Miller BJ (2018) Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull 44: 75-83.
[Crossref] [Google Scholar] [PubMed]
- Kanchanatawan B, Sirivichayakul S, Thika S, Ruxrungtham K, Carvalho AF, et al. (2017) Physio-somatic symptoms in schizophrenia: Association with depression, anxiety, neurocognitive deficits and the tryptophan catabolite pathway. Metab Brain Dis 32: 1003-1016.
[Crossref] [Google Scholar] [PubMed]
- Anderson G, Maes M (2017) Interactions of tryptophan and its catabolites with melatonin and the alpha 7 nicotinic receptor in central nervous system and psychiatric disorders: Role of the aryl hydrocarbon receptor and direct mitochondria regulation. Int J Tryptophan Res 10: 1178646917691738.
[Crossref] [Google Scholar] [PubMed]
- de Hert MARC, Schreurs V, Vancampfort D, van Winkel RUUD (2009) Metabolic syndrome in people with schizophrenia: A review. World Psychiatry 8: 15-22.
[Crossref] [Google Scholar] [PubMed]
- Malhotra N, Grover S, Chakrabarti S, Kulhara P (2013) Metabolic syndrome in schizophrenia. Indian J Psychol Med 35: 227-240.
[Crossref] [Google Scholar] [PubMed]
- Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, et al. (2010) International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 62: 343-380.
[Crossref] [Google Scholar] [PubMed]
- Ganguly S, Coon SL, Klein DC (2002) Control of melatonin synthesis in the mammalian pineal gland: The critical role of serotonin acetylation. Cell Tissue Res 309: 127-137.
[Crossref] [Google Scholar] [PubMed]
- Macchi MM, Bruce JN (2004) Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol 25: 177-195.
[Crossref] [Google Scholar] [PubMed]
- Reiter RJ (1991) Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr Rev 12: 151-180.
[Crossref] [Google Scholar] [PubMed]
- Chrustek A, Olszewska-Slonina D (2021) Melatonin as a powerful antioxidant. Acta Pharm 71: 335-354.
[Crossref] [Google Scholar] [PubMed]
- Barrett P, Conway S, Morgan PJ (2003) Digging deep-structure-function relationships in the melatonin receptor family. J Pineal Res 35: 221-230.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi