Review Article, J Clin Exp Oncol Vol: 6 Issue: 5
Mcl-1 is a Gate Keeper Regulating Cell Death in Cancer Cells
Yongqiang Chen1 and Spencer B Gibson1,2*
1Research Institute in Oncology and Hematology, CancerCare Manitoba, Canada
2Department of Biochemistry and Medical Genetics, Faculty of Health Sciences, University of Manitoba, Canada
*Corresponding Author : Spencer B Gibson
Department of Biochemistry and Medical Genetics, Faculty of Health Sciences, University of Manitoba, Winnipeg, MB R3E 0V9, Canada
Tel: 2047872051
Fax: 2047872190
E-mail: spencer. gibson@umanitoba.ca
Received: August 08, 2017 Accepted: August 16, 2017 Published: August 23, 2017
Citation: Chen Y, Gibson SB (2017) Mcl-1 is a Gate Keeper Regulating Cell Death in Cancer Cells. J Clin Exp Oncol 6:5. doi: 10.4172/2324-9110.1000197
Abstract
Selectively inducing cancer cells to death is the goal of cancer therapy. The discovery of B-cell lymphoma 2 (Bcl-2) family members regulating apoptotic cell death of cancer cells revealed new targets for cancer therapy. Bcl-2 family members can be classified into pro-apoptotic members and anti-apoptotic members among which myeloid cell leukemia 1 (Mcl-1) plays unique roles in regulating cell death and survival in cancer cells. Mcl-1 has a short half-life due to its degradation by multiple E3 ubiquitin-ligases. Under hypoxic conditions, Mcl-1 is up-regulated by activation of growth factor receptor, EGFR promoting cell survival whereas, prolonged/severe hypoxia leads to deactivation of EGFR and Mcl-1 degradation by E3 ubiquitin -ligase FBW7 contributing to cell death. Furthermore, cancer cells upregulated Mcl-1 contributing resistance to chemotherapeutic treatment such as by inhibitors of other antiapoptotic Bcl-2 members Bcl-2, Bcl-xL and Bcl-w. Therefore, Mcl- 1 might be the key player in pro-survival Bcl-2 family members in regulating cancer cell death. This encourages the on-going active development of Mcl-1 specific inhibitors for cancer treatment.
Keywords: Myeloid cell leukemia 1 (Mcl-1); B-cell lymphoma 2 (Bcl-2); Cell death; Autophagy; Hypoxia
Introduction
Cancer is a devastating deadly disease causing millions of death worldwide. Chemo- and radio- therapies have been used to kill cancer cells. Unfortunately, cancer cells frequently develop resistance to these treatments. Many pro-cell survival proteins have been shown to contribute to rapid proliferation and treatment resistance of cancer cells, among which the myeloid cell leukemia 1 (Mcl-1) has received intensive attention in recent years. Mcl-1 is an important anti-apoptotic protein belonging to the B-cell lymphoma 2 (Bcl-2) family that includes anti-apoptotic members such as Bcl-2, Bcl-xL, Bcl-w and Mcl-1, and pro-apoptotic members such as Bax, Bak, Bim, Bid and BNIP3 [1]. The expression of Mcl-1 can be transcriptionally regulated by many signal transduction pathways such as MEK/ERK, p38 MAPK, PI3K/AKT, JAK/STAT, and MAPK/Elk-1 [2,3]. Mcl-1 can also be modified post-transcriptionally by alternative splicing and phosphorylation. The alternative splicing of Mcl-1 gene generates three splice variants including the full length Mcl-1, Mcl-1 short (Mcl-1S) which only has a BH3 domain, and Mcl-1extra short (Mcl-1ES) which results from the first exon splicing and losses the PEST motif [3-8] (Figure 1). Different from the full length anti-apoptotic Mcl-1, Mcl-1S and Mcl-1ES play a pro-apoptotic role [4,5,7,9]. The phosphorylation of Mcl-1 might have different outcomes. Mcl-1 phosphorylation by TPK-induced ERK activation was observed in viable cells whereas the taxol- or okadaic acid -induced Mcl-1 phosphorylation was found in dying cells [3]. Mcl-1 plays an important role in various types of cancer. It protects cancer cells from death although its overexpression does not increase cell proliferation [10]. In this review, we discuss the recent progress made in studying the mechanistic role of MCL-1 in cancer cell death.
Types of cell death
The aim of cancer therapy is to selectively kill cancer cells making it essential to know how cancer cells die. There are three main types of cell death; apoptosis, necrosis and autophagic cell death. Apoptosis is a type of programmed cell death (Type I programmed cell death) characterized by cell shrinkage, plasma membrane blebbing, chromatin condensation, DNA fragmentation, formation of apoptotic bodies and caspase activation. Necrosis is an unregulated cell death caused by an accidental damage to cells by extracellular insults. Autophagic cell death is induced by prolonged or high level of autophagy in cells leading to irreversible digestion of cellular structures leading to death. Here, autophagy refers to macroautophagy which has being studied most extensively and is characterized by the formation of a doublemembraned structure autophagosome. Autophagosome fuses lysosome into autolysosome where intracellular materials are degraded.
In recent years, studies of cancers and other diseases has revealed other cell death pathways which include necroptosis, pyroptosis, entosis, mitotic catastrophe and ferroptosis. Necroptosis is a programmed form of necrosis which activation involves the kinase activity of RIP1 [11]. Pyroptosis is a type of caspase 1-dependent programmed cell death that is activated by inflammatory stimuli and featured by rapid rupture of plasma membrane [12]. Entosis refers to the death of one cell engulfed by another cell [13]. Mitotic catastrophe is a type of cell death induced by abnormal entry of cells into mitosis [14]. Ferroptosis is a type of cell death induced by the activity of oxidized form of iron (ferric iron) and subsequent generation of reactive oxygen species [15].
Mcl-1 is unique among Bcl-2 family protein members
Bcl-2 family protein members include anti-apoptotic members such as Bcl-2, Bcl-xL Bcl-w and Mcl-1, and pro-apoptotic members such as Bax, Bak, and BH3-only members such as Bim, Bid and BNIP3 [1] (Figure 1). Similar to Bcl-2, Bcl-xL and Bcl-w, Mcl-1 has three Bcl-2 homology (BH) domains (BH1-BH3) and the carboxyterminal hydrophobic transmembrane (TM) domain (Figure 1). These anti-apoptotic members perform their pro-survival role through protein-protein interactions with pro-apoptotic members by inserting the BH3 helix of a pro-apoptotic protein into the groove formed by the BH1, BH2 and BH3 helices of an anti-apoptotic protein [16]. Compared to other Bcl-2 family members, Mcl-1 has a distinctive large N-terminus containing PEST sequences which is not required for BH3 binding but plays a role in Mcl-1 regulation and degradation [1,16-19]. It might be possible that this region is involved in an undiscovered function of Mcl-1. The pro-apoptotic members Bax and Bak oligomerize to induce mitochondrial outer membrane permeabilization (MOMP) leading to release of apoptotic factors such as cytochrome c to induce cell death [20]. The BH3-only members induce cell death either by activating Bax and Bak or by inhibiting anti-apoptotic members [17,20].
Mcl-1 has a high affinity of BH3 peptide-binding specificity for Bax, Bak, Bim, Bid, Puma and Noxa compared to other Bcl- 2 pro-survival family members indicating that cancer cells might use Mcl-1 as the most efficient guardian against stresses such as chemotherapeutic drugs [17]. This is supported by the finding that cancer cells over-express Mcl-1 to resist the treatment by ABT-737, a small molecule inhibitor antagonizing the three pro-survival proteins Bcl-2, Bcl-xL and Bcl-w [17,21,22].
Mcl-1 has a short half-life of less than 4 h [16]. Our recent studies demonstrated that when U87 cells were treated under hypoxia for a 72-h time course, Mcl-1 protein level increased up to 2 fold at 4 h, when cells did not die, and then dramatically decreased to about 10% at 72 h, when 40% of cells died [23]; in contrast, the levels of Bcl-xL [23] and Bcl-2 [24] did not change significantly over the 72-h time course. It is possible that other pro-survival Bcl-2 members such as Bcl-2, Bcl-xL and Bcl-w protect cells from death when Mcl-1 protein level dropped.
Pro-cell survival mechanisms of Mcl-1
Mcl-1 is known to play a pro-cell survival role. It has been shown to inhibit apoptosis and autophagic cell death (Figure 2). Mcl-1 inhibits apoptosis by binding to the apoptosis effectors tBid, Bim, Puma, and Bak [18,25] and by inhibiting Bax function at mitochondria independent of the interaction between Mcl-1 and Bax [26]. Disruption of the interaction between Mcl-1 and these apoptosis effectors led to apoptosis. For example, the BH3 mimetic pan Bcl-2 small molecule inhibitor Obatoclax inhibits the interaction between Mcl-1 and Bak to induce apoptotic cell death [27].
Although autophagy plays a pro-cell survival role in most stress conditions such as starvation, its over-activation can lead to cell death which is called autophagic cell death [28]. The pro-cell survival function of autophagy can be shifted to a pro-cell death role through the inhibition of an autophagy suppressor [24,29]. Mcl-1 was demonstrated to inhibit autophagy (macroautophagy) by interacting with the autophagy protein Beclin 1 preventing the formation of autophagosome [30-32]. Autophagic cell death is expected to be induced by the loss of Mcl-1/Beclin 1 interaction. This is supported by the report that sorafenib and SC-59 induced autophagic cell death by destroying the interaction between Mcl-1 and Beclin 1 [32].
Necrosis occurs independent of Mcl-1. Interestingly, Bax and Bak mediated necroptosis led to reduction of Mcl-1 protein [33]. The role of Mcl-1 in other emerging types of cell death is unknown. Based upon its role in apoptosis and autophagy, it is reasonable to suggest the Mcl-1 will have similar pro-survival functions against these types of cell death. This is currently under active investigation.
Pro-cell death mechanisms of Mcl-1S and Mcl-1ES
The full length Mcl-1 protein has a role in anti-apoptosis and antiautophagic cell death. However, the Mcl-1 splice variants Mcl-1S and Mcl-1ES are pro-apoptotic (Figure 2). Mcl-1S plays its pro-apoptotic role by forming dimerization with and antagonizing Mcl-1 to effect downstream caspase [4]. Mcl-1ES induced mitochondrial apoptosis that is Mcl-1 dependent but independent of Bax and Bak [9]. This provides a counter balance to Mcl-1 expression in cells regulating cell death. The roles of Mcl-1S and Mcl-1ES in other types of cell death are still unknown.
Mcl-1 up-regulation in cancer cells
Analysis of published datasets indicates that the mRNA level of Mcl-1 is upregulated in human glioblastoma tissues [23] compared to that in normal human brain tissues. This is consistent with the fact that Mcl-1 protein is over expressed in various types of cancers [10]. The reasons for this up regulation is multifaceted but generally are believed to be due to increased transcription through growth factor receptors. We and others have demonstrated that over-expression of epidermal growth factor receptor (EGFR) family members increased both mRNA and protein levels of Mcl-1. This upregulation is sufficient to protect cells from both death receptor and DNA damage induced apoptosis. Furthermore, the mRNA levels of Mcl-1 in various types of human tumor tissues are significantly higher than that of Bcl-2, Bcl-xL or Bcl-w when normalized to corresponding normal tissues (Table 1).
| Type of tumor | A | B | C | D | E | F | |
|---|---|---|---|---|---|---|---|
| Dataset | TCGA Brain | TCGA Ovarian | TCGA Breast | Taylor Prostate 3 | Peng Head-Neck | Biewenga Cervix | |
| Number of cancer patients | 542 | 586 | 76 | 131 | 57 | 40 | |
| Normal tissue (total number) | Brain (10) |
Ovary (8) |
Breast (61) |
Prostate Gland (29) | Oral Cavity (22) | Cervix uteri (5) | |
| Fold (mRNA) | Mcl-1 | 5.085 | 2.734 | 1.355 | 1.296 | 1.228 | 1.656 |
| Bcl-2 | -1.037 | 1.058 | -2.017 | -1.375 | -1.038 | 1.138 | |
| Bcl-xL | -1.013 | 1.325 | 1.49 | 1.023 | 1.18 | 1.079 | |
| Bcl-w | -5.513 | -1.329 | 1.058 | -1.095 | -1.121 | 1.064 | |
| P value | Mcl-1 | 7.23E-08 | 0.002 | 2.00E-04 | 6.39E-05 | 7.00E-03 | 2.00E-03 |
| Bcl-2 | 0.802 | 0.095 | 1 | 1 | 0.735 | 0.026 | |
| Bcl-xL | 0.565 | 3.34E-05 | 1.59E-09 | 2.73E-01 | 8.42E-04 | 2.67E-01 | |
| Bcl-w | 1 | 0.996 | 0.162 | 0.998 | 0.977 | 0.142 | |
Table 1: The mRNA levels of Mcl-1, Bcl-2, Bcl-xL and Bcl-w in some types of human tumor tissues compared to that in corresponding normal tissues. Data were extracted from Oncomine database.
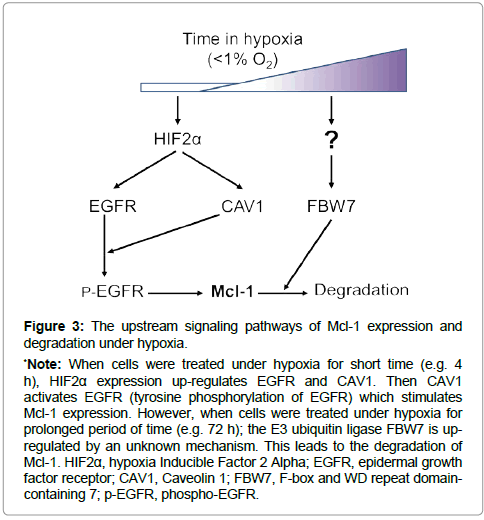
Most in vitro studies in the literature have been performed in atmospheric condition of greater than 20% oxygen. In contrast, the in vivo condition in different tissues of the human body is a hypoxic environment with various oxygen concentration ranging from 1.1% in the superficial region of skin to 9.5% in the kidney [34]. Furthermore, the oxygen concentration in solid tumors is much lower than that in the corresponding normal tissue [35]. Therefore, it is a vital topic to investigate the biology of cancer cells under hypoxia. It is well known that hypoxia can promote tumorigenesis. The hypoxiainducible factors (HIFs) can stimulate both cell survival and cell death pathways depending upon the context [36]. The mRNA and protein levels of Mcl-1 were reported to increase by hypoxia treatment in the human hepatoma cell line HepG2 [37]. Our recent study showed that the protein level of Mcl-1 was increased when glioma (U87) cells were treated under hypoxia (less than 1% of oxygen) for short time (4 h) [23]. Knockdown of Mcl-1 promoted cell death supporting that the upregulated Mcl-1 plays a pro-cell survival role. The upstream mechanism of Mcl-1 elevation in hypoxia can be attributed by the lipid raft protein caveolin-1 (CAV1) and epidermal growth factor receptor (EGFR) (Figure 3). Hypoxia induces the expression of hypoxia-inducible factor 2 alpha (HIF2α) leading to the upregulation of CAV1 and EGFR proteins [38,39]. CAV1 activates EGFR tyrosine kinase in a ligand-independent manner [39]. Then EGFR activation stimulates Mcl-1 expression possibly via the MAPK-Elk-1 signaling [2], protecting cells from hypoxia-induced cell death.
Mcl-1 degradation
Regulation of Mcl-1 expression goes beyond mRNA and protein levels. Mcl-1 protein is rapidly degraded. Indeed, cell death is elevated by the degradation of Mcl-1 when its transcription level of Mcl-1 remains unchanged. Mcl-1 degradation is induced by the ubiquitinproteasome dependent-and independent- pathways [18,40]. This degradation is regulated by multiple E3 ubiquitin-ligases including Noxa, MULE (Mcl-1 Ubiquitin Ligase E3), β-TrCP (beta transducincontaining protein), FBW7, Trim17 and CDC20 [40]. Interestingly, a deubiquitinase in the USP family, USP9X (ubiquitin specific peptidase 9 X-linked), can antagonize the ubitiquin degradation of Mcl-1 [40,41]. The protein level of Mcl-1 decreases with prolonged treatment of cells under hypoxia [23,42,43] (Figure 3). When cancer cells underwent prolonged hypoxia treatment, EGFR de-activation led to less Mcl-1 protein expression [23]. Furthermore, increased expression of FBW7 led to more Mcl-1 protein to be degraded [23]. These dual effects caused rapid loss of Mcl-1 protein during prolonged hypoxia, promoting cell death. The roles of other E3 ubiquitin ligases, deubiquitinase, and ubiquitin-independent degradation pathways in Mcl-1 degradation in hypoxia and other stress conditions need to be further investigated.
*Note: When cells were treated under hypoxia for short time (e.g. 4 h), HIF2α expression up-regulates EGFR and CAV1. Then CAV1 activates EGFR (tyrosine phosphorylation of EGFR) which stimulates Mcl-1 expression. However, when cells were treated under hypoxia for prolonged period of time (e.g. 72 h); the E3 ubiquitin ligase FBW7 is upregulated by an unknown mechanism. This leads to the degradation of Mcl-1. HIF2α, hypoxia Inducible Factor 2 Alpha; EGFR, epidermal growth factor receptor; CAV1, Caveolin 1; FBW7, F-box and WD repeat domaincontaining 7; p-EGFR, phospho-EGFR.
Figure 3: The upstream signaling pathways of Mcl-1 expression and degradation under hypoxia.
Therapeutic targeting Mcl-1 for cancer treatment
Since a pro-survival Bcl-2 family protein counteracts apoptosis by binding to the BH3 region of a pro-apoptotic protein such as Bax and Bak [16], BH3 mimetic anti-cancer drugs have being actively developed. For example, ABT-737 and its derivative ABT- 263 (Navitoclax) bind to Bcl-2, Bcl-xL and Bcl-w [16]. ABT-199 (Venetoclax), an engineered version of ABT-263, was more recently developed to selectively bind Bcl-2 [44].
Mcl-1 has been shown to contribute to tumorigenesis, metastasis, and resistance to traditional and targeted therapies [45-47]. Some effective drugs induce cell death in cancer cells by degrading Mcl- 1 [48-52]. Chemotherapeutic drugs are more effective in killing Mcl-1-dependent leukemia cells than in killing Bcl-2-dependent counterparts [48]. However, upregulation of Mcl-1 has been used by cancer cells to resist treatments such as by BCL-2/BCL-xL inhibitors [45]. In contrast, Mcl-1 splicing variants Mcl-1S and Mcl-1ES antagonize Mcl-1 to play a pro-apoptotic role in cancer cells. This provides rationale to develop drugs that inhibit Mcl-1 interactions allowing cancer cells dependent on Mcl-1 expression to die.
The development of selective Mcl-1 inhibitors targeting proteinprotein interactions between Mcl-1 and apoptosis effectors such as BIM and BAK are under active development [16,45]. It is worthy to mention that the small-molecule Mcl-1 inhibitor, AMG 176, has been the first of its kind to be put into clinical development (Phase I clinical trial) whereas many other potential small-molecule Mcl-1 inhibitors are still in preclinical development stage [45]. Due to the ability of Mcl-1 to alter its expression through alteration in transcription, alternative splicing and degradation, the development of Mcl-1 inhibitors could be used in combination with inhibition of other Bcl- 2 family members to prevent drug resistance in cancer.
The current drug development of Mcl-1 small-molecule inhibitors are mainly focusing on targeting apoptotic cell death. Future drug development should also target the roles of Mcl-1 in other cell death pathways such as the interaction between Mcl-1 and Beclin 1 in an autophagic cell death, especially when cancer cells become resistant to apoptosis. In addition, effective drugs that target the degradation of Mcl-1, would also be effective at killing cancer cells [48-52]. E3 ubiquitin-ligases Noxa, MULE, β-TrCP, FBW7, Trim17 and CDC20 are involved in Mcl-1 degradation [23,40]. By understanding the relative roles of these enzymes in Mcl-1 degradation, novel drug targets could be developed. Indeed, development of drugs targeting the deubiquitinase of Mcl-1 such as USP9X show signs of being a promising strategy [10].
Conclusion
Mcl-1 plays a vital role in promoting cell survival and inhibiting chemotherapeutic drugs-induced cell death in cancer cells. Compared to other anti-apoptotic Bcl-2 family members such as Bcl-2, Bcl-xL and Bcl-w, Mcl-1 is more robustly up-regulated in various cancer tissues in related to corresponding normal tissues. Furthermore, Mcl- 1 is upregulated by hypoxic environment and in drug resistant cancer cells. On the other hand, some effective chemotherapeutic drugs or targeted therapies against Bcl-2 family members led to degradation of Mcl-1 or disruption of the interaction between Mcl-1 and an apoptotic effector. This suggests that Mcl-1 is a gate-keeper regulating cell death and survival through its expression level and interactions. Developing specific Mcl-1 inhibitors that disrupt Mcl-1 interactions, or induce its degradation, could be effective alone or in combination with chemotherapy or pro-survival Bcl-2 family member inhibitors in overcoming drug resistance in cancer.
References
- Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13: 1899-1911.
- Booy EP, Henson ES, Gibson SB (2011) Epidermal growth factor regulates Mcl-1 expression through the MAPK-Elk-1 signalling pathway contributing to cell survival in breast cancer. Oncogene 30: 2367-2378.
- Craig RW (2002) MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia 16: 444-454.
- Bae J, Leo CP, Hsu SY, Hsueh AJ (2000) MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem 275: 25255-25261.
- Bingle CD, Craig RW, Swales BM, Singleton V, Zhou P, et al. (2000) Exon skipping in Mcl-1 results in a bcl-2 homology domain 3 only geneproduct that promotes cell death. J Biol Chem 275: 22136-22146.
- Giménez-Cassina A, Danial NN (2015) Regulation of mitochondrial nutrient and energy metabolismby BCL-2 family proteins. Trends Endocrinol Metab 26: 165-175.
- Kim JH, Sim SH, Ha HJ, Ko JJ, Lee K, et al. (2009) MCL-1ES, a novel variant of MCL-1, associates with MCL-1L and induces mitochondrial cell death. FEBS Lett 583: 2758-2764.
- Morciano G, Pedriali G, Sbano L, Iannitti T, Giorgi C, et al. (2016) Intersection of mitochondrial fission and fusion machinery with apoptoticpathways: Role of Mcl-1. Biol Cell 108: 279-293.
- Kim JH, Bae J (2013) MCL-1ES induces MCL-1L-dependent BAX- and BAK-independent mitochondrial apoptosis. PLoS One 8: e79626.
- Quinn BA, Dash R, Azab B, Sarkar S, Das SK, et a. (2011) Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs 20: 1397-1411.
- Zhou W, Yuan J (2014) Necroptosis in health and diseases. Semin Cell Dev Biol 35: 14-23.
- Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99-109.
- Kroemer G, Perfettini JL (2014) Entosis, a key player in cancer cell competition. Cell Res 24: 1280-1281.
- Mc Gee MM (2015) Targeting the Mitotic Catastrophe Signaling Pathway in Cancer. Mediators Inflamm 2015: 146282.
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, et al. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060-1072.
- Belmar J, Fesik SW (2015) Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacol Ther 145: 76-84.
- Ertel F, Nguyen M, Roulston A, Shore GC (2013) Programming cancer cells for high expression levels of Mcl1. EMBO Rep 14: 328-336.
- Thomas LW, Lam C, Edwards SW (2010) Mcl-1; the molecular regulation of protein function. FEBS Lett 584: 2981-2989.
- Packham G, Stevenson FK (2005) Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology 114: 441-449.
- Cosentino K, García-Sáez AJ (2017) Bax and Bak Pores: Are We Closing the Circle? Trends Cell Biol 27: 266-275.
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, et al. (2006) Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10: 375-388.
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, et al. (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10: 389-399.
- Chen Y, Henson ES, Xiao W, Shome E, Azad MB, et al. (2016) Bcl-2 family member Mcl-1 expression is reduced under hypoxia by the E3 ligase FBW7 contributing to BNIP3 induced cell death in glioma cells. Cancer Biol Ther 17: 604-613.
- Chen Y, Henson ES, Xiao W, Huang D, McMillan-Ward EM, et al. (2016) Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy 12: 1029-1046.
- Bose P, Rahmani M, Grant S (2012) Coordinate PI3K pathway and Bcl-2 family disruption in AML. Oncotarget 3: 1499-1500.
- Germain M, Milburn J, Duronio V (2008) MCL-1 inhibits BAX in the absence of MCL-1/BAX Interaction. J Biol Chem 283: 6384-6392.
- Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, et al. (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A 104: 19512-19517.
- Henson E, Chen Y, Gibson S (2017) EGFR Family Members' Regulation of Autophagy Is at a Crossroads of Cell Survival and Deathin Cancer. Cancers (Basel) 9: E27.
- Chen Y, Gibson SB (2017) How Autophagy Kills Cancer Cells? SM Clin Med Oncol 1: 1002.
- Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, et al. (2007) Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy 3: 561-568.
- Germain M, Nguyen AP, Le Grand JN, Arbour N, Vanderluit JL, et al. (2011) MCL-1 is a stress sensor that regulates autophagy in a developmentally regulated manner. EMBO J 30: 395-407.
- Tai WT, Shiau CW, Chen HL, Liu CY, Lin CS, et al. (2013) Mcl-1-dependent activation of Beclin 1 mediates autophagic cell death induced by sorafenib and SC-59 in hepatocellular carcinoma cells. Cell Death Dis 4: e485.
- Karch J, Kanisicak O, Brody MJ, Sargent MA, Michael DM, (2015) Necroptosis Interfaces with MOMP and the MPTP in Mediating Cell Death. PLoS One 10: e0130520.
- Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15: 1239-1253.
- McKeown SR (2014) Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol 87: 20130676.
- Loor G, Schumacker PT (2008) Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ 15: 686-690.
- Piret JP, Minet E, Cosse JP, Ninane N, Debacq C, et al. (2005) Hypoxia-inducible factor-1-dependent overexpression of myeloid cell factor-1 protects hypoxic cells against tert-butyl hydroperoxide-induced apoptosis. J Biol Chem 280: 9336-9344.
- Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, et al. (2007) Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci U S A 104: 13092-13097.
- Wang Y, Roche O, Xu C, Moriyama EH, Heir P, et al. (2012) Hypoxia promotes ligand-independent EGF receptor signaling via hypoxia-inducible factor-mediated upregulation of caveolin-1. Proc Natl Acad Sci USA 109: 4892-4897.
- Mojsa B, Lassot I, Desagher S (2014) Mcl-1 ubiquitination: unique regulation of an essential survival protein. Cells 3: 418-437.
- Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, et al. (2010) Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463: 103-107.
- Brunelle JK, Shroff EH, Perlman H, Strasser A, Moraes CT, et al. (2007) Loss of Mcl-1 protein and inhibition of electron transport chain together induce anoxic cell death. Mol Cell Biol 27: 1222-1235.
- Shroff EH, Snyder C, Chandel NS (2007) RoleofBcl-2family membersinanoxiainducedcell death. CellCycle 6: 807-809.
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, et al. (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19: 202-208.
- Chen L,Fletcher S (2017) Mcl-1inhibitors: apatentreview. Expert Opin Ther Pat 27: 163-178.
- Wang S,Jiang Y,Liu J,Zhao Y,Xiang C,et al. (2014) Revisiting the role of MCL1 in tumorigenesis of solid cancer: gene expression correlates with antiproliferative phenotype in breast cancer cells and its functional regulatory variants are associated with reduced cancer susceptibility. Tumour Biol 35: 8289-8299.
- Young AI, Law AM, Castillo L, Chong S, Cullen HD, et al. (2016) MCL-1 inhibition provides a new way to suppress breast cancer metastasis and increase sensitivity to dasatinib. Breast Cancer Re 18: 125.
- Brunelle JK, Ryan J, Yecies D, Opferman JT, Letai A (2009) MCL-1 dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts. J Cell Biol 187: 429-442.
- Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, et al. (2011) SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471: 104-109.
- Podar K, Gouill SL, Zhang J, Opferman JT, Zorn E, et al. (2008) ApivotalroleforMcl-1inBortezomib-inducedapoptosis. Oncogene 27: 721-731.
- Tong J, Wang P, Tan S, Chen D, Nikolovska-Coleska Z, et al. (2017) Mcl-1Degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res 77: 2512-2521.
- Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, et al. (2011) Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 andFBW7. Nature 471: 110-114.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi