Research Article, J Clin Nutr Metab Vol: 3 Issue: 1
Malnutrition Indicators which is More Predictive? Nutrition Risk Index (NRI) or Malnutrition Universal Screening Tool (MUST)
Daradkeh G1,2,3*, Essa MM1,2, Al-Mashaani A4, Al-Adawi S2,5, Arabawi S4, Amiri R4 and Al-Barashdi J4
1Department of Food Science and Nutrition, College of Agricultural and Marine Sciences, Sultan Qaboos University, Sultanate of Oman
2Ageing and Dementia Research Group, Sultan Qaboos University, Sultanate of Oman
3Hamad Medical Corporation, Qatar
4Khoula Hospital, Ministry of Health, Sultanate of Oman
5Department of Behavioral Medicine, College of Medicine and Health Sciences, Sultan Qaboos University, Sultanate of Oman
*Corresponding Author : Ghazi Daradkeh
Department of Dietetics and Clinical Nutrition, Al-Khor Hospital, Hamad Medical Corporation, Qatar
Tel: +974 5526 4714
E-mail: gdaradkeh@hamad.qa
Received: June 18, 2018 Accepted: January 26, 2019 Published: February 04, 2019
Citation: Daradkeh G, Essa MM, Al-Mashaani A, Al-Adawi S, Arabawi S, et al. (2019) Malnutrition Indicators Which is More Predictive? Nutrition Risk Index (NRI) or Malnutrition Universal Screening Tool (MUST). J Clin Nutr Metab 3:1.
Abstract
Background: Traumatic brain injury (TBI) is a major cause of disability, death and economic cost. Malnutrition is one of the common factors at the time hospital admission and tends to worsen during hospitalization, among TBI.
Methods: This study was conducted in an outpatient neurosurgery clinic at Khoula Hospital (National Trauma Center)–Muscat–Oman. 77 TBI adult patients, aged 18-65 years, males and females were included in this study. Patients were invited to participate in an anonymous survey. This invitation was extended during routine outpatient visits.
Results: The majority of patients were males (85.9%) with 6.1:1 male to female ratio. Most of the patients (75%) were aged between 18-30. 46.5% of subjects were classified as mild TBI while 12.7% and 40.8% were classified as moderate and severe TBI respectively using Glasgow Coma Scale. Motor vehicle accidents were the most common cause of TBI (91.7%), followed by falls from height (8.3%), (28.1%) of patients were underweight BMI<18.5 kg/m² while (16.9%) and (7.1%) were overweight and obese respectively.
Conclusion: NRI is more predictive malnutrition assessment tool than MUST in TBI patients. The higher sensitivities of NRI (92.9%) mean it is the better screening tool because there is a possibility that only 5%-7% of patients who may be malnourished were not correctly identified. Early recognition and management may help decrease hospital lengths of stay, readmission rates and associated healthcare costs.
Keywords: Malnutrition; Nutrition risk index; Malnutrition Universal screening tool; Traumatic brain injury
Introduction
Malnutrition is a major health problem among admitted patients in hospitals. The prevalence of malnutrition is 23% among hospitalized inpatients [1]. Malnourished patients have increased hospital length of stay (LOS), on average malnourished patients spending 4.5 days longer in the hospital compared to well-nourished ones and increased readmission rates and are more likely to be discharged to a long term care or rehabilitation facility [2].
The increased hospital length of stay can triple healthcare costs from $9,485 for the average hospitalized patient to $26,944 for malnourished ones [3]. In developing countries malnutrition is reported to be the leading cause of disease burden with high morbidity and mortality rates. Malnutrition and its implications have been documented in hospitalized patients by Butterworth [4]. 30%- 50% of hospitalized patients are described as malnourished or at a risk of malnutrition, in North America [5-7].
Poor nutritional status is positively related with impaired wound healing, increased post-operative complications and mortality [8-10]. To explore the association between malnutrition and various chronic diseases such as cancer, infections and chronic kidney diseases [11-16]. Several studies have been carried out. It showed a relationship between the length of hospital stay and nutritional status.
Traumatic brain injury (TBI) is a major cause of disability, death and economic cost [17,18]. TBI induces inflammatory and hormonal responses that increase the metabolic processes and alter nutrition requirements. Following TBI, the increased energy expenditure and nitrogen excretion mandates adequate nutritional support to provide the optimal milieu for neurological and systemic recovery [19-21]. The hypermetabolic nature of post-traumatic brain injury state makes adequate nutritional support critical. Maintenance of adequate nutritional intake has been shown to have a significant impact on outcomes after TBI [22]. Inadequate nutrition support for TBI patients may result in malnutrition and muscle wasting [23]. This cachexia increases the duration of rehabilitation and length of hospitalization, increases difficulties in mobility, functional rehabilitation, and promoted the development and exacerbation of medical complications [24], as well as neurobehavioral impairments.
To prevent the catabolic state and muscle protein breakdown as a result of increased energy demands, accurate assessment and estimation of the energy and protein needs of TBI patients are a critical component in nutritional treatment and support. With multiple injuries, hyper metabolism increases in a variable degree, and some studies have reported that the mean resting metabolic rate increases by 117%-175% of the healthy resting rate [25-27]. After 2-3 weeks of injury, 120%-145% of energy expenditure is required for TBI patients [28-32]. Furthermore, it was reported that adequate calories intake are essential for reducing sequelae of TBI [33]. Early identification of malnutrition is important to improving outcomes and overall nutritional status of patients [34]. Traditional measures of nutrition status such as laboratory (i.e. serum albumin and prealbumin) and anthropometric measures (i.e. body mass index and percentage weight loss) are beneficial in identifying malnutrition; however, they are not enough and can delay the recognition of malnutrition, especially in TBI patients [34,35].
Two screening tools, the Malnutrition Universal Screening Tool (MUST) and Nutritional Risk Index (NRI), have shown some potential to be reliable methods of evaluating the nutritional status of TBI patients. The Nutritional Risk Index (NRI) [36], is an assessment tool uses objective measurements (serum albumin and percent usual body weight) rather than subjective measurements to determine nutritional risk in hospitalized patient populations [37,38]. It has been successfully modified from its original form to be used in various patient groups [38]. The NRI was originally derived from the serum albumin concentration and the ratio of present to usual weight. The NRI uses objective measurements to calculate a score from the following formula: (1.519 x serum albumin + 41.7 x current weight/ usual body weight) [39]. From NRI values, we defined four grades of nutrition-related risk: (i) severe risk (NRI<83.5); (ii) moderate risk (NRI 83.5-97.5); (iii) mild risk (NRI 97.5-100); (iv) No risk (NRI >100). The NRI cut-off values were determined according to weight losses of 5%, 10% or 20%. The weight loss norms of 5% and 10% have already been validated by the European Society of Parenteral and Enteral Nutrition (ESPEN) Guidelines for Nutritional Screening [40].
Malnutrition Universal Screening Tool, or (MUST) [41], is a screening tool originally developed by the Malnutrition Advisory Group for the British Association of Parenteral 8 and Enteral Nutrition. It has shown strength for application with adult patients across all healthcare settings including TBI [42]. MUST is a five-step screening tool to identify patients who are with no risk, moderate risk and high risk of malnutrition when MUST score was 0,1 and ≥ 2 respectively. One benefit of the MUST tool is that it guides the user to either seek immediate nutrition consultation for high risk patients, or to observe medium risk patients upon hospital admission.
Early recognition of malnutrition by healthcare providers could lead to early intervention, decreased morbidity and mortality, and decreased healthcare costs and LOS [43,44].
The current study aimed to compare between the effectiveness of NRI and MUST as assessment tool to detect malnutrition among TBI patients.
Methods
The Ethics Committee of our Institution approved this study and patients’ written informed consent was obtained (MERC/11/03). This study was conducted in an outpatient neurosurgery clinic at Khoula Hospital (National Trauma Center)–Muscat–Oman. 77 TBI adult patients, aged 18-65 years, males and females were included in this study. Patients were invited to participate in an anonymous survey. This invitation was extended during routine outpatient visits.
The exclusion criterion included: pre-injury psychiatric or neurological history other than those resulting from a TBI, non-Omani patients and those who were known to have cognitive impairments that would preclude completion of the protracted assessment.
Demographic and anthropometric measures
Demographic information, including age, sex, and education level, marital and smoking status were collected using a structured questionnaire. Weight was measured in kilogram to the nearest 0.1 kg using a digital weighing scale (Seca 208, Vogal and Halke, Germany) Height was measured to the nearest 0.5 cm by using a stadiometer protocol adapted from Lohman et al. [45] with a vertical measuring scale fixed to a metal bar connected to weighing scale. Body Weight (BW) change was calculated as: (current BW in kilograms - ideal BW in kilograms)/ideal BW × 100. For patients who were unable to stand, height was estimated by using knee height equation as “stature=85.10+1.73 x knee height-0.11 x age” for males, “stature=91.45+1.53 x knee height-0.16 x age” for females [46], and by ulna length for males, height (cm)=4.605U+1.308A+28.003, and for females, height (cm)=4.459U+1.315A+31.485 [47], and by demi– span for Males: height (cm)=(1.40 × demi-span in cm)+57.8 and for Females: height (cm)=(1.35 × demi-span in cm)+60.0 [48]. The mean height of the three measurements was considered in calculations. Body Mass Index (BMI) was calculated as wt. (kg)/ ht. (m2), and the cutoff points of the World health organization were used [49].
Laboratory measures
Blood samples for serum albumin, and serum creatinine levels were drawn on admission. Albumin was measured by immunonephelometry (normal range 3.5-5.5 g/dL). An albumin level of less than 3.5 g/dL was set as the lower limit of normal in our study [50,51].
Statistical analysis
Graph Pad Prism (version 6.0) was used for statistical analysis. Means and Standard Deviations (using t-tests for two means, one way Anova was used to compare between groups), two sided statistical significance was set at α ≤ 0.05 and Proportions were compared by using chi-square test.
Results
77 patients in the age group 18-65, with a mean age of 27.3 years that fulfilled the eligibility criteria were enrolled in the study. The majority of patients were males (85.9%) and (14.1%) were females, with 6.1:1 male to female ratio. Most of the patients (75%) were aged between 18-30. 46.5% of subjects were classified as mild TBI while 12.7% and 40.8% were classified as moderate and severe TBI respectively using Glasgow Coma Scale. Motor vehicle accidents were the most common cause of TBI (91.7%), followed by falls from height (8.3%), the most important injuries as a result of fall resulting for fainting among diabetic patients and others with ENT problems results in loss of balance. 28.1% of patients were underweight BMI<18.5 kg/m2 while (16.9%) and (7.1%) were overweight and obese respectively (Table 1).
| Characteristics | n | % |
|---|---|---|
| Gender Male Female |
61 10 |
85.9 14.1 |
| Body Mass Index (kg/m²) Underweight BMI<18.5 Normal weight BMI 18.5-24.9 Overweight BMI 25-29.9 Obese BMI = 30 |
20 34 12 5 |
28.1 47.9 16.9 7.1 |
| Severity of Trauma (Glasgow Coma Scale) Mild (GCS = 13) Moderate (GCS 9-12) Severe (GCS = 8) |
33 9 29 |
46.5 12.7 40.8 |
| TBI cause Vehicle accident Fall from height |
71 6 |
92.2 7.8 |
Table 1: Demographic and anthropometric characteristics of TBI Patients.
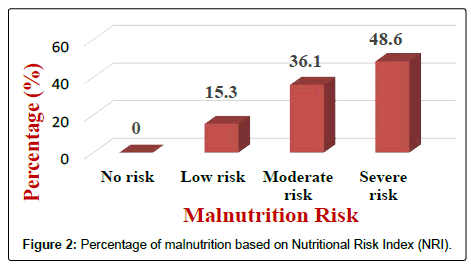
Results of the NRI analysis indicated 84.7% were at moderate and severe malnutrition risk, compared with 72.3% detected as severe and moderate risk of malnutrition by using MUST. As expected the BMI values were higher in the No Risk group (26.8 ± 7 kg/m2) as compared to Mild Risk (23.7 ± 4 kg/m2), Moderate Risk (21.6 ± 5 kg/m2) or Severe Risk groups (20 ± 7 kg/m2) with P<0.5 in both tools (Figure 1). The patients were divided into four groups according to NRI scores identified as No Risk group (n=0), Mild Risk group (15.3%), Moderate Risk group (36.1%) and Severe Risk group (48.6%) (Figure 2). While based on MUST, malnutrition scores identified as mild risk (27.7%), moderate risk (30.6%) and severe risk (41.7%) (Figure 3). Moderate and severe malnutrition were more predicted by NRI compared by MUST (48.6%, 41.7%) and (36.1%, 30.6%) respectively. While mild risk of malnutrition was more predicted by MUST than NRI (27.7%, 15.3%) respectively (Figure 4).
Discussion
The NRI was developed by the Veterans’ Affairs Total Parenteral Nutrition Cooperative Study Group to determine nutritional risk in the postsurgical patient population [52]. The NRI uses the patient’s serum albumin, and the ratio of current body weight to ideal body weight to predict a patient’s malnutrition status. The score is calculated as follows: 1.5 × serum albumin+41.7 × current Weight/ ideal body weight. A score of >100 means there is no evidence of malnutrition, 97.5-100 indicates mild malnutrition, 83.5-97.5 means moderate malnutrition, and <83.5 signify severe malnutrition [52]. The MUST was originally developed by the Malnutrition Advisory Group for the British Association of Parenteral and Enteral Nutrition [53]. MUST is a five-step tool that is easy to use and usually takes 3-5 min to complete. It evaluates BMI score, recent weight loss, and acute disease, then assigns an overall numerical risk [53]. A score of 0=low risk, 1=medium risk, and ≥ 2=high risk. Based on the MUST score appropriate management guidelines are provided. A score of 0 requires no intervention. Patients with a score of 1 require close dietary intake monitoring to evaluate for necessary supplements. A score of 2 or more requires immediate nutritional evaluation by a dietitian.
Laboratory values of albumin are useful in identifying malnutrition in the general and TBI populations, but it has a few limitations in their accuracy. Albumin concentrations can be affected by hydration, renal function, and the presence of infection or inflammation [54]. Albumin more accurately identifies chronic malnutrition. Aside from pre albumin and albumin, there is currently a lack of literature that compares other laboratory trends and trends in co-morbidities among malnourished TBI patients. Subjective nutritional screening tools can be easy, rapid and inexpensive methods of identifying malnutrition risk and prevalence among patients.
Our findings showed that NRI is more effective to predict TBI patient’s with moderate and severe malnutrition risk (84.7%) compared to MUST (72.3%). These findings are consistence with Al-Najjar and Clark [53] findings, who reported that NRI was a uni-variable predictor of mortality (chi-square 25, p<0.001), and an independent predictor of outcome in multivariable analysis (chi-square 12, p<0.001; [52]. In another study, NRI was found to be the most significant predictor of all-cause mortality and readmission rates [55]. Implementing the use of NRI or MUST on admission for TBI patients may help identify the presence of malnutrition earlier so that the malnourished may be referred to a dietitian for appropriate nutritional intervention earlier.
Conclusion
NRI is more predictive malnutrition assessment tool than MUST in TBI patients. The higher sensitivities of NRI (92.9%) mean it is the better screening tool because there is a possibility that only 5%-7% of patients who may be malnourished were not correctly identified. Noting the negative impacts of malnutrition on the patient, early recognition and management may help decrease hospital lengths of stay, readmission rates and associated healthcare costs.
References
- Chima CS, Barco K, Dewitt ML, Maeda M, Teran JC, et al. (1997) Relationship of nutritional status to length of stay, hospital costs, and discharge status of patients hospitalized in the medicine service. J Am Diet Assoc 97: 975-978.
- Gout BS, Barker LA, Crowe TC (2009) Malnutrition identification, diagnosis and dietetic referrals: are we doing a good enough job? Nutr Diet 66: 206-211.
- Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A, et al. (2010) American Society for Parenteral and Enteral Nutrition. Malnutrition diagnoses in hospitalized patients: United States, 2010. Jpen-Parenter Enter 38: 186-195.
- Butterworth CE Jr (1974) The skeleton in the hospital closet. Nutr Today 9: 4-8.
- Edington J, Boorman J, Durrant ER, Perkins A, Giffin CV, et al. (2000) Prevalence of malnutrition on admission to four hospitals in England. Clin Nutr 19: 191-195.
- Bruun LI, Bosaeus I, Bergstad L, Nygaard K (1999) Prevalence of malnutrition in surgical patients: evaluation of nutritional support and documentation. Clin Nutr 18: 141-147.
- Constans T, Bacq Y, Bréchot JF, Guilmot JL, Choutet P, et al. (1992) Protein-energy malnutrition in elderly medical patients. J Am Geriatr Soc 40: 263-268.
- Giner M, Laviano A, Meguid MM, Gleason JR (1996) In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutr 12: 23-29.
- Bidlack WR (1996) Interrelationships of food, nutrition, diet and health: the National Association of State Universities and Land Grant Colleges White Paper. J Am Coll Nutr 15: 422-433.
- He J, Gu D, Wu X, Reynolds K, Duan X, et al. (2005) Major causes of death among men and women in China. New Engl J Med 353: 1124-1134.
- Paccagnella A, Morello M, Da Mosto MC, Baruffi C, Marcon ML, et al. (2010) Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer 18: 837-845.
- August DA, Huhmann MB (2009) American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. ASPEN clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. Jpen-Parenter Enter 33: 472-500.
- Nassar MF, El-Batrawy SR, Nagy NM (2009) CD95 expression in white blood cells of malnourished infants during hospitalization and catch-up growth. E Mediterr Health J.
- Ivers LC, Cullen KA, Freedberg KA, Block S, Coates J, et al. (2009) HIV/AIDS, undernutrition, and food insecurity. Clin Infect Dis 49: 1096-1102.
- Cupisti A, Saba A, D’Alessandro C, Meola M, Panicucci E, et al. (2009) Dimethylarginine levels and nutritional status in hemodialysis patients. J Nephrol 22: 623-629.
- Bellizzi V, Di Iorio BR, Brunori G, De Nicola L, Minutolo R, et al. (2010) Assessment of nutritional practice in Italian chronic kidney disease clinics: a questionnaire- based survey. J Ren Nutr 20: 82-90.
- Ghajar J (2000) Traumatic brain injury. Lancet 356: 923-929.
- Gururaj G (2002) Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res 24: 24-28.
- Clifton GL, Robertson CS, Grossman RG, Hodge S, Foltz R, et al. (1984) The metabolic response to severe head injury. J Neurosurg 60: 687-696.
- Deutschman CS, Konstantinides FN, Raup S, Thienprasit P, Cerra FB (1986) Physiological & metabolic response to isolated closed head injury. Part 1: Basal metabolic state: Correlation of metabolic and physiological parameters with fasting and stressed controls. J Neurosurg 64: 89-98.
- Young B, Ott L, Norton J (1985) Metabolic & nutritional sequelae in the non- steroid treated head injury patient. Neurosurgery 17: 784-791.
- Charrueau C, Belabed L, Besson V, Chaumeil JC, Cynober L, et al. (2009) Metabolic response and nutritional support in traumatic brain injury: evidence for resistance to nutrition. J Neurotrauma 26: 1911-1920.
- Denes Z (2004) The influence of severe malnutrition on rehabilitation in patients with severe head injury. Disabil Rehabil 26: 1163-1165.
- Brandi LS, Santini L, Bertolini R, Malacarne P, Casagli S, et al. (1999) Energy expenditure and severity of injury and illness indices in multiple trauma patients. Crit Care Med 27: 2684-2689.
- Faisy C, Guerot E, Diehl JL, Labrousse J, Fagon JY (2003) Assessment of resting energy expenditure in mechanically ventilated patients. Am J Clin Nutr 78: 241-249.
- Plank LD, Hill GL (2000) Sequential metabolic changes following induction of systemic inflammatory response in patients with severe sepsis or major blunt trauma. World J Surg 24: 630-638.
- Clifton GL, Robertson CS, Choi SC (1986) Assessment of nutritional requirements of head injured patients. J Neurosurg 64: 895-901.
- Fried RC, Dickerson RN, Guenter PA, Stein TP, Gennarelli TA, et al. (1989) Barbiturate therapy reduces nitrogen excretion in acute head injury. J Trauma 29: 1558-1564.
- Young B, Ott L, Norton J, Tibbs P, Rapp R, et al. (1985) Metabolic and nutritional sequelae in the nonsteroid treated head injury patient. Neurosurgery 17: 784-791.
- Weekes E, Elia M (1996) Observations on the patterns of 24-hour energy expenditure changes in body composition and gastric emptying in head-injured patients receiving nasogastric tube feeding. J Parenter Enteral Nutr 20: 31-37.
- Borzotta AP, Pennings J, Papasadero B, Paxton J, Mardesic S, et al. (1994) Enteral versus parenteral nutrition after severe closed head injury. J Trauma 37: 459-468.
- Robertson CS, Clifton GL, Grossman RG (1984) Oxygen utilization and Cardiovascular function in head-injured patients. Neurosurgery 15: 307-314.
- Hatton J, Ziegler TR (1998) Nutritional support of the neurosurgical patient, The Practice of Neurosurgery, USA.
- Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A, et al. (2014) Malnutrition diagnoses in hospitalized patients: United States, 2010. J Parenter Enteral Nutr 38: 186-195.
- Araújo JP, Lourenço P, Rocha-Gonçalves F, Ferreira A, Bettencourt P (2011) Nutritional markers and prognosis in cardiac cachexia. Int J Cardiol 146: 359-363.
- Veterans Affairs Total Parenteral Nutrition Cooperative Study Group (1991) Perioperative total parenteral nutrition in surgical patients. N Engl J Med 325: 525-532.
- Buzby GP, Knox LS, Crosby LO, Eisenberg JM, Haakenson CM, et al. (1988) Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr 47: 366-381.
- Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, et al. (2005) Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 82: 777-783.
- Aziz EF, Javed F, Pratap B, Musat D, Nader A, et al. (2011) Malnutrition as assessed by Nutritional Risk Index is associated with worse outcome in patients admitted with acute decompensated heart failure: An ACAP-HF data analysis. Heart Int 6: 3-8.
- Kondrup JE, Allison SP, Elia M, Vellas B, Plauth M (2003) ESPEN guidelines for nutrition screening 2002. Clin Nutr 22: 415-421.
- Elia M (2003) Screening for malnutrition: A multidisciplinary responsibility. Development and Use of the Malnutrition Universal Screening Tool (‘MUST’) for Adults. Red ditch: BAPEN, United Kingdom.
- Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, et al. (2004) Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’(‘MUST’) for adults. Br J Nutr 92: 799-808.
- Elia M (2009) The economics of malnutrition. Nestle Nutr 12: 29-40.
- Faramarzi E, Mahdavi R, Mohammad-Zadeh M, Nasirimotlagh B (2013) Validation of Nutritional Risk Index method against Patient-Generated Subjective Global Assessment in screening malnutrition in colorectal cancer patients. Chin J Cancer Res 25: 544-548.
- Stratton RJ, King C, Elia M (2004) Simple cost-analysis of changes in hospital length of stay with oral nutritional supplements from randomized controlled trials. Clin Nutr 23: 906-915.
- Lohman T, Roche A, Martorell R (1988) Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books, USA.
- Cheng HS, See LC, Shieh YH (2001) Estimating stature from knee height for adults in Taiwan. Chang Gung Med J 24: 547.
- Gauld LM, Kappers J, Carlin JB, Robertson CF (2004) Height prediction from ulna length. Dev Med Child Neurol 46: 475-480.
- Bassey EJ (1986) Demi-span as a measure of skeletal size. Ann Hum Biol 13: 499-502.
- Organization WHO (2011) World Health Organization Global Database on Body Mass Index 2012, USA.
- Corti MC, Guralnik JM, Salive ME, Sorkin JD (1994) Serum albumin level and physical disability as predictors of mortality in older persons. JAMA 272: 1036-1042.
- Incalzi RA, Landi F, Pagano F, Capparella O, Gemma A, et al. (1998) Changes in nutritional status during the hospital stay: a predictor of long-term survival. Aging 10: 490-496.
- Al-Najjar Y, Clark AL (2012) Predicting outcome in patients with left ventricular systolic chronic heart failure using a Nutritional Risk Index. Am J Cardiol 109: 1315-1320.
- Elia M, Malnutrition Advisory Group (2010) The ‘MUST’ Report: Nutritional screening of adults, a multidisciplinary responsibility. British Association of Parenteral and Enteral Nutrition, USA.
- Beck FK, Rosenthal TC (2002) Pre albumin: A marker for nutritional evaluation. Am Fam Physician 65: 1575-1578.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi