Research Article, J Chem Appl Chem Eng Vol: 1 Issue: 2
Ligand Field and Counter Anion Effects on the Thermal Stability of Copper(II) Complexes of 1,2-Di(imino-4’-antipyrinyl)ethane and 4-N-(4’-antipyrylmethylidene) aminoantipyrine
Madhu NT1,2*, Knittl EK2, Tesfay GA1, Bekele GM3, Sirak TB1 and Linert W2*
1Department of Industrial Chemistry, Addis Ababa Science and Technology University, Addis Ababa, P. O. Box.16417, Ethiopia
2Institute of Applied Synthetic Chemistry, Vienna University of Technology, Getreidemarkt 9/163-AC, A-1060 Vienna, Austria
3Department of Chemistry, University of Hawassa, Sidama, P. O. Box.05, Ethiopia
*Corresponding Authors : Nayathuparambil Thomas Madhu
Department of Industrial Chemistry, Addis Ababa Science and Technology University, Addis Ababa, PO Box 16417, Ethiopia
Tel: +43-1-58801-15350
Fax: +43-1-5816668 16299
E-mail: ntmadhu@hotmail.com
Wolfgang Linert
Institute of Applied Synthetic Chemistry, Vienna University of Technology, Getreidemarkt 9/163-AC, A-1060 Vienna, Austria
Tel: +43-1-58801-163613
Fax: +43-1-58801-9-163613
E-mail: wolfgang.linert@tuwien.ac.at
Received: December 07, 2017 Accepted: December 26 2017 Published: December 30,2017
Citation: Madhu NT, Esther TK, Tesfay GA, Bekele GM, Sirak TB, et al. (2017) Ligand Field and Counter Anion Effects on the Thermal Stability of Copper(II) Complexes of 1,2-Di(imino-4’-antipyrinyl)ethane and 4-N-(4’-antipyrylmethylidene) aminoantipyrine. J Chem Appl Chem Eng 1:2. doi: 10.4172/2576-3954.1000110
Abstract
A comparative thermolytic investigation on copper(II) complexes with two antipyrine Schiff base derivatives, namely 1,2-Di(imino-4’-antipyrinyl)ethane (GA) and 4-N-(4’-antipyrylmethylidene) aminoantipyrine (AA), with different counter anions like perchlorate,nitrate, chloride, and bromide has been done by thermogravimetric and derivative thermogravimetric analysis. The present investigation reveals that the counter anions and the ligand field play a big role on the thermal stability of the copper (II) complexes of both Schiff base derivatives. For the present complexes, the thermal decomposition mechanism has been investigated according to Šatava, which follows a ‘random nucleation with one nucleus on each particle, i.e. the Mampel model’.
Keywords: Copper(II)complexes;1,2-Di(imino-4’-antipyrinyl)ethane; 4-N-(4’-antipyrylmethylidene) Aminoantipyrine; Thermal decomposition; Mechanism
Introduction
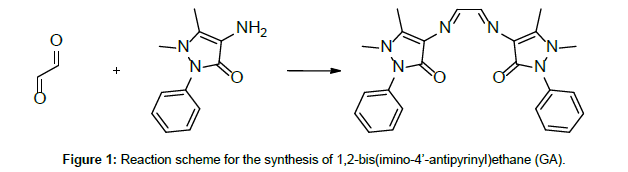
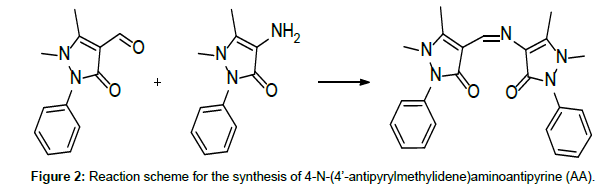
Due to their easy modification and flexible complexing nature, antipyrine Schiff base ligands have drawn the attention of many investigators [1-4]. On top of this, antipyrine derivatives and their metal complexes have interesting pharmacological properties [5]. A search through the literature reveals few reports on thermal studies on antipyrine Schiff base metal complexes [6]. Our earlier publications on the thermolytic investigations of antipyrine Schiff bases with first row transition metals revealed that the counter ions play a major role on the thermal stability of the complexes [7-12]. Here, in the present investigation, in addition to the counter anionic variation, the ligand field effect has also taken into consideration on the thermal characteristics of the complexes. Thus, a comparative thermolytic investigation on copper(II) complexes with two different antipyrine Schiff base ligands, namely 1,2-Di(imino-4’-antipyrinyl)ethane(GA) and 4-N-(4’-antipyrylmethylidene)aminoanti-pyrine(AA) (Figures 1 and 2) has been done with a variety of counter anions (perchlorate, nitrate, chloride, and bromide) [13].
Experimental
Both, the Schiff bases 1,2-bis(imino-4’-antipyrinyl)ethane(GA) and 4-N-(4’-antipyrylmethylidene)aminoantipyrine (AA), and their copper(II) complexes, were prepared and characterised as previously described [4]. For the preparation of the ligands GA and AA, simple condensation reactions were carried out, using 4-aminoantipyrine and the respective aldehydes glyoxal and 4-antipyrine carboxaldehyde. (Figures 1 and 2) [4]. Thermogravimetric analysis was carried out on a Delta Series TGA 7 thermal analyser in a nitrogen atmosphere, using 10 mg of sample for each measurement with a heating rate of 10°C/ min. The thermal decomposition mechanism of the complexes was done according to Šatava [14], using a computer program in QBasic.
Results and Discussion
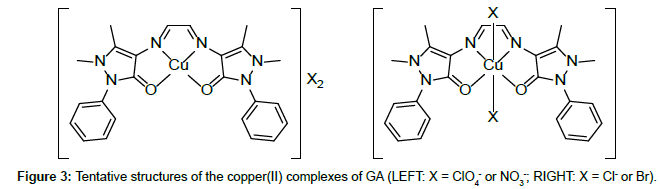
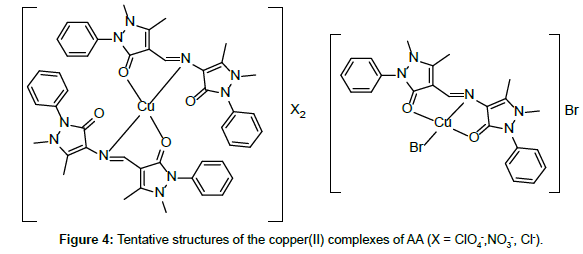
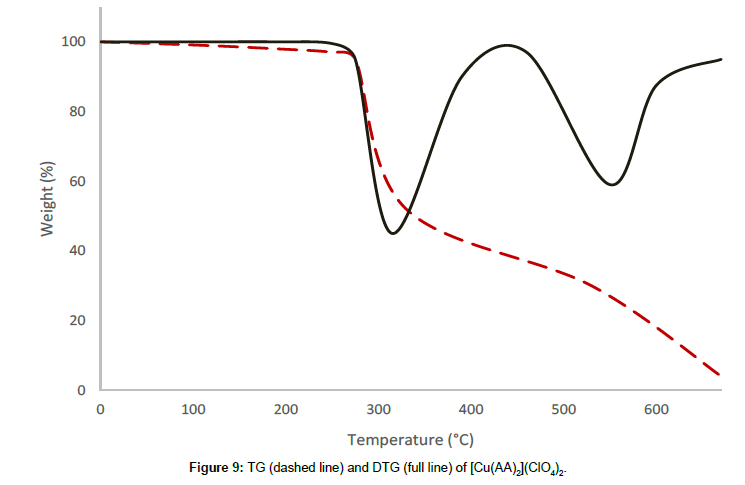
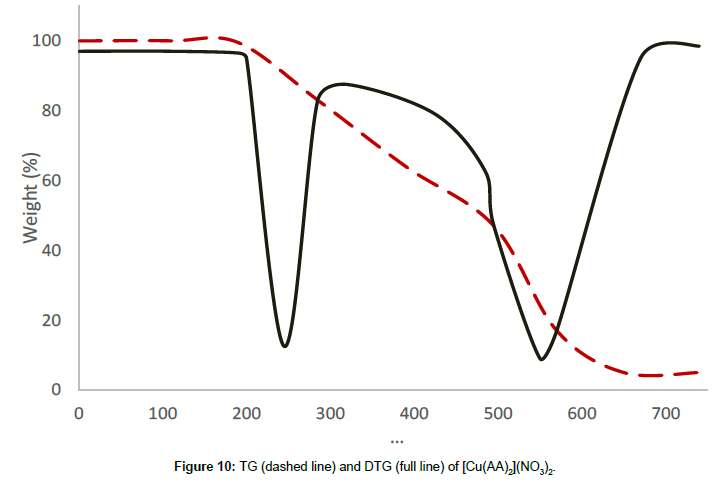
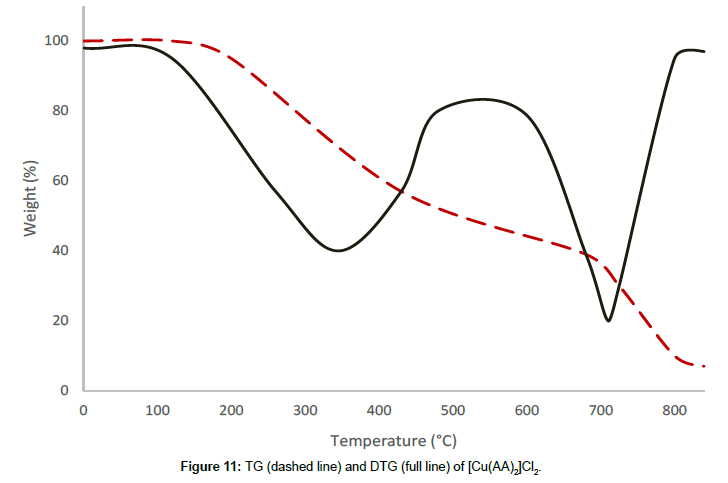
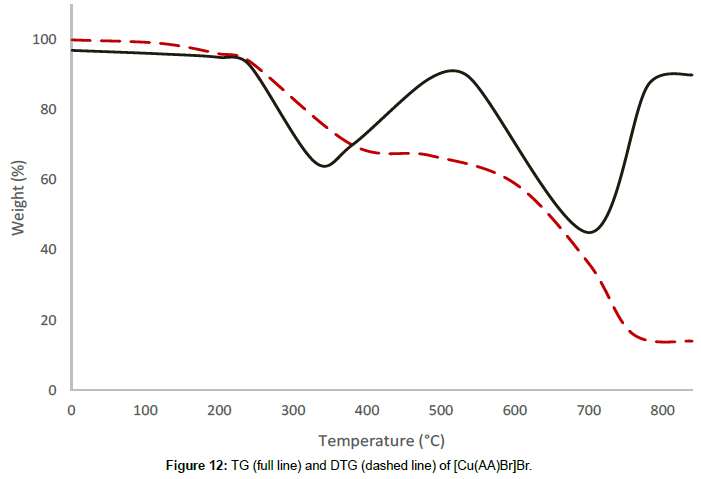
Molar conductance in non-aqueous solvents, molar magnetic moments, infrared spectra and electronic and EPR spectral data [4] were determined for all eight copper(II) complexes in the present study and can therefore be formulated as [Cu(GA)](ClO4)2, [Cu(GA)] (NO3)2, [Cu(GA)Cl2] and [Cu(GA)Br2] with the ligand GA, while the AA complexes are of the formulae [Cu(AA)2](ClO4)2, [Cu(AA)2] (NO3)2, [Cu(AA)2]Cl2 and [Cu(AA)Br]Br. A tetradentate coordination via both carbonyl oxygen atoms and both azomethine nitrogen atoms occur in all the copper(II) complexes of GA. The counter anions perchlorate and nitrate remain uncoordinated, that is why square planar complexes are generated, while the halides coordinate to the metal centre, thus, forming coordination compounds with octahedral geometries (Figure 3). In the case of copper (II) complexes of AA, the coordinating nature of the ligand varies, using different counter anion. AA behaves as a neutral bidentate ligand, coordinating through one carbonyl oxygen and the azomethine nitrogen for the complexes with perchlorate, nitrate and chloride anions. In contrast to that, AA coordinates in a tridentate fashion for copper coordination compounds with bromide anions, through both carbonyl oxygens and the azomethine nitrogen, and one bromide anion, thus, forming a square planar geometry in the final coordination compound. In fact, square planar geometries have been assigned for all synthesized complexes of AA (Figure 4). The thermal analysis of all eight complexes is shown in the (Figures 5-12).
Phenomenological aspects
The phenomenological behaviour of all copper (II) complexes is described in the Tables 1 and 2.
| Complex | Stage of decomposition | TG plateau (°C) | DTG peak (°C) | Mass loss (%) |
|---|---|---|---|---|
| [Cu(GA)](ClO4)2 | I | 137-266 | 220 | 30.90 |
| II | 266-513 | 350 | 18.75 | |
| III | 513 | - | continuous | |
| [Cu(GA)](NO3)2 | I | 228-464 | 245 | 43.57 |
| II | 464-611 | 561 | 44.28 | |
| [Cu(GA)Cl2] | I | 178-427 | 348 | 38.08 |
| II | 427 | - | continuous | |
| [Cu(GA)Br2] | I | 197-492 | 297 | 33.33 |
| II | 492-893 | 684 | 32.74 |
Table 1: Phenomenological data for the thermal decomposition of copper (II) complexes of GA.
| Complex | Stage of decomposition | TG plateau (°C) | DTG peak (°C) | Mass loss (%) |
|---|---|---|---|---|
| [Cu(AA)2](ClO4)2 | I | 190-328 | 314 | 43.47 |
| II | 328-615 | 564 | 43.11 | |
| [Cu(AA)2](NO3)2 | I | 206-464 | 245 | 46.37 |
| II | 464-615 | 561 | 46.10 | |
| [Cu(AA)2]Cl2 | I | 181-489 | 347 | 41.10 |
| II | 489-807 | 718 | 41.01 | |
| [Cu(AA)Br]Br | I | 186-549 | 330 | 32.78 |
| II | 549-719 | 698 | 32.17 |
Table 2: Phenomenological data for the thermal decomposition of copper (II) complexes of AA.
Complexes of GA
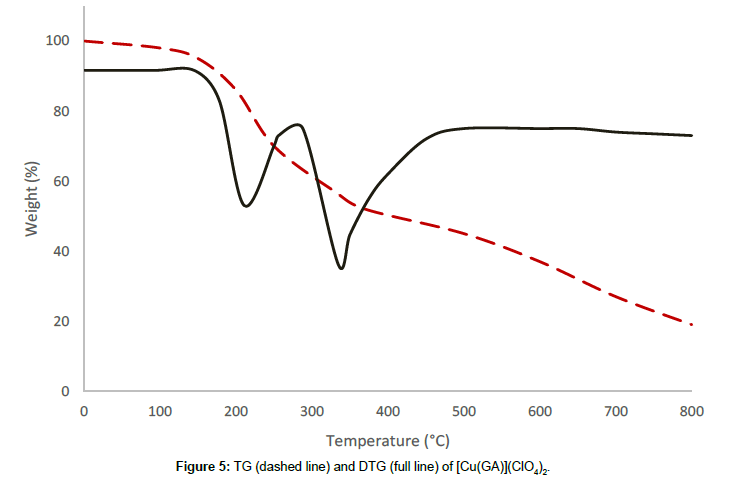
The perchlorate complex with the formula [Cu(GA)](ClO4)2, decomposes in three stages. The absence of small molecules like water or solvent in the complex is confirmed by its thermal stability till 137°C. The first decomposition stage occurs in the temperature range of 137°C-266°C with a DTG-peak at 220°C, accompanied with a mass loss of 30.90%, which corresponds to the removal of half of the ligand molecule [10]. The partial removal of the ligand at this stage is confirmed by the infrared spectrum of the residue at 266°C. A mass loss of 18.75% at a temperature range of 266°C-513°C occurs in the second stage, owing to the conversion of two perchlorates into chlorides. The DTG-peak corresponding to this stage is observed at 350°C. the third stage, which starts at 513°C, is a continuous one. The steady mass loss, observed in this stage, may be due to the removal of the remaining part of the ligand molecule together with the volatilisation of the residue of anhydrous cupric chloride [13] above 620°C.
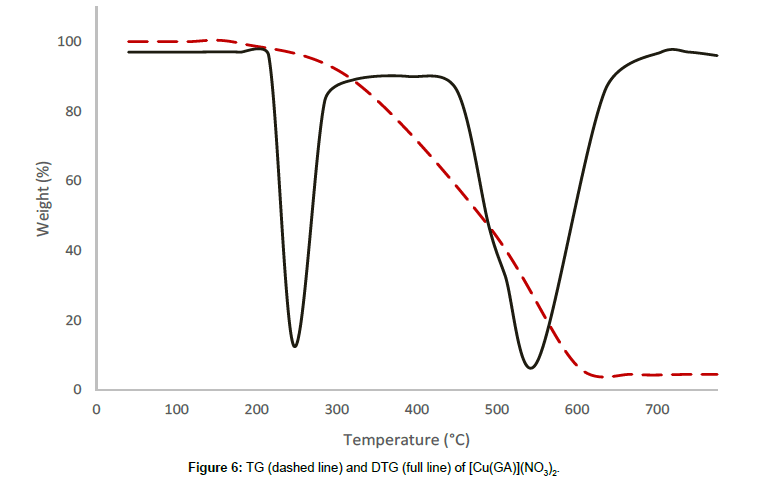
[Cu(GA)](NO3)2, exhibits a two stage decomposition pattern in the temperature range of 228°C-611°C. In this complex, there is no mass loss observed until 228°C, revealing that neither the water molecules nor solvent are present in this complex. The first stage starts at 228°C and comes to an end at 464°C with the DTG-peak at 245°C. At this stage, a mass loss of 43.57% could be observed, which can be attributed to the decomposition of half of the ligand molecule and one of the nitrate anions. The infrared spectrum of the residue at 464°C reveals the presence of GA and a nitrate ion, indicating the partial removal of the ligand and the nitrate ion at this stage. The thermal decomposition between 464°C and 611°C corresponds to the second stage with a mass loss of 44.28%, which can be attributed to the loss of the remaining half of GA and one nitrate ion with a DTGN peak at 561°C. The decomposition is completed at 611°C and the final residue is qualitatively proved to be anhydrous copper oxide.
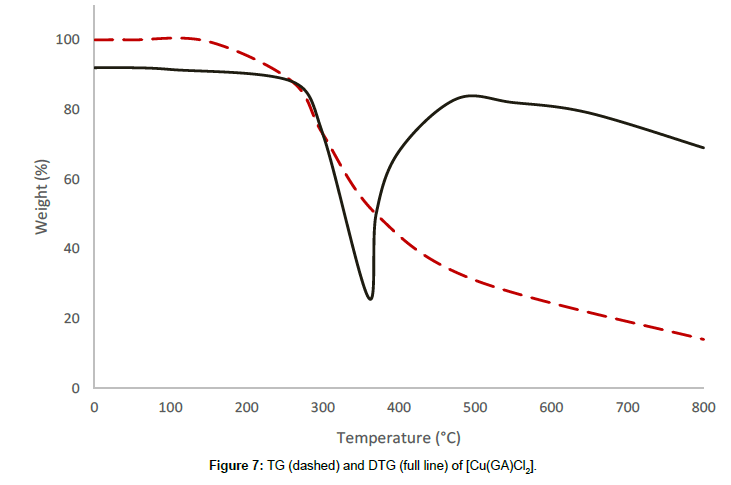
The complex with the formula [Cu(GA)Cl2] undergoes a two-stage decomposition pattern with no mass loss until 178°C, revealing the absence of either water or solvent molecules in this complex. The first decomposition stage starts at 178°C and ends at 427°C, which shows a DTG-peak at 348°C. The mass loss of 38.08% at this stage is due to the decomposition of half of the molecule of GA in this complex. The partial removal of the ligand at this stage is confirmed by the infrared spectrum of the residue at 427°C. The thermal decomposition, which begins at 427°C, corresponds to the second stage and happens to be a continuous one. The steady mass loss observed at this stage, may be due to the expulsion of the remaining part of the ligand molecule, together with the volatilisation of the residue of anhydrous cupric chloride [13] above 620°C.
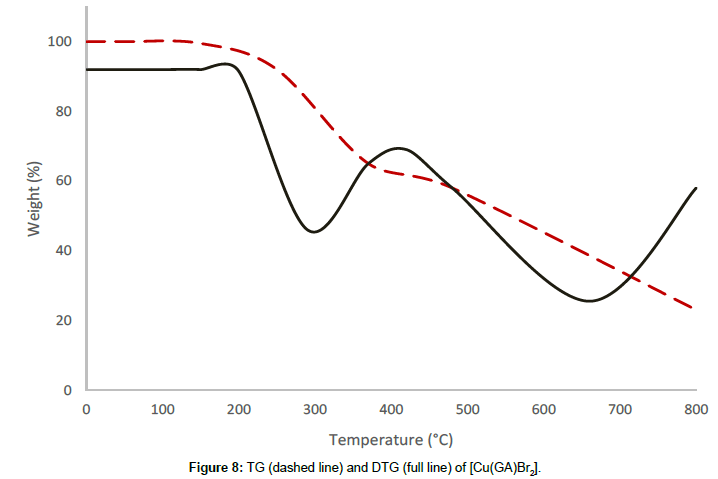
The bromide complex [Cu(GA)Br2] undergoes a two-stage decomposition process. The thermal stability of the bromide complex till 197°C reveals the absence of small molecule like water or solvent molecules in this complex. The decomposition process between 197°C and 492°C corresponds to the first decomposition stage with a mass loss of 33.33% and is due to the removal of half of the molecule of GA of the complex. The infrared spectrum of the residue after this stage reveals the presence of GA, indicating only a partial removal of the ligand at this stage. A mass loss of 32.74% occurs in the second stage between 492°C and 893°C, owing to the decomposition of the remaining half of the ligand molecule. The rate of mass loss is found to have maximas at 297°C and 684°C, respectively for the first and the second stages of the decomposition, indicated by their DTG-peaks. The final product was found to be anhydrous cupric bromide, confirmed by qualitative analysis.
Complexes of AA
A two stage decomposition has been observed for the complex [Cu(AA)2](ClO4)2, in the temperature range of 190°C and 615°C. There is no mass loss observed till 190°C, proving the absence of either water or solvent molecules in this complex. The thermal decomposition between 190°C and 328°C corresponds to the first decomposition stage with a mass loss of 43.47%, which is attributed to the decomposition of a molecule of AA and the conversion of one perchlorate anion into chloride. The rate of mass loss has its maximum at 314°C, which is indicated by the respective DTG-peak for this stage. The second decomposition stage is between 328°C and 615°C with a DTG-peak at 564°C, and a mass loss of 43.11%, which can be attributed to the removal of the remaining molecule of AA and the conversion of the second perchlorate anion into chloride. The decomposition is completed at 615°C, yielding anhydrous cupric chloride as the final residue, which is confirmed by qualitative analysis.
[Cu(AA)2](NO3)2, the nitrate complex, decomposes in two stages in the temperature range of 206°C-615°C, without any mass until 206°C, revealing the absence of either water or solvent molecules in this sample. The first decomposition stage starts at 206°C and comes to an end at 464°C with the DTG-peak at 245°C, and a mass loss of 46.37%, which can be attributed to the decomposition of a molecule of AA and a nitrate anion. The thermal decomposition process between 464°C and 615°C corresponds to the second decomposition stage with a DTG peak at 561°C and a mass loss of 46.10%, which originates in the decomposition of the remaining nitrate anion and the ligand molecule. The decomposition is completed at 615°C and the final residue is qualitatively proved to be anhydrous cupric oxide.
[Cu(AA)Br]Br undergoes two decomposition stages. The thermal decomposition of the complex in the range of 186°C-549°C corresponds to a mass loss of 32.78% and is due to the decomposition of half a molecule of the ligand AA. This is confirmed by the analysis of the residue at 549°C by infrared spectroscopy. The second decomposition stage of between 549°C and 719°C corresponds to a mass loss of 32.17% and can be attributed to the removal of the remaining half of the ligand molecule. The maximum rate of mass loss is at 330°C and 698°C, respectively, for the first and the second decomposition stages, indicated by the corresponding DTG-peaks. The decomposition is completed at 719°C, leaving the residue of anhydrous cupric bromide as the final residue, confirmed by qualitative analysis.
Structure and thermal stability correlation
From the thermograms of the complexes of GA, it can be concluded that even, the ligand was the same in all complexes Cu(GA)X2 (where X = ClO4-, NO3 -, Cl- and Br-), the nature of the decomposition pattern was completely different. The perchlorate complex follows a three-stage decomposition process, while the rest of the complexes decompose in two stages. Among this series of complexes of GA, the perchlorate complex is the least stable, while the nitrate-complex the most stable. The following order can made concerning these complexes stability:
Nitrate > Bromide > Chloride > Perchlorate
The complexes of AA, even though the molecular composition of the complex, [Cu(AA)Br]Br, with the bromide-anion was different from the other complexes of this series, [Cu(AA)2](ClO4)2, [Cu(AA)2](NO3)2, and[Cu(AA)2]Cl2, undergo a two stage decomposition process. The complex with the nitrate-anion was found to be the most stable, while the complex with the chloride-anion the least stable among this series. The following order concerning the complexes’ stability can be done:
Nitrate > Perchlorate > Bromide > Chloride
The difference in the thermal stability of the complexes of GA and AA reveals that the anion might have a remarkable influence on the thermal stability of the complexes [7-12]. By comparing the thermal stability of all coordination compounds, a general trend cannot be found, since the ligand field also plays an enormous role on the thermal stability of the complexes.
Mechanistic aspects
The assignments of the mechanism are based on the assumption that the form of g(α) depends on the reaction mechanism. In the present study, nine forms of g(α), according to Šatava [14] are used to enunciate the thermal decomposition mechanism in each decomposition stage of the coordination compounds of GA and AA. For all of the nine forms of g(α), the correlation coefficients were calculated (Tables 3 and 4) and the form of g(α), for which the correlation has a maximum value, is chosen as the mechanism of the reaction. In the present case, the highest value of the correlation coefficient is obtained in all stages of the decomposition for:
| No. | Form of g (a) | Correlation coefficient (r) | ||||||
|---|---|---|---|---|---|---|---|---|
| Perchlorate complex | Nitrate complex | Chloride complex | Bromide complex | |||||
| Stage I | Stage II | Stage I | Stage II | Stage I | Stage I | Stage II | ||
| 1 | a2 | -0.9809 | -0.9762 | -0.9722 | -0.9981 | -0.9879 | -0.9618 | -0.9633 |
| 2 | a+(1-a)ln(1-a) | -0.9892 | -0.9886 | -0.9870 | -0.9995 | -0.9943 | -0.9791 | -0.9815 |
| 3 | [1-(1-a)1/3]2 | -0.9712 | -0.9001 | -0.9250 | -0.9966 | -0.9704 | -0.8721 | -0.8225 |
| 4 | [1-(2/3)a]-(1-a)2/3 | -0.9922 | -0.9928 | -0.9919 | -0.9986 | -0.9963 | -0.9857 | -0.9878 |
| 5 | -ln(1-a) | -0.9990 | -0.9998 | -0.9996 | -0.9816 | -0.9993 | -0.9992 | -0.9995 |
| 6 | [-ln(1-a)]1/2 | -0.9986 | -0.9997 | -0.9995 | -0.9770 | -0.9988 | -0.9978 | -0.9984 |
| 7 | [-ln(1-a)]1/3 | -0.9977 | -0.9988 | -0.9990 | -0.9705 | -0.9983 | -0.9902 | -0.9904 |
| 8 | 1-(1-a)1/2 | -0.9923 | -0.9916 | -0.9916 | -0.9973 | -0.9957 | -0.9821 | -0.9842 |
| 9 | 1-(1-a)1/3 | -0.9956 | -0.9964 | -0.9968 | -0.9938 | -0.9981 | -0.9911 | -0.9926 |
Table 3: Correlation coefficients calculated using the nine forms of g (α) for copper (II) complexes of GA.
| No. | Form of g (a) | Correlation coefficient (r) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Perchlorate complex | Nitrate complex | Chloride complex | Bromide complex | ||||||
| Stage I | Stage II | Stage I | Stage II | Stage I | Stage II | Stage I | Stage II | ||
| 1 | a2 | -0.9067 | -0.9733 | -0.9985 | -0.9824 | -0.9800 | -0.9747 | -0.9713 | -0.9920 |
| 2 | a+(1-a)ln(1-a) | -0.9496 | -0.9853 | -0.9987 | -0.9899 | -0.9904 | -0.9863 | -0.9854 | -0.9957 |
| 3 | [1-(1-a)1/3]2 | -0.7761 | -0.9568 | -0.9980 | -0.9769 | -0.9452 | -0.9529 | -0.9139 | -0.9886 |
| 4 | [1-(2/3)a]-(1-a)2/3 | -0.9677 | -0.9896 | -0.9990 | -0.9926 | -0.9938 | -0.9905 | -0.9901 | -0.9969 |
| 5 | -ln(1-a) | -0.9990 | -0.9990 | -0.9993 | -0.9993 | -0.9997 | -0.9991 | -0.9993 | -0.9988 |
| 6 | [-ln(1-a)]1/2 | -0.9978 | -0.9985 | -0.9992 | -0.9989 | -0.9992 | -0.9988 | -0.9981 | -0.9986 |
| 7 | [-ln(1-a)]1/3 | -0.9929 | -0.9977 | -0.9989 | -0.9989 | -0.9978 | -0.9985 | -0.9953 | -0.9982 |
| 8 | 1-(1-a)1/2 | -0.9593 | -0.9899 | -0.9989 | -0.9931 | -0.9929 | -0.9907 | -0.9881 | -0.9972 |
| 9 | 1-(1-a)1/3 | -0.9827 | -0.9946 | -0.9990 | -0.9960 | -0.9970 | -0.9950 | -0.9942 | -0.9980 |
Table 4: Correlation coefficients calculated using the nine forms of g (α) for copper (II) complexes of AA.
g(α) = -ln(1–α)
This corresponds to ‘random nucleation with one nucleus on each particle’, representing the ‘Mampel model’[7-12], irrespective of the ligand field, or counter-ionic effects.
Acknowledgment
We are grateful to Addis Ababa Science and Technology University for the support, which has facilitated the completion of this manuscript.
References
- Alaudeen M, Abraham A, Radhakrishnan PK (1995) Synthesis and antibacterial activity of rare earth perchlorate complexes of 4-(2′-hydroxynaphthylazo) antipyrine. J Chem Sci 107: 123-126.
- Alice CJ, Prabhakaran CP (1990) Palladium(II) complexes of Schiff bases derived from 5-amino-2,4-(1H, 3H) pyrimidinedione (5-aminouracil) and 1,2-dihydro-1,5-dimethyl-2-phenyl-4-amino-3H-pyrazol-3-one. Transit Met Chem 15: 449-453.
- Madhu NT, Radhakrishnan PK, Grunert M, Weinberger P, Linert W(2003) Antipyrine and its Derivatives with First Row Transition Metals. Rev Inorg Chem 23: 1-24.
- Madhu NT, Radhakrishnan PK (2001) Copper (II) complexes of 1,2-di(imino-4′-antipyrinyl)ethane and 4-n-(4′-antipyrylmethylidene) aminoantipyrine. Synth React Inorg Met Org Chem 31: 315-330.
- Gilman AG, Goodman LS, Gilman A (1980) The Pharmacological Basis of Therapeutics.(6thedn), Macmillan Publishing Co, New York, USA.
- Vinodkumar CR, Nair MKM, Radhakrishnan PK (2000) Thermal studies on lanthanide nitrate complexes of 4-n-(2′-furfurylidene) aminoantipyrine. J Therm Anal Calorim 61: 143-149.
- Madhu NT, Radhakrishnan PK, Grunert M, Weinberger P, Linert W(2003) A thermal decomposition study on cobalt (II) complexes of 1, 2-di (imino-4′-antipyrinyl) ethane. Thermochim Acta 400: 29-36.
- Madhu NT, Radhakrishnan PK, Grunert M, Weinberger P, Linert W(2003) Synthesis and thermal studies on iron(III) complexes of 4- N-(4′-antipyrylmethylidene) aminoantipyrine with varying counter ions. Thermochim Acta 407: 73-84.
- Madhu NT, Radhakrishnan PK,Williams E, Linert W(2005) Thermal decomposition studies on cobalt(II) complexes of 4-N-(4’-antipyrylmethylidene) aminoantipyrine with varying counter ions. J Therm Anal Calorim 79: 157–161.
- Madhu NT, Radhakrishnan PK, Linert W (2006) Thermal decomposition study on nickel(ii) complexes of 1,2-(diimino-4’-antipyrinyl)ethane with varying counter ions. J Therm Anal Calorim 84: 607-611.
- Madhu NT, Radhakrishnan PK, Linert W (2007) Thermolytic investigations on nickel(II) complexes of 4-N-(4′-antipyrylmethylidene) aminoantipyrine with various counterions. Int J Chem Kinet 39: 53-58.
- Madhu NT, Knittl ET, Fekadu K, Feleke H, Abuyea K, et al. (2017) Thermal Decomposition Studies on Iron(III) Complexes of 1,2-Bis(imino-4’- antipyrinyl) ethane with Varying Counter Ions. J Chem Appl Chem Eng 1:1.
- J. A. Dean (1976) Lange’s Hand Book of Chemistry. (13thedn), 421 McGraw-Hill, New York, USA.
- Satava V (1971) Mechanism and kinetics from non-isothermal TG traces. Thermochim Acta 2: 423.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi