Research Article, J Plant Physiol Pathol Vol: 11 Issue: 6
Leaf Spot Severity in Forage and Seed-Transmitted Exserohilum rostratum in Different Plant Phenological Stages
Patrícia Resplandes Rocha dos Santos, Rosângela Ribeiro de Sousa, Marcela Cristina Agustini Carneiro da Silveira, Paulo Henrique Tschoeke, Gil Rodrigues dos Santos*
Federal University of Tocantins, Campus Gurupi, Gurupi, Brazil
*Corresponding Author: Gil Rodrigues dos Santos
Federal University of Tocantins, Campus Gurupi, Gurupi, Brazil
E-mail: gilrsan@uft.edu.br
Received date: 31 December, 2021, Manuscript No. JPPP-21-50659;
Editor assigned date: 06 January, 2022, PreQC No. JPPP-21-50659 (PQ);
Reviewed date: 18 January 2022, QC No JPPP-21-50659;
Revised date: 25 January 2022, Manuscript No. JPPP-21-50659 (R);
Published date: 31 January, 2022, DOI: 10.37532/jppp.2021.10(1).284
Abstract
In order to obtain a successful pasture management, it is essential to know mainly the material to be sown, the local edaphic characteristics, and the disease epidemiology in these areas. Besides identifying the causal agent, the quantification of damage caused by pathogens is essential for the adoption of effective control measures. In this sense, seed transmission of fungi, which is the main transport mechanism in forage plants, is still little studied, as well as the severity and damage caused by the main diseases affecting plants. Thus, the objective of this work was to study the severity and progress of Exserohilum rostratum. The fungus was inoculated at different phenological stages of Brachiaria decumbens cv. Basilisk and Panicum maximum cv. Mombasa. The transmission of this pathogen from infected plants to seeds was also evaluated. The pathogen was inoculated in the plants at the following stages: Vegetative I; Vegetative II; Booting I; Booting II; Reproductive I; and Reproductive II. Severity was evaluated using a grade scale ranging from no symptoms to plants with more than 50% of affected leaf area. In order to evaluate the transmission of E. rostratum in seeds produced by the inoculated plants, the blotter test was used. E. rostratum was pathogenic to B. decumbens cv. Basilisk and P. maximum cv. Mombasa. Basilisk plants were susceptible in all phenological stages, with higher severity from the Vegetative II stage and greater progress between the second and tenth days after inoculation, coinciding with the seed-plant transmission of the fungal inoculum from this point on. For the cultivar Mombasa, higher severity of the disease during Vegetative stages I and II was observed, with the greatest progress of the disease between the second and fourth day after inoculation. Significant transmission of the inoculum from plants to seeds was observed from the Booting I stage.
Keywords: Brachiaria decumbens, Panicum maximum, Epidemiology, Helminthporporosis, Disease progress
Introduction
Brazil is a country that has a vast territorial extension and whose climatic conditions are excellent for the practice of agricultural activities [1]. With approximately 159 million hectares of pasture [2], the grasses Brachiaria sp. and Panicum maximum occupy a prominent position in the formation of these areas.
Over the past few years, the Brazilian livestock sector has undergone modifications by seeking technologies such as the improvement of forage plants, the replacement of nutrients in the soil, and the combat of pests, diseases, and invasive plants, consequently qualifying pasture management [3]. Such technologies enable to increase the forage yield of both seeds and dry biomass and also increase plant persistence in the field without the need to incorporate new areas for pasture formation [4]. The problem arising from breeding is usually that the selection aims to increase forage yield to the detriment of disease resistance.
Based on the development of technical knowledge of forage management, knowing the epidemiology and disease management is essential for the success of the sustainable exploitation of the crop in the field, as pathogenic species of Bipolaris, Curvularia, Exserohilum, Fusarium, Phoma, and fungi in stored seeds, such as Aspergillus, Penicillium, and Rhizopus have been reported to cause damage to the quality and establishment of forage plants [5-7]. Among these fungi, the species of Bipolaris, Curvularia, Drechslera, and Exserohilum represent a group of phytopathogens that severely attack forage grasses frequently, being known as dematiaceous hyphomycetes for their production of asexual spore pigments [8,9]. These phytopathogens are responsible for significant losses in plant yield. The symptoms caused by this group of fungi are generally leaf lesions caused by destruction of photosynthetic tissues, which can progress to necrosis and premature leaf death [10,11], limiting seed and forage formation.
It is known that, besides identifying the causal agent, quantifying the damage caused by these pathogens and diagnosing the period in which the plants are most susceptible to the disease are essential for the adoption of effective control measures, as these pathogens cause a reduction of dry biomass production and seeds with low sanitary quality. It was found that there are few studies on the severity and progress of diseases affecting forage grasses, especially on the ability of fungi to transmit from plants to the seeds produced. Knowledge of these aspects is very important to determine efficient control strategies.
Thus, the objective of this work was to study the severity and progress of leaf spot caused by Exserohilum rostratum inoculated in different phenological stages of Brachiaria decumbens cv. Basilisk and Panicum maximum cv. Mombasa, as well as to study the seed transmission of this pathogen.
Material and Methods
Seed sample and incidence of Exserohilum rostratum
Commercial samples of seeds of B. decumbens cv. Basilisk and P. maximum cv. Mombasa were obtained in the agricultural market, with both produced in the 2015/16 crop season. In order to check the primary incidence of E. rostratum in the seeds, the blotter test method [12] was used with 200 seeds per sample in four replicates of 50 seeds without prior disinfestation. The seeds were distributed in sterile plastic Gerbox boxes with two layers of filter paper sterilized and moistened with distilled water, and sterilized at a ratio of 2.5 times the mass of the dry substrate. The Gerbox boxes were placed in an incubation chamber under 12 h photoperiod and temperature of 25 ± 2°C for seven days [12]. After the incubation period, the analysis of fungi present in the seeds was performed using a stereomicroscope, through which the morphological characteristics of the fungal structures were visualized. Genus-level identification was conducted based on specialized literature, such as Ellis [13], Barnett and Hunter [14], and Watanabe [15].
Plant obtaining, Exserohilum inoculation, and treatments
Seeds of the cultivars were sown in pots of 15 L capacity of substrate consisting of the mixture of sand and soil in the 1:1 ratio, with both being previously autoclaved for one hour at 120°C. The seeds were treated with imidacloprid (150 g a.i./L) + thiodicarb (450 g a.i./L) at a dose of 300 mL c.p./100 kg of seeds and metalaxyl-M (10 g a.i./L) + fludioxonil (25 g a.i./L) at a dose of 200 ml c.p./100 kg of seeds. Seed samples were packed in plastic bags, where the chemical treatment was conducted. The fungicide and insecticide was applied to the seeds, the plastic bags were inflated, and then seeds were stirred in order to allow better distribution of the product [16].
The pathogen was obtained from the sanitary analysis of Brachiaria sp. seeds, in which monosporic isolates were obtained and grown in PDA (potato-dextrose-agar) medium, and maintained in an incubation chamber under 12-h photoperiod and a temperature of 25 ± 2°C for seven days for inoculation reproduction. The isolates were subjected to molecular analysis for species identification. Molecular identification was conducted through DNA extraction [17], being subjected to polymerase chain reaction (PCR) followed by sequencing (Sanger). The identification was conducted by the Laboratory of Applied Molecular Biology of the Instituto Biológico - São Paulo, Brazil. After molecular analysis, the etiological agent that caused leaf spots in forage was identified and associated with the species Exserohilum rostratum (teleomorph Setosphaeria rostrata) (MT755902), with their respective fragments deposited on Gen Bank.
The experimental design was completely randomized with seven treatments in six replicates. After seedling emergence, the application of treatments was conducted using a manual sprinkler, with leaves being sprayed on their abaxial and adaxial faces, with suspension of conidia in the concentration of 1 x 106 conidia mL-1 of E. rostratum. The inoculation was conducted in six phenological stages of the plants, in which conidia suspension was applied only when the plant had reached the following pre-established moments: Vegetative I (30 days after emergence - DAE); Vegetative II (60 DAE); Booting I (Beginning of inflorescence development); Booting II (Full development of inflorescence); Reproductive I (green seeds); Reproductive II (seed maturation); and Control (plants without inoculation of the pathogen).
After inoculation, the plants remained in a dark humid chamber for 36 h and were then transferred to a natural environment to monitor the severity and progress of the disease. The tissues with symptoms were taken to the laboratory and subjected to superficial disinfestation to isolate the pathogens in PDA medium in order to confirm Koch’s Postulates [18]. During the experimental period, the data of maximum and minimum temperature, relative air humidity, and precipitation were recorded to observe the influence of climatic conditions on the development of the pathogen in the plants. Climatic data were obtained from the Automatic Station located in the municipality of Gurupi, Tocantins, Brazil (11° 43’ 45” latitude, 49° 04’ 07” longitude, altitude of 278 m).
Evaluation of symptons of Exserohilum rostratum
After 48h of pathogen inoculation, evaluations of disease severity were conducted at two-day intervals. After five evaluations, the frequency was reduced, with seven-day intervals. Ten evaluations were conducted in total and the symptoms were evaluated using the grade scale described by Santos et al., [19], in which the plant as a whole (all leaves) was considered, where: 0 - absence of symptoms; 1 - less than 1% of affected leaf area; 3 - between 1 and 5% of affected leaf area; 5 - between 6 and 25% of affected leaf area affected; 7 - between 26 and 50% of affected leaf area; 9 - more than 50% of affected leaf area. Subsequently, the severity scores were converted into percentages of diseased leaf area by the midpoint of each grade according to the number of evaluations for each trial, which was based on the onset of the disease. At the end of the evaluations, severity data were converted into an area under disease progress curve (AUDPC), according to the method described by Shaner and Finney [20].
Seed-plant transmission of Exserohilum rostratum
After inoculation of E. rostratum in the respective phenological stages Vegetative I and II, Booting I and II, and Reproductive I and II in both cultivars (previous subtopic), the completion of the plant cycle was awaited for seed harvesting. Plant seeds without inoculation were also collected, representing the control. A manual harvesting method was used in which all inflorescences were cut before the beginning of seed removal and placed on a lined and disinfected surface for drying. The seeds that came off the inflorescences were collected and taken to the laboratory for analysis.
Samples with 200 seeds of each treatment were submitted to the sanity test by the blotter test method [12]. The experimental design was completely randomized (CRD) with four replicates in a 7x2 factorial scheme, with seven treatments (phenological stage) of seeds submitted or not to disinfestation [12]. After the incubation period, a morphological analysis of the fungi present in the seeds was performed using a stereomicroscope in order to determine the existence of seeds infected with E. rostratum with the help of specialized literature, such as Watanabe [15].
Statistical analysis
Quantitative data (disease progression) were submitted to regression analysis and qualitative data were subjected to analysis of variance, with mean comparison by Tukey and Scott-Knott test at 5% probability. For the analysis, the SISVAR software was used [21].
Results
During conduction of the tests, which were exposed to the natural environment condition, little variation of the climatic characteristics was verified, except for the rains that occurred in all months, although with more voluminous precipitation between November and December, decreasing in the following months (Figure 1). This condition was favorable to the development of leaf spots. The average maximum and minimum temperatures of 32.2°C and 22.2°C, respectively, and relative humidity of 78.5% were also recorded.
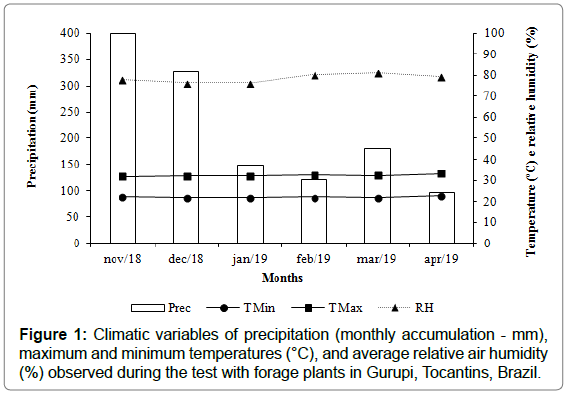
Figure 1: Climatic variables of precipitation (monthly accumulation - mm), maximum and minimum temperatures (°C), and average relative air humidity (%) observed during the test with forage plants in Gurupi, Tocantins, Brazil.
Regarding the initial sanitary analysis of the seeds, before the seeds were treated for sowing in pots, there was no incidence of Exserohilum sp. Thus, it was ensured that the experiment was conducted free of natural infection caused by the fungus. The genera Bipolaris, Cladosporium, Curvularia, Fusarium, Penicillium, Phoma, Phyllosticta, Pyrenochaeta, and Trichoderma were found associated with the seeds of cultivars Basilisk and Mombasa (Table 1). However, it is worth mentioning, as aforementioned in the methodology, that the seeds were treated with fungicide and insecticide in order to obtain plants aimed to the study of the severity and progress of E. rostratum, preventing the emergence of other diseases.
cv. BasiliskPanicum maximum
cv. MombasaBipolaris31.010Cladosporium318.5Curvularia11.54.5Fusarium47.5Penicillium0.50.5Phoma26.57Phyllosticta03.5Pyrenochaeta9.53Trichoderma10
1Mean of four replicates, with each replicate consisting of 50 seeds.
Table 1: Fungal incidence in Basilisk and Mombasa seed samples.
The temporal progress of leaf spot caused by E. rostratum in B. decumbens cv. Basilisk and P. maximum cv. Mombasa was represented in Figure 2. In both forages, the initial symptoms of the disease appeared after two days of inoculation, regardless of the phenological stage in which it was applied. This indicates that the climatic conditions were favorable for pathogen infection. As expected, the plants used as controls remained healthy until the end of the cycle.
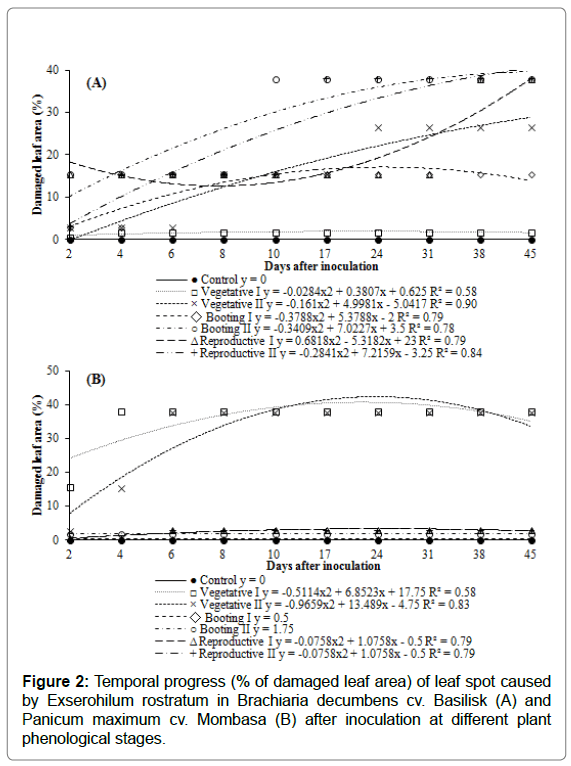
Figure 2: Temporal progress (% of damaged leaf area) of leaf spot caused by Exserohilum rostratum in Brachiaria decumbens cv. Basilisk (A) and Panicum maximum cv. Mombasa (B) after inoculation at different plant phenological stages.
For Basilisk (Figure 2A), only the plants inoculated during the Vegetative I stage (30 DAE) presented affected leaf area below 2% over time. The other phenological stages presented an increase in disease severity until 45 days after the inoculation of E. rostratum, ranging between 15% and 38% of the affected leaf area. The plants with the greatest sensitivity to pathogen infection were inoculated at the Booting II and Reproductive I stages, presenting 15% of affected leaf area 48 hours after inoculation and progressing to 38% of affected leaf at 45 days after inoculation. Plants inoculated during the Reproductive II stage, despite finishing seed production, were also very sensitive to the fungus, with 15% severity at four days after inoculation, progressing to 38% at 17 days, and stabilizing the disease until the end of the evaluation. It is important to note that these forage grasses are of perennial cycle. Thus, they continue to till and emit new leaves after seed production, being still susceptible to the pathogen, and new infections may arise.
The cultivar Mombasa (Figure 2B) presented higher tolerance to E. rostratum severity in the Booting I and II and Reproductive I and II stages, in which it was infected by the pathogen (affected leaf area below 3%) but showed little or no disease progress until 45 days after inoculation. In Vegetative stages I (30 DAE) and II (60 DAE), the plants were more susceptible to the pathogen, with affected leaf area of 15% and 3%, respectively, at two days after inoculation, with progress after this period.
The disease severity, expressed by the AUDPC and considering inoculation at different phenological stages of B. decumbens cv. Basilisk and P. maximum cv. Mombasa is shown in Figures 3A and 3B, respectively.
It was observed that, for the cultivar Basilisk, when inoculation was conducted in the Vegetative stage II up to the Reproductive stage II, it resulted in greater disease progress in relation to the Vegetative stage I. The AUDPC of Vegetative stage I did not differ statistically from the plants used as control (Figure 3A).
When analyzing the AUDPC of the cultivar Mombasa, greater progress in disease severity was observed in Vegetative stages I and II. Although the stages of Booting I to Reproductive II did not differ statistically, it was considered that Booting I and II were significantly equal to the control (Figure 3B).
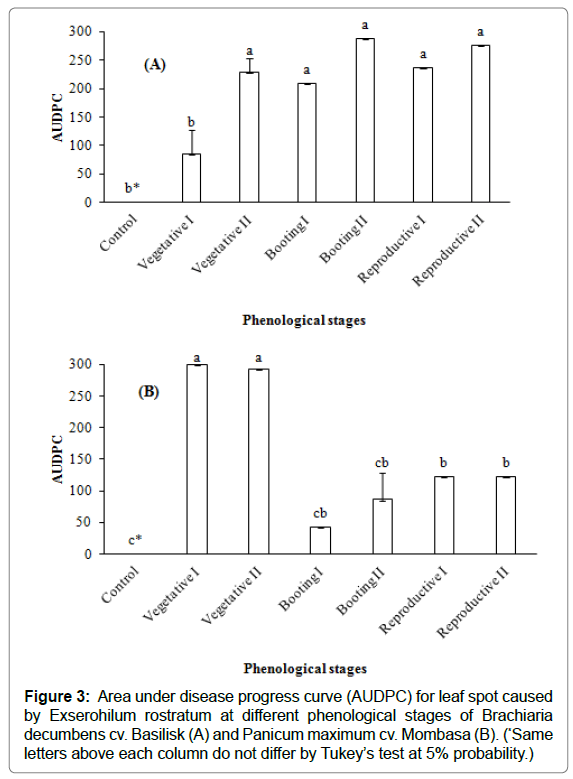
Figure 3: Area under disease progress curve (AUDPC) for leaf spot caused by Exserohilum rostratum at different phenological stages of Brachiaria decumbens cv. Basilisk (A) and Panicum maximum cv. Mombasa (B). (*Same letters above each column do not differ by Tukey’s test at 5% probability.)
Symptoms caused by E. rostratum in B. decumbens cv. Basilisk leaves started with small leaf spots of reddish-brown color (Figure 4A and 4B), resulting in coalescence, drying, and progressive leaf necrosis (Figure 4A). The characteristic symptoms of E. rostratum are elongated, elliptical lesions that are gray or brown colored and varying in length, occurring initially in older leaves. Figures 4C and 4D show the signs of the pathogen after incubation of the leaf tissue infected, with the presence of reproductive structures (conidia). In the field, under favorable conditions to the development of the pathogen, this sporulation usually occurs (Figure 4C) and conidia dispersal occurs by wind or rain/irrigation water, spreading the pathogen to other areas.
Figure 4: Progress of leaf spot caused by Exserohilum rostratum in Brachiaria decumbens cv. Basilisk: Evolutionary symptoms of the disease with coalescence of lesions followed by necrosis and drying of the limbus (A); Initial symptoms of the disease (B); Signs of the pathogen in the leaf tissue (C); Fungal conidium (D). Photo: Own authorship.
The main means of survival, dissemination, and introduction of pathogens in new areas of cultivation is through seeds, which come mostly from sick plants. In this sense, for each treatment, seeds were collected to check the phenological stage in which seed-plant transmission was observed (Table 2).
1WD-Without seed disinfestation; DS - With seed disinfestation. 2Means of four repetitions. Means followed by the same lowercase letter in the column and uppercase letter in the line do not differ by Scott-Knott test at 5% probability.
Table 2: Incidence of Exserohilum rostratum in seeds produced by plants inoculated at different times (phenological stages).
Basilisk plants inoculated in the Vegetative II, Booting I and II, and Reproductive I and II stages produced seeds infected with E. rostratum, with those being the periods in which the greatest disease severity was observed. The seeds were analyzed with and without superficial disinfestation, a technique that allows the elimination of contaminating microorganisms present on the outer shell of a seed (tegument). The seeds from plants inoculated in the phenological stages mentioned above, without disinfestation, presented statistically higher incidence of E. rostratum than disinfected seeds. However, even in a lower incidence, the possibility of pathogen transmission was not discarded, with emphasis on the seeds in which E. rostratum was inoculated during the Reproductive II stage, which had 27% transmission (Table 2).
The cultivar Mombasa, which presented higher disease severity during the Vegetative I and II stages when inoculated, produced seeds originating from these inoculation stages with the lowest percentages and incidence of the fungus, with or without superficial disinfestation. The highest seed-plant transmission rates were observed during the Reproductive I and II stages (86% and 67% - WD) and with statistically lower transmission rates for disinfected seeds (48% and 53% - DS) (Table two).
Discussion
Leaf spots caused by dematiaceous fungal infections can cause large losses in agricultural activity [22-25] as a result of the decrease or total destruction of the plant photosynthetic area. Conditions such as a susceptible host, a virulent pathogen, and a favorable environment are the key factors for infection and for the development of these diseases to occur [26,27].
As observed, under average temperature of 27°C, relative air humidity of approximately 80%, and frequent precipitation, the forages B. decumbens cv. Basilisk and P. maximum cv. Mombasa were susceptible to E. rostratum, although the disease severity ranged according to the phenological stages in which the pathogen was inoculated. According to the Köppen classification, the climate of the study region is Aw, defined as hot and humid tropical with a rainy season in summer [28], resulting in favorable weather conditions for fungal infection during the conduction of the experiment, as there was predominance of high temperatures and high relative humidity.
Regarding disease severity and production of infected seeds, areas where the disease occurs more severely do not always favor high levels of seed infection. On the other hand, in areas where the disease is manifested with less intensity, high levels of seed infection can be detected, which are more associated with the stage of crop development, making it more susceptible or not to seed infection [29-31].
In this study, the highest severity of E. rostratum in the cultivar Basilisk was observed from the Vegetative II to Reproductive II stages, coinciding with higher levels of seed transmission and infection. On the other hand, the highest severity of E. rostratum in the cultivar Mombasa was observed during Vegetative stages I and II, and the highest seed infection from the Booting I stage. Thus, it was found that the most susceptible period of these forages to the pathogen did not necessarily coincide with the period of greatest susceptibility to seed infection. Vechiato et al., [32], who analyzed seeds of the species B. decumbens, B. brizantha, and P. maximum, reported a significant incidence of E. rostratum, although they did not test the disease pathogenicity and severity.
For the cultivar Basilisk with inoculation of E. rostratum from the Booting II until Reproductive II stages and for the cultivar Mombasa with inoculation in the Vegetative I and II stages, leaf spot severity was verified in the first four days, with relatively high values of affected leaf area (between 15% to 38%). The multiple lesions that occur in the plant leaf tissue, when in a favorable environment, develop very quickly, suggesting the action of fungal toxins involved in the infection process [33], corroborating with reports of the presence of a nonspecific toxin, monocerine, in the water-soluble phytotoxic compounds of E. turcicum [34,35] and E. rostratum [36]. Robeson and Strobel [37] isolated the monocerin of E. turcicum and reported that the compound demonstrated phytotoxic activity on the growth of Sorghum halepense and Cucumis sativus. However, more works relating the role that the monocerin present in Exserohilum sp. plays in the process of plant infection are needed.
Sick plants tend to produce seeds infected by the pathogen. This fact was demonstrated in the present study and also by other authors [31,38]. Thus, it can be inferred that among the consequences resulting from the plant-seed transmission of fungi, it is possible to mention the spread of pathogens by the seeds to exempt areas [7,39] and the reduction of the physiological quality of seeds, which may reduce germination and emergence [40-42]. In addition, when contaminated seeds are aimed to consumption, such as grains, they can present harmful toxins to animals and humans [43,44].
According to Neergaard [29], the occurrence of a disease whose causal agent comes from the seed depends on the three following main factors: inoculum quantity in the seeds, transmission rate, and its rate of progress in the field. Picinini and Fernandes [45] reported that lots of barley seeds presenting infection levels above 5% of Bipolaris sorokiniana (dematiaceous) were sufficient to produce the inoculum necessary for the development of an epidemic in the field under favorable conditions. Assuming this condition in this study, in which Brachiaria and Panicum seeds presented infection of 27% and 53%, respectively, their use for the formation of new pasture areas would result in a large production of inoculum and infection of the plants with E. rostratum.
The incidence of E. rostratum decreases seed quality in several aspects regarding sanitary and visual quality and their production, whether by forage plants or other crops of agricultural importance [46]. According to Silva et al., [42], seeds infected with E. rostratum germinate less due to the colonization of the inner seed tissues by the pathogen, such as the pericarp, endosperm, or even the embryo. It is likely that E. rostratum infects the inner seed tissues because even after disinfestation its detection associated with the seeds is possible [47].
In this study, E. rostratum transmission was observed. In addition, even after the disinfestation of Brachiaria and Panicum seeds, incidence of the fungus in the seeds was observed. Reis and Casa [48] discussed the association of B. sorokiniana with barley seeds, in which the fungus located internally in the seed in the form of mycelium in the pericarp and endosperm would result in higher transmission rates, as the pathogen has higher efficiency to survive and subsequently move to the root and shoot organs of plants. Thus, B. decumbens cv. Basilisk and P. maximum cv. Mombasa seeds produced and evaluated in this study could be potential vectors of dissemination and transmission of E. rostratum.
E. rostratum has also been reported to cause severe leaf spots in other grasses such as maize and rice [42,49] and even in other cultivated plants, such as açaí, coconut, beans, and fishtail palm [50,51]. In addition, forage grass species have been very susceptible to dematiaceous fungi that cause leaf spots, such as Bipolaris sp., Curvularia sp., and Helmintosporium sp., among others [7,11,52], which demonstrates the need for the development of resistant cultivars and/or phytosanitary products for the adequate management of the disease. Most grass-infecting dematiaceous hyphomycetes have similar disease cycles [8], initiating infection by older leaves by conidia produced from infected tissues or crop residues, with repeated cycles [53,54] and in severe cases, plant weakening, with loss of vigor and emergence.
After analyzing the temporal progress of the disease caused by E. rostratum and seed-plant transmission in the forage cultivars Basilisk and Mombasa, the need to establish control measures was emphasized mainly in fields of seed production, in which there is E. rostratum incidence, especially considering the phenological stages of the plants most susceptible to the disease. Therefore, the production of forage seeds with sanitary quality control is essential for the success of the seed production sector in Brazil.
Acknowledgements
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Post-Graduate Program in Plant Production of the Federal University of Tocantins.
References
- Silva GM, Silva FF, Viana PT, Rodrigues ESO, Moreira CN, et al. (2016a) Evaluation of tropical forages: Review. Vet Medand Anim Sci 10:190-196.
- IBGE (2017) Brazilian institute of geography and statistics. Agricultural Census: Preliminary results. Rio de Janeiro, 7.
- Dias-Filho MB (2010) Grazing cattle production on the agricultural frontier. Belém, PA: Embrapa Eastern Amazon.
- Dias-Filho MB (2016) Pasture use for beef cattle production in Brazil: Past, present and future. Belém, PA: Embrapa Eastern Amazon.
- Pratt RG (2006) Johnsongrass, Yellow foxtail, and broadleaf signalgrass as new hosts for six species of Bipolaris, Curvularia, and Exserohilum pathogenic to Bermudagrass. Plant Disease 90:538.
- Marchi CE, Fernandes CD, Bueno ML, Batista MV, Fabris LR (2010) Fungi associated to commercial seed of braquiaria grass. Biological Institute Archive 77:65-73.
- Santos GR, Tschoeke PH, Silva LG, Silveira MCAC, Reis HB, et al. (2014) Sanity analysis, transmission and pathogenicity off fungi associated with forage plant seeds in tropical regions of Brazil. J Seed Sci 36:54-62.
- Smiley RW, Dernoeden RH, Clarke BB (1992) Compendium of Turfgrass diseases. American Phytopathological Society Press.
- Pratt RG (2000) Diseases caused by dematiaceous fungal pathogens as potential limiting factors for production of bermudagrass on swine effluent application sites. Agron J 92:512-517.
- Lima LM, Pozza EA, Torres HN, Pozza AAA, Salgado M et al. (2010) Relationship between nitrogen/potassium with Phoma spot and nutrition of coffee seedlings cultivated in nutrient solution. Trop Plant Pathol 35:223-228.
- Tavanti TR, Takada J, Ribeiro LFC, Moraes SRG, Pedreira CE (2016) Occurrence of Bipolaris maydis leaf spot on tanzania guineagrass in the north region of the Mato Grosso state. J Agri-environ Sci 14:82-85.
- Brasil (2009) Ministry of agriculture, livestock and supply. Seed Sanitary Analysis Manual/Ministry of Agriculture, Livestock and Supply. Agricultural Defense Secretariat, Brasília: Map/ACS.
- Ellis MB (1971) Dematiaceous Hyphomycetes. Commonwealth Mycological Institute Kew.
- Barnett HL, Hunter BB (1998) Illustrated genera of imperfect fungi. APS Press.
- Watanabe T (2010) Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. CRC Press.
- Marchi CE, Fernandes CD, Jerba VF, Vechiato MH, Trentin RA, et al. (2006) Treatment of seeds of Marandu grass to control Fusarium spp. and other soil fungi. Biological 68:596-598.
- Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11-15.
- Alfenas AC, Mafia RG (2007) Métodos em Fitopatologia. UFV.
- Santos GR, Café-Filho AC, Leão FF, César M, Fernandes LE (2005) Disease progress and crop losses due to watermelon gummy stem blight. Brazilian Horticulture 23:230-234.
- Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox Wheat. Phytopathology 67:1051-1056.
- Ferreira DF (2014) Sisvar: a guide for its bootstrap procedures in multiple comparisons. Sci Agrotech 38:109-112.
- Sharma K, Goss EM, Dickstein ER, Smith ME, Johnson JA, et al. (2014) Exserohilum rostratum: Characterization of a cross-kingdom pathogen of plants and humans. Plos One 6:9.
- Mourão DSC, Pereira TFS, Souza DJ, Chagas Junior AF, Dalcin MS, et al. (2017) Essential oil of Cymbopogon citratus on the control of the Curvularia leaf spot disease on maize. Medicines 20:4.
- Farag MF, Attia FM (2020) A first record of Exserohilum rostratum as a new pathogen causing bean blight in Egypt. J Plant Pathol Microbiol 11:496.
- Sun X, Qi X, Wang W, Liu X, Zhao H, et al. (2020) Etiology and symptoms of maize leaf spot caused by Bipolaris spp. in Sichuan, China. Pathogens 9:3.
- Guini R, Hamada E, Bettiol W (2011) Impacts of climate change on diseases of important crops in Brazil. Jaguariúna: Embrapa Environment.
- Cota LV, Silva DD, Costa RV (2013) Helmintosporiosis caused by Exserohilum turcicum in corn crop. Embrapa Corn and Sorghum-Circular Technique (INFOTECA-E).
- Hargreaves GH, Samani ZA (1985) Reference crop evapotranspiration from temperature. Appl Engineer Agri 1:96-99.
- Neergaard P (1979) Seed pathology. (2edtn), McMillan.
- Lima EF, Carvalho JMFC, Carvalho LP, Costa JN (1985) Transport and transmissibility of Colletotrichum gossypii var. cephalosporioides through cotton seeds. Brazil Phytopathol 10:99-109.
- Araujo AE (2008) Detection and plant-seed transmission of Colletotrichum gossypii South var. cephalosporioides Costa: effect of levels of incidence in the seed and chemical control of the aerial part on the progress of cotton ramulosis. Luiz de Queiroz College of Agriculture.
- Vechiato MH, Aparecido CC, Fernandes CD (2010) Frequency of fungi in commercialized seed lots from Brachiaria and Panicum. São Paulo: Biological Institute.
- Brunings AM, Datnoff LE, Palmateer AJ, Locke JC, Krause CR (2009) Exserohilum leaf spot on tiger grass. Plant Health Progress 10.
- Bashan B, Levy Y (1992) Differential response of sweet corn cutlivars to phytotoic water-soluble compounds from culture filtrates of Exserohilum turcicum. Plant Dis 76:451-454.
- Cuq F, Herrmann-Gorline S, Klaebe A, Rossignol M, Petitprez M (1993) Monocerin in Exserohilum turcicum isolates from maize and a study of its phytotoxicity. Phytochemistry 34:1265-1270.
- Borges FC, Araújo RNM, Ferreira LRS, Marinho AMR, Marinho PSB et al. (2011) Monocerin isolated from the biomass of the fungus Exserohilum rostratum associated with the leaves of Bauhinia guianensis. Annual meeting of the Brazilian Chemical Society, 34.
- Robeson DJ, Strobel GA (1982) Monocerin, a phytotoxin from Exserohilum turcicum (Drechslera turcica). Agri Biol Chem 46:2681-2683.
- Tomasini TD, Soman JM, Silva JC, Inowe MA, Maringoni AC (2017) Plant-seed transmission of Curtobacterium flaccumfaciens pv. flaccumfaciens in bean cultivars. Summa Phytopathologica 43.
- Marchi CE, Fernandes CD, Anache FC, Jerba VF, Fabris LR (2008) Chemo and thermotherapy in seeds and fungicide application in Brachiaria brizantha as strategies in charcoal management. Summa Phytopathologica 34:321-325.
- Farias CJ, Del Ponte EM, Lucca-Filho O, Pierobon CR (2005) Helminthosporiosis-causing fungi associated with black oat (Avena strigosa Schreb) seeds. Braz J Agrosci 11:57-61.
- Martins CC, Melo PAFR, Pereira FECB, Anjos Neto AP, Nascimento LC (2017) Sanity quality of Brachiaria brizantha cv. Marandu and Xaraés seeds harvested in different states in Brazil. Biosci J33:1431-1440.
- Silva WR, Moreira-Nuñez V, Maich SLP, Gaviria-Hernández V, Gonçalves VP, et al. (2019) Physiological changes of rice seeds in the presence of Exserohilum rostratum. 30:1-10.
- Lins JLF, Silva JM, Silva LP, Santos TMC, Santos EL (2014) Occurrence of field fungi and storage in ingredients and swine feed. Green J Agroecol Sustain Develop 9:14-20.
- Hemckmeier D, Galindo CM, Melchioretto E, Gava A, Casa RT (2018) Claviceps purpurea and Bipolaris sp. as a cause of ergotism in cattle in the state of Santa Catarina. Braz Vet Res 38:875-882.
- Picinini EC, Fernandes JM (1999) Barley diseases. Diseases in winter cereals - Epidemiological aspects and control. Deep step.
- Silva FJA, Maich SLP, Meneses PR, Bellé C, Barros DR, et al. (2016b) First report on Exserohilum rostratum pathogenicity causing brown spot to rice in Brazil. Plant Dis 100.
- Kusai NA, Azmi MMZ, Zainudim NAI, Yusof MT, Razak AA (2016) Morphological and molecular characterization, sexual reproduction, and pathogenicity of Setosphaeria rostrata isolates from rice leaf spot. Mycologia 105:905-914.
- Reis EM, Casa RT (1998) Winter cereal seed pathology. Passo Fundo: North Village.
- Tsai JN, Tsai WH, Chen JL (2001) Pathogenicity of Exserohilum rostratum on corn and weeds in the corn fields. Plant Pathol Bullet 10:181-186.
- Cúndom MA, Gutiérrez AS, Cejas P, Cabrera MG (2006) Exserohilum rostratum Pathogenicity of Caryota mild na Argentina. Summa Phytopathologica 32:277-279.
- Poltronieri LS, Verzignassi JR, Benchimol RL, Freire FCO (2008) First record of Exserohilum rostratum (Setosphaeria rostrata anamorph) causing leaf spots in açaí palm in Brazil. Summa Phytopathologica 34:195.
- Favoreto L, Santos JM, Calzavara AS, Lara LA (2011) Phytosanitary study, multiplication and taxonomy of nematodes found in forage grass seeds in Brazil. Braz Nematolo 35:1-2.
- Vargas Junior JM (1994) Management of Turfgrass Diseases. CRC Press.
- Couch HB (1995) Diseases of Turfgrasses. Krieger Pub Co.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 



