Research Article, J Fashion Technol Textile Eng Vol: 11 Issue: 5
Herbal Antimicrobial Finishing of Cotton and Khadi Fabric Using Moringa (Moringa oleifera) Leaves Extract
Komal Dwivedi*, Ekta Sharma and Nargis Fatima
Department of Textiles and Apparel Designing, Sam Higginbottom University of Agriculture, Uttar Pradesh, India
*Corresponding Author: Komal Dwivedi
Department of Textiles and Apparel
Designing, Sam Higginbottom University of Agriculture, Uttar Pradesh, India
E-mail: softydwivedi@gmail.com
Received date: 31 October, 2022, Manuscript No. JFTTE-22-78683; Editor assigned date: 02 November, 2022, PreQC No. JFTTE-22-78683 (PQ); Reviewed date: 16 November, 2022, QC No. JFTTE-22-78683; Revised date: 24 January, 2023, Manuscript No. JFTTE-22-78683 (R); Published date: 31 January, 2023, DOI: 10.4172/2329-9568.1000286
Citation: Dwiv edi K, S harm a E , Fatima N (2023) Herbal Antimicrobial Finishing of Cotton and Khadi Fabric Using Moringa (Moringa Oleifera) Leaves Extract. J Fashion Technol Textile Eng 11:1
Abstract
Microbes can be found almost everywhere in the environment including in textile materials. Natural fibers are especially prone to attack by microorganisms that cause staining and bad odor along with deterioration of strength and other mechanical properties. Consumers are now increasingly aware of healthy and hygienic lifestyles and there is an expectation for a wide range of textile products with antimicrobial properties. In the present study, the antimicrobial finish was imparted to cotton and khadi fabrics using Moringa leaf (Moringa Oleifera) extract through direct and microencapsulation methods to improve the antimicrobial properties of the fabric. Microcapsules were prepared by using Moringa Oleifera extract as core material and chitosan as wall material and these were applied on fabric using pad dry cure method. Both treated and untreated samples were subjected to various tests including SEM, FTIR and antimicrobial efficacy carried out by quantitative and qualitative methods in terms of bacterial reduction and durability of antimicrobial activity against washing. The treated samples showed satisfactory antimicrobial activity against gram positive (Staphylococcus aureus) and gram negative (Klebsiella pneumoniae) bacteria. The deposition of microcapsules was observed in the SEM analysis and the active compounds of Moringa leaves (Moringa Oleifera) extract were also confirmed by FTIR spectroscopy. Microencapsulated herbal samples showed higher resistance to microbes even after 10 wash cycles than directly treated samples.
Keywords: Antimicrobial finish; Chitosan; Moringa; Microencapsulation
Introduction
Textile materials especially cotton and khadi, are the most preferred fabrics for garment production due to their comfort property and absorbency, however, these fabrics offers an ideal environment for the growth of microbes that causes an obnoxious odor causes staining and also spreading skin diseases. In recent years there has been an increasing awareness of consumers towards health and hygiene coupled with the harmful effects of synthetic based finishing treatments on fabrics for antimicrobial activity. Consumers are looking for safer alternatives to synthetic chemicals and environmentally friendly processes. Thus, herbal extracts are the best alternatives. Herbal extracts from stem, leaves, flowers fruits and seeds of diverse species of plants exhibits antimicrobial properties and can be used as textile finishing agents either in the crude form or as microcapsules to enhance the durability of finishing treatment. Microencapsulation is a rapidly expanding technology with greater applicability in textiles in recent years.
Microencapsulation may be defined as a micro packaging technique, where in a active core material is encapsulated in a polymer shell of limited permeability. It is a physiochemical technique that provides textiles with resistance to microorganisma and insects.
Moringa belongs moringaceae family; Moringa oleifera tree has approximately 5 mh to 10 mh eight and found in all over the world, as every part of Moringa is used for cerain nutritional and medicinal purpose. Besides being a good source of protine, vitamins, oils, fatty acids, micro-macro minerals elements and various phenolics, it is also reported for its anti-inflammatory, antimicrobial, antioxidant, anticancer, cardiovascular, hepatoprotective, anti-ulcer, diuretic, antiurolithiatic and antihelminitic properties. Its multiple pharmaceuticals effects are capitalized as therapeutic remedy for various diseases in traditional medicinal system [1].
In the present study we attempted to apply Moringa leaf extract to cotton and khadi fabric to impart antimicrobial properties [2]. Two methods that is direct and microencapsulation were used for this purpose and both treated and untreated fabrics were subjected to various tests to analyze the efficacy of antimicrobial finishing.
Materials and Methods
The aim of the investigation for this study was to utilize Moringa leaves extract for antimicrobial finishing of cotton and khadi fabrics by direct and microencapsulation technique and to evaluate the treated and untreated samples. The research methodology adopted to carry out the present research has been bifurcated in the following heads and sub-heads.
Raw material used
Fabrics: Cotton and khadi fabrics were used for the application of antimicrobial finish as these fabrics are cellulosic in nature and offer an ideal condition for microbial growth. The EPI of cotton fabric was 104 and PPI 77 and for khadi fabric EPI was 42 and 35 PPI.
Plant material
Fresh leaves of Moringa (Moringa Oleifera) were collected and its extract was used as core material for antimicrobial finish on selected cotton and khadi fabrics (Table 1).
| S.No. | Chemicals used | Purposes |
|---|---|---|
| 1 | Chitosan | Wall material of microcapsules |
| 2 | Distilled water | Dissolving the chitosan |
| 3 | Silicon oil | Making emulsion in the formation of microcapsules |
| 4 | Citric acid | Cross linking agent |
| 5 | Amylase | Desizing agent |
| 6 | Na2CO3 | Scouring agent |
| 7 | H2O2 | Bleaching agent |
Table 1: Chemicals used with their purposes.
Pre-treatment of fabrics
Before applying antimicrobial finish on cotton and khadi fabrics, the entire length of fabric was desized, scoured and bleached using standardized recipes.
Desizing
Desizing is generally done to remove starch or sizing material from the gray fabric to make the fabric more absorbent without undergoing any chemical or physical damage [3]. The recipe given was used for desizing of cotton and khadi fabric.
10 gms of amylase enzyme solution per 1000 ml of water was prepared maintaining the material: Liquor ratio 1:50. It was heated to 50°C-60°C. Cotton and khadi fabrics were dipped into the solution separately and stirred gently [4]. Then fabrics were kneaded and squeezed and finally rinsed under tap water and then dried in sunlight.
Bleaching and scouring
Scouring and bleaching were carried out simultaneously by using the recipe given. A detergent solution containing 3 gm of NaOH, 5 gm of Na2CO3, 1 gm of wetting agent (soap) and 3 gm of H2O2 added in 1000 ml of water. Separate solutions were prepared for both cotton and khadi fabrics maintaining the material: Liquor ratio 1:50. Solutions were heated to 100°C and cotton and khadi fabrics were dipped into prepared solution separately and stirred gently to about 60 minutes [5]. After pretreatment the fabrics were thoroughly washed with hot water and finally rinsed with cold water. Then samples were allowed for drying in sunlight and after that kept in dry place [6].
Method of extraction of plant material: Moringa leaves were plucked, sorted and washed with water to remove dust particles and were dried under shade at room temperature. After that crushed into small pieces and stored in air tight containers for extraction. The soxhlet apparatus was used for extraction process. In this sequence dried and crushed leaves were weighted 20 gms by electronic weighing balance; some glass wool was added into soxhlet tube as filtering material then weighted crushed leaves were added into soxhlet tube and tube was fixed with round bottom glass. Finally 300 ml of methanol was added as solvent and fixed in the soxhlet extractor for 3 hours at 60°C temperature. After 3 hours, extract was filter into a beaker incubated at 40°C till required consistency was achieved and finally stored in refrigerator at 4°C for formation of microcapsule as core material.
Fabric treatment with plant extract
Direct application method: Methanol extract of the Moringa leaves was directly applied on cotton fabric by pad dry cure method. 500 ml of methanol extract and 6% of citric acid as cross linking agent were taken and stirred completely. Samples were immersed in stirred extract of Moringa leaves for 30 minutes. After that samples were taken out and padded on padding mangle at a pressure of 2.5 psi with 2 dips and nips to give a wet pick up of 85%. Drying and curing was carried out in hot air oven which was used for drying, condensation and fixation. The samples were dried and cured at 102°C for 3 minutes.
Microencapsulation method
Formation of microcapsules: 2.5 g of chitosan (as a wall material in microencapsulation) was dissolved in 100 ml of distilled water with 1% acetic acid by mechanical stirring and kept overnight till a clear solution was obtained. Silicon oil (0.5 ml) and 2 g of core material (extract of Moringa leaves) were slowly added in this solution with continuous stirring for making an emulsion. Stirring was further continued for another 15 minutes. Coacervation of chitosan was done by addition of sodium hydroxide (1 g in 100 ml). Stirring was stopped and the mixture was allowed to rest for a period of 30 minutes at a temperature of 0°C-5°C in refrigerator for the formation of microcapsules. After that solution was kept at 5°C-10°C for 24 hours, filtered and washed thrice with distilled water [7].
Application of microcapsules on fabric
For the application of microcapsules, a solution of filtered microcapsules and distilled water was prepared and samples were immersed in microcapsule solution for 30 minutes then padded through padding mangle at a pressure of 2.5 psi. Treated samples were dried at 80°C for 5 minutes in hot air oven to cure the samples. The cured samples were evaluated for antimicrobial efficacy against gram positive (Staphylococcus aureus) and gram negative (Klebsiella pneumonia) microorganism.
Functional analysis of finish
Quantitative bacterial reduction test for antimicrobial reduction: Assessment of quantitative bacterial reduction was carried out by AATCC test method 100:2014 for both treated and control samples. Samples showing antimicrobial activity were evaluated quantitatively. Treated and control samples were inoculated with test organisms (Staphylococcus aureus and Klebsiella pneumonia) [8]. After incubation, the bacteria were eluted from the swatches by shaking in known amount of neutralizing solution. The number of bacteria present in this liquid was determined and the percentage reduction by the treated specimen was calculated. The bacterial counts were reported as the number of bacteria per sample (swatches in jar) not as the number of bacteria per ml of neutralizing solution. ‘0’ counts at 100 dilution was reported as “less than 100”. The percentage Reduction (R) of bacteria by the specimen treatments was calculated using the following formula;
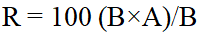
Where, A is the number of bacteria recovered from the inoculated treated test specimen swatches in the jar incubated over the desired contact period and B, the number of bacteria recovered from the inoculated treated test specimen swatches in the jar immediately after inoculation (at ‘0’ contact time) [9].
Qualitative method for testing presence or absence of antimicrobial activity
Qualitative assessment of antimicrobial activity was carried out by AATCC 147-2004 test method. Sterilized nutrient agar cooled to 47°C ± 2°C (117°C ± 4°C) was dispensed by pouring 15 ml ± 2 ml into each standard (15 mm × 100 mm) flat bottomed petridis. Agar was allowed to get firm before inoculating. Inoculum was prepared by transferring 1.0 ml ± 0.1 ml of a 24 h broth culture into 9.0 ml ± 0.1 ml of sterile distilled water contained in a small flask. It was mixed well using appropriate agitation. Using a 4 mm inoculating loop, one loopful of the diluted inoculum was loaded and transferred to the surface of the sterile agar plate by making five streaks approximately 60 mm in length, spaced 10 mm apart covering the central area of standard petri dish without refilling the loop. It was taken care that no surface breakage should take place while making the streaks.
FTIR
The FTIR spectra of cotton, khadi and extract of Moringa were measured using Perkin Elmer FT-IR Spectrometer (SPECTRUM TWO). The wave number range was 450 cm-1-4000 cm-1 and the resolution was 1 cm-1.
Surface morphology of treated, untreated and washed samples was observed under “Carl Zeiss EVO 18 Special” at a magnification of 50X to 2500,000X on and at acceleration voltage of 20 KV. Before the assessment, all the samples were sputtered under vacuum with gold.
Wash durability of treated fabric
After the evaluation of functional analysis of treated samples, washing fastness was done for treated samples to evaluate the durability of antimicrobial property against washing using AATCC 100 test method up to 10 cycles wash cycles.
Results and Discussion
The results obtained from the study as well as relevant discussion have been summarized under following heads.
Result of quantitative bacterial reduction
Assessment of quantitative bacterial reduction was carried out by AATCC 100:2004 test method and the results are reported in Table 2. In AATCC 100:2004 test method the treated sample show the bacterial reduction of samples in percentage.
| S. No. | Sample | Test organism | No. of bacteria per sample (CFU) | Bacteria reduction in % | ||
|---|---|---|---|---|---|---|
| ‘0’ hrs (B) | ‘24’ hrs (A) | |||||
| 1. | Control (untreated) | Cotton | Staphylococcus aureus | 1.35 × 105 | 1.44 × 107 | Nil |
| Klebsiella pneumonia | 1.41 × 105 | 1.27 × 107 | Nil | |||
| Khadi | Staphylococcus aureus | 1.38 × 105 | 1.55 × 107 | Nil | ||
| Klebsiella pneumonia | 1.47 × 105 | 1.26 × 107 | Nil | |||
| 2. | Samples treated with Moringa extract using microencapsulation method | Cotton | Staphylococcus aureus | 1.82 × 105 | 0.105 × 105 | 94.23 |
| Klebsiella pneumonia | 1.20 × 105 | 0.086 × 105 | 92.83 | |||
| Khadi | Staphylococcus aureus | 1.54×105 | 0.065 × 105 | 95.77 | ||
| Klebsiella pneumonia | 1.67 × 105 | 0.089 × 105 | 94.67 | |||
| 3. | Samples treated with Moringa extract using direct application method | Cotton | Staphylococcus aureus | 1.42 × 105 | 0.065 × 105 | 95.42 |
| Klebsiella pneumonia | 1.12 × 105 | 0.037 × 105 | 96.91 | |||
| Khadi | Staphylococcus aureus | 1.32 × 105 | 0.045 × 105 | 96.59 | ||
| Klebsiella pneumonia | 1.56 × 105 | 0.058 × 105 | 96.28 | |||
Table 2: Assessment of bacterial reduction.
Results clearly reveal that both the cotton and khadi fabric samples treated with Moringa extract showed good antimicrobial properties against gram positive (Staphylococcus aureus) and gram negative (Klebsiella pneumonia) microorganism. In case of direct application methods both the samples (cotton and khadi) showed higher percentage of antimicrobial reduction against both the organisms (95.77% to 96.91%) as extract was applied directly on the fabric surface. On the other hand percentage of antimicrobial efficacies against both the organisms in microencapsulation application method found less compared to direct application method [10].
Result of qualitative method for assessing presence or absence of antimicrobial activity
Qualitative assessment of antimicrobial activity was carried out under AATCC 147-2004 test method and the results are reported in Table 3.
| Sample | Test organism | Zone of inhibition (mm) | Result of anti-microbial activity | |||
|---|---|---|---|---|---|---|
| Zone of inhibition | Growth of specimen | |||||
| Control (untreated) | Cotton | Staphylococcus aureus | No zone | Present | Antimicrobial activity Absent | |
| Klebsiella pneumonia | No zone | Present | Antimicrobial activity Absent | |||
| Khadi | Staphylococcus aureus | No zone | Present | Antimicrobial activity Absent | ||
| Staphylococcus aureus | No zone | Present | Antimicrobial activity Absent | |||
| Treated with Moringa extract | Cotton | Direct application | Staphylococcus aureus | No zone | Absent | Antimicrobial activity present |
| Klebsiella pneumonia | No zone | Absent | ||||
| Microcapsulation | Staphylococcus aureus | No zone | Absent | Antimicrobial activity present | ||
| Klebsiella pneumonia | No zone | Absent | ||||
| Khadi | Direct application | Staphylococcus aureus | No zone | Absent | Antimicrobial activity present | |
| Klebsiella pneumonia | No zone | Absent | ||||
| Microcapsulation | Staphylococcus aureus | No zone | Absent | Antimicrobial activity present | ||
| Klebsiella pneumonia | No zone | Absent | ||||
Table 3: Assessment of presence or absences of bacteria (zone of inhibition).
It was observed that both untreated samples (cotton and khadi) did not show antimicrobial properties hence microbial growth was observed on these samples. However, treated samples showed presence of antimicrobial property thereby no microbial growth was found on these samples.
Qualitative test was found to be good for testing the main agent or treated fabrics, providing the antibacterial agent used were capable of leaching out. It is observed from that all the treated fabrics showed antimicrobial activity against gram positive (Staphylococcus aureus) and gram negative (Klebsiella pneumonia) microorganism whereas untreated fabrics showed the growth of microorganisms which means antibacterial activity was not found in treated samples.
In case of both the treated fabrics (i.e. cotton and khadi) there was no zone of inhibition for both the organisms. The zone of inhibition that indicates that Moringa leaves extract not only prevents the growth of microbes on the fabrics but also leaches out the bacteria.
FTIR
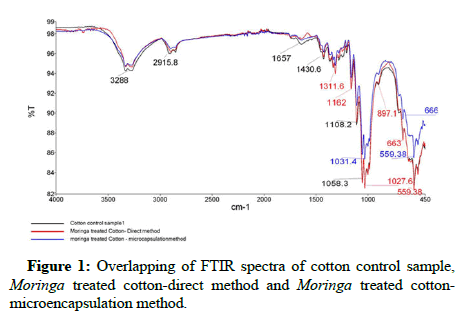
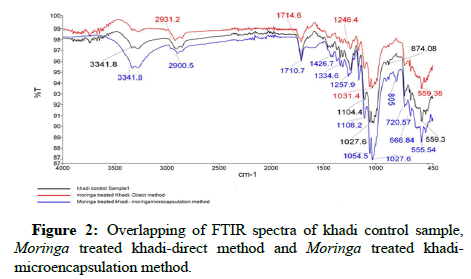
FTIR test of control and treated samples were performed and the results. The overlapping spectrum of cotton and khadi control sample treated directly and sample treated with microencapsulation method are shown. Figure 1 reveals the presence of typical group of Moringa Oleifera on the surface of treated samples. The peaks 3288 cm-1 observed in direct application as well as in microencapsulation application which indicates the occurrence of stretch free vibration type alcohol with O-H, 2915.8 cm-1 stretch and 1430.6 cm-1 peaks represent alkanes and alkyls acid with C-H and O-H correspondingly. The presence of C-H stretch organic amine is confirmed with the presence of 1031.4 cm-1 band. Similarly Figure 2 also reveals the presence of typical group of Moringa Oleifera on the surface of treated samples. Peaks 3341.8 cm-1 indicates the occurrence of stretch free vibration type alcohol with O-H, 2900.8 cm-1 and 1710.7 cm-1 represents organic aromatic group and carbonyl acid with C=C and O-H correspondingly. The presence of functional group like organic (=C-H bending), carbonyl acid (C-O stretch), organic (alkane; C-H stretch) and carbonyl (amide N-H stretch) are confirmed which indicate the presence of phenolic group on treated samples and indicating be reason for antimicrobial efficacy.
SEM (Scanning Electron Microscope)
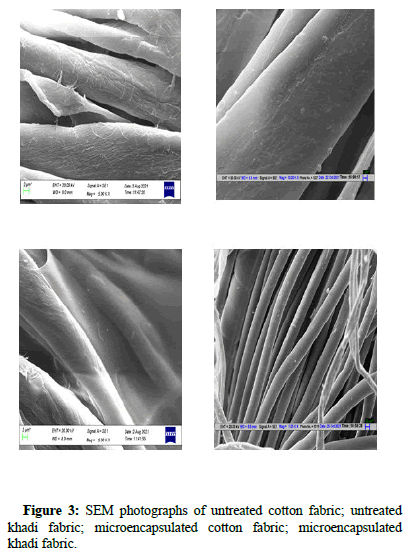
Both treated and untreated cotton and khadi samples were analyses using SEM test and the results are shown in Figure 3. From the plate no. 1, it was observed that both untreated samples (cotton and khadi) showed smooth and clean surface because of pretreatment process all impurity including starch get washed off. However treated samples plate no. 1, showed rough and uneven surface due to deposition of microcapsules made by chitosan as wall material.
Wash durability of treated samples
Wash durability test was performed on both cotton and khadi fabrics treated with Moringa leaves extract through direct and microencapsulation method as using standard test method AATCC 100 and the results are reported in Table 4.
| Washing cycles | Efficacy of bacterial reduction (in Percentage) | |||||||
|---|---|---|---|---|---|---|---|---|
| Direct method | Microencapsulation method | |||||||
| Cotton | Khadi | Cotton | Khadi | |||||
| Sa | Kp | Sa | Kp | Sa | Kp | Sa | Kp | |
| 0 | 98 | 96.3 | 96.5 | 97.2 | 97 | 98.3 | 96 | 97.2 |
| 1 | 48 | 52 | 19 | 36 | 95 | 93 | 94 | 93 |
| 3 | 26 | 31.1 | 21 | 18 | 88 | 89 | 87 | 88 |
| 5 | 14 | 16.2 | 13 | 13 | 79 | 82 | 81 | 84 |
| 7 | 11 | 10.9 | 6.2 | 6.9 | 65 | 73 | 73 | 75 |
| 10 | 2.3 | 3.2 | 3.2 | 3 | 52 | 64 | 65 | 63 |
| Note: Sa-Staphylococcus aureus, Kp-Klebsiella pneumonia | ||||||||
Table 4: Wash durability of treated samples.
The result of washing durability of finishing was observed and it was found that in both the methods all the treated samples showed higher efficacy of bacterial reduction. However on subsequent washing the efficiency of treated fabrics for bacterial reduction get decreased. It was observed that decrease in efficacy of bacteria reduction was more in directly treated as compared to microencapsulated samples. This may be due to the reason that finish is applied directly on fabric while in microencapsulation process make bonding with fibre that make it more resistant to washing.
Conclusion
Microencapsulation of herbal extract (Moringa leaves) has been done successfully by phase coacervation method using herbal extract as core material and wall material chitosan as well followed by its application on to fabric using pad dry cure and direct method. It is found that cotton and khadi fabric treated with direct application, sample showed higher percentage of antimicrobial efficacy in both the application method against Klebsiella pneumonia and Staphylococcus aureus. The wash durability test comparing microencapsulated and direct applied herbal extract for both cotton and khadi fabric revealed that microencapsulated samples retained their activity up to 10 wash cycles.
Acknowledgment
The research scholar wishes to thank to NITRA (Northern India Textile Research Association) Ghaziabad for their support and cooperation. The whole experimental process such as pretreatment, extraction, formation of microcapsules, application of microcapsules on substrate, physical testing, antimicrobial evaluation and its washing fastness was done in NITRA lab.
References
- Farooq F, Rai M, Tiwari A, Khan AA, Farooq S (2012) Medicinal properties of Moringa oleifera: An overview of promising healer. J Med Plant Res 6:4368-4374.
- Holme I (2002) Durable freshness through antimicrobial finishes. Text magazine 30:13-16.
- Morais DS, Guedes RM, Lopes MA (2016) Antimicrobial approaches for textiles: From research to market. Materials 9:498.
[Crossref] [Google Scholar] [PubMed]
- Mucha H (2002) Antimicrobial finishes and modifications. Melliand Int 8:148-151.
- Nelson G (2001) Microencapsulation in textile finishing. Rev Prog Color 31:57-64.
- Tepe B, Donmez E, Unlu M, Candan F, Dafererera D, et al. (2004) Antimicrobial and antioxidative activities of the essential oils and methanol extracts of salvia cryptantha (Montbret et Aucher ex Benth) and Salvia multicaulis (Vahl). Food Chem 84:519-525.
- Montazer M, Afjeh MG (2007) Simultaneous x-linking and antimicrobial finishing of cotton fabric. J Appl Polym Sci 103:178-185.
- Chandrasekar S, Vijayakumar S, Rajendran R (2014) Application of chitosan and herbal nanocomposites to develop antibacterial medical textile. Biomed Aging Pathol 4:59-64.
- Singh N, Yadav M, Khanna S, Sahu O (2017) Sustainable fragrance cum antimicrobial finishing on cotton: Indigenous essential oil. Sustain Chem Pharm 5:22-29.
- Subramani K, Shanmugam BK, Rangaraj S, Murugan V, Srinivasan S, et al. (2020) Functional and antimicrobial properties of herbal nanocomposites from Piper betle plant leaves for enhanced cotton fabrics. J Coat Technol Res 17:1363-1375.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi