Research Article, J Pharm Drug Deliv Res Vol: 12 Issue: 6
Hepatoprotective Pharmacological Examination of Plant Albizia lebbeck Linn on Thioacetamide Provoked Hepatotoxicity in Rodents
Arun Kumar Maurya*, Sabra Banu, Nahid Parveen, Taniya Rawat and Sushmita Rana
Department of Pharmacy, J B Institute of Engineering and Technology, Dehradun, India
- *Corresponding Author:
- Arun Kumar Maurya
Department of Pharmacy,
J B Institute of Engineering and Technology,
Dehradun,
India;
E-mail: Arunmedi4u@gmail.com
Received date: 30 May, 2023, Manuscript No. JPDDR-23-100678;
Editor assigned date: 02 June, 2023, PreQC No. JPDDR-23-100678 (PQ);
Reviewed date: 16 June, 2023, QC No. JPDDR-23-100678;
Revised date: 31 July, 2023, Manuscript No. JPDDR-23-100678 (R);
Published date: 07 August, 2023, DOI: 10.4172/2325-9604.1000249
Citation: Maurya AK, Banu S, Parveen N, Rawat T, Rana S (2023) Hepatoprotective Pharmacological Examination of Plant Albizia lebbeck Linn on
Thioacetamide Provoked Hepatotoxicity in Rodents. J Pharm Drug Deliv Res 12:6.
Abstract
Introduction: Liver diseases such as jaundice, cirrhosis and fatty liver are very common worldwide. There are many factors for the development of these diseases, one of the important factors being the use of drugs. Drug induced liver injury is a major health problem that challenges not only health care professionals but also the pharmaceutical industry and drug regulatory agencies. Hence the present study was designed to evaluate the hepato-protective activity of Albizia lebbeck leaf extracts in rats.
Objective of study: Collection and authentication of Albizia lebbeck Linn. (Mimosaceae) plant leaves. Extraction of plant material using various solvents of increasing order of polarity ether, alcohol and water. Evaluation of hepatoprotective activity of Albizia lebbeck extracts. To investigate extracts for their antioxidant property.
Methodology: Thioacetamide induced liver damage in rats model was used to evaluate hepato-protective activity of Albizia lebbeck Linn and following parameters were considered.
Parameters: Liver weight, Aspartate Amino Transferase (AST), Alanine Amino Transferase (ALT), Alkaline Phosphatase (ALP), total bilirubin, direct bilurubin, total protein, serum albumin, serum sodium, serum potassium and clotting time.
Oxygen Reactive Absorbing Concentration (ORAC), 1,1-Diphenyl-2-Picryl Hydrazyl (DPPH) and 2,2’-Azinobis-3-ethyl- Benzothiozoline-6-Sulphonic acid (ABTS) methods used for determination of antioxidant activity for the extracts which have shown significant hepatoprotective activity.
Results: In control animals, thioacetamide induces liver damage and hence caused elevation of serum concentration of AST, ALT, ALP, total bilurubin, direct bilurubin and increase in liver weight. Thioacetamide treatment reduced serum concentration of total protein, serum albumin, sodium, potassium and also prolonged clotting time in control animals. Administration of standard drug silymarin, ethanolic extract and aqueous extract significantly reduced serum concentration of AST, ALT, ALP, total bilurubin, direct bilurubin, and liver weight and also significantly reduced clotting time in their respective groups as compared to control animals.
Conclusion: The results of above study suggested that ethanolic and aqueous extracts of Albizia lebbeck possess significant hepatoprotective activity in thioacetamide induced liver damage in rats whereas pet ether extract of Albizia lebbeck doesn’t possess hepato protective activity in the above mentioned model.
The results obtained in determination of antioxidant activity suggesting one of the possible mechanism i.e. free radical scavenging mechanism which is required for above therapeutic activity.
Keywords: Albizia lebbeck Linn; Leaves contains alkaloids; Kaemferol; Quercetin; Caffeic acid
Introduction
Liver is main organ of biliary system and it is one of the vital organs involved in various responsibilities required to maintain important homeostasis of the body. It has got its own importance in the physiological system. Metabolism of ingested substances like lipids, carbohydrates, proteins, blood coagulation and immunomodulation are the primary functions of the liver [1].
Liver damage is always associated with cellular necrosis, increase in tissue lipid peroxidation and depletion in the tissue Glutathione (GSH) levels. In addition serum levels of many biochemical markers like Aspartate Amino Transaminase (AST), Alanine Amino Transaminase (ALT), triglycerides, cholesterol, bilirubin, alkaline phosphatase are elevated [2].
There are several reasons are known to cause moderate to severe hepatic complications. Few liver complications emerge out as result of socially unaccepted life style of the individuals. On the other hand, some other liver toxicities result due to unavoidable circumstances. Irrespective of the reason for the toxicity, the subject has to suffer from several systemic complications, which push the subject towards the danger edge of the life.
Indeed, drug induced liver toxicity is the leading cause of actue liver failure in the United States, according to the Food and Drug Adminitration (FDA). There is an ever increasing need of an agent which could protect it from such damage [3]. Whatever is the reason for hepatotoxicity, immediate medical attention and treatment is essential to avoid jeopardy outcomes.
As per survey made by World Health Organisation (WHO), about 18,000 people die every year due to liver diseases. The common ailments of liver are cirrhosis, cholestasis, hepatitis, portal hypertension, hepatic encephalopathy, fulminant hepatic failure and certain tumors like hepatoma. It is estimated that two billion people around the world are infected with hepatitis B. About 350 million of these have the chronic form of the disease [4].
In modern medicine for the treatment of liver toxicity, corticosteroids and immune-suppressants are commonly used allopathic medicines. But, these drugs are associated with adverse effects such as immunosuppression and bone marrow depression. Further, the success rate of treating liver diseases is disappointing. Attempts are being made globally to get scientific evidences for these traditionally reported herbal drugs [5].
In spite of phenomenal growth of modern medicine, there are few synthetic drugs available for the treatment of hepatic disorders. However there are several herbs/herbal formulations claimed to have possess beneficial activity in treating hepatic disorders [6]. But they need to be validated in the light of science to ensure their ability to conserve therapeutic effectiveness in the formulation form.
This is one of the reason why people even in developed countries turning towards complementary and alternative medicine. Hence medicinal plants are staging a comeback and herbal ‘renaissance’ is happening all over the globe. The herbal products today symbolise safety in contrast to the synthetics that are regarded as unsafe to human and environment [7].
The natural plants show anti-oxidant activity for instance alkaloids, quercitin, tocopherol, flavonoids, coumarins and more generally phenolic compounds widespread in the plant kingdom [8]. The scavenging of free radicals by antioxidants could reduce the fibrosis in tissues [9].
About 600 commercial preparations with claimed liver protecting activity are available all over the world. About 100 Indian medicinal plants belonging to 40 families are used for herbal formulation. A few reports on the hepatoprotective activity are cited here, e.g. Apium graveolens linn. (Umbelliferae), Boerrhavia diffusa linn. (Nyctaginaceae), Euphorbia antisyphilitica (Euphorbiaceae), Rubia cordifolia (Rubiaceae), Solanum lyratum (Solanaceae) [10].
Albizia lebbeck linn leaves contains alkaloids, kaemferol, quercetin, caffeic acid, mature leaves contain keto acids and showed antioxidant properties, have not been scientifically investigated for evaluation of hepato-protective activity. Hence the present study was designed to investigate Albizia lebbeck Linn. Leaves extracts for its hepato proectivetivity against thioacetamide induced hepatic damages in rats.
Materials and Methods
Collection and authentication of drug
The plant leaves of Albizia lebbeck Linn were collected in Dhanvantri vana, Dehradun university, Bangalore and authenticated by Dr. Siddamallaiah, regional research institute, Dehradun.
Preparation of plant extract
The plant leaves were shade dried and were powdered, the coarse powder was subjected to extraction with petroleum ether and alcohol (70%) individually in soxhlet apparatus and also drug powder was subjected to aqueous extraction.
Chemicals, reagents and drug
• Standard drug Silymarin was obtained from Sigma-aldrich chemcal
Pvt. Ltd., Delhi
• Thioacetamide, hepatotoxin was obtained from Sigma-aldrich
chemical Pvt. Ltd., Dehradun.
Preliminary qualitative tests
The extracts were subjected to preliminary qualitative phytochemical investigation. The various tests and reagents used are given below and observations are recorded in Table 1.
| Groups | Drug |
|---|---|
| Group-1 | Saline (2 ml/kg) |
| Group-2 | Thioacetamide (TAA) (100 mg/kg b. w., s.c.) as a 2% w/v solution in water for injection on day 1st and then normal saline for 21 days. |
| Group-3 | Thioacetamide (TAA) (100 mg/kg b. w., s.c.) as a 2% w/v solution in water for injection on day 1st and then silymarin (25 mg/kg per day, p. o.) for 21 days. |
| Group-4 | Thioacetamide (TAA) (100 mg/kg b .w., s.c.) as a 2% w/v solution in water for injection on day 1st and then ether extract of A. lebbeck (low dose) for 21 days. |
| Group-5 | Thioacetamide (TAA) (100 mg/kg b .w., s.c.) as a 2% w/v solution in water for injection on day 1st and then ether extract of A. lebbeck (high dose) for 21 days |
| Group-6 | Thioacetamide (TAA) (100 mg/kg b .w., s.c.) as a 2% w/v solution in water for injection on day 1st and then ethanolic extract of A. lebbeck (low dose) for 21 days. |
| Group-7 | Thioacetamide (TAA) (100 mg/kg b .w., s.c.) as a 2% w/v solution in water for injection on day 1st and then ethanolic extract of A. lebbeck (high dose) for 21 days. |
| Group-8 | Thioacetamide (TAA) (100 mg/kg b .w., s.c.) as a 2% w/v solution in water for injection on day 1st and then aqueous extract of A. lebbeck (low dose) for 21 days. |
| Group-9 | Thioacetamide (TAA) (100 mg/kg b .w., s.c.) as a 2% w/v solution in water for injection on day 1st and then aqueous extract of A. lebbeck (high dose) for 21 days. |
Table 1: Tween 80 was obtained from nice chemicals Dehradun.
Detection of carbohydrates
Extracts were dissolved individually in 5 ml of distilled water and filtered. The filtrates were used to test the presence of carbohydrates.
Molisch’s test
Filtrates were treated with 2 drops of alcoholic α-naphthol solution in a test tube and 2 ml concentrated sulphuric acid was added carefully along the sides of the test tubes. Formation of violet ring at the junction indicates the presence of carbohydrates.
Results and Discussion
Preliminary phytochemical screening
Leaves extracts of Albizia lebbeck were selected for the present study. About 500 g of dried leaves were powdered and extracted with petroleum ether, ethanol and water separately. The nature of extracts and their extractive value are as follows.
Effect on ALT: Thioacetamide administration caused significant elevation of serum alanine transaminase by inducing hepatic damage in control animals as compared to normal animals while, standard drug silymarin treatment reduced alt concentration in animals of standard group and those were equivalent to normal. There was dose dependent significant reduction in serum alt levels in plant extracts EEAL and AEAL treated animals as compared to control group but in animals treated with peal the SGPT level was similar to control group.
Effect on AST: The treatment with thioacetamide caused liver damage which in turn shown significant elevation of serum aspartate transaminase in control animals when compared to normal animals while, silymarin administration reduced serum AST levels in animals of standard group. The treatment with plant extracts EEAL and AEAL significantly reduced concentration of AST in animals of respective groups in dose dependent manner as compared to control group but there was no significant variation in AST concentration in peal treated animals compared to control group.
Effect on ALP: The liver damage induced by thioacetamide treatment resulted into significant elevation of serum enzyme alkaline phosphatase in control animals when compared to normal animals. But in animals administered with standard drug, there was significant decrease of ALP concentration compared to control group. The treatment with EEAL and AEAL significantly reduced ALP level in animals of respective groups in dose dependent manner as compared to control group but there was no significant variation in ALP concentration in PEAL treated animals compared to control group.
Effect on total bilurubin: The total bilurubin concentration was found to increase in animals with liver damage by thioacetamide (i.e., control group). In standard group, silymarin administration reduced total bilurubin and animals treated with EEAL and AEAL have exhibited dose dependent significant reduction in total bilurubin compared to control group. But PEAL didn’t show reduction in total bilurubin levels and it was similar to positive control group.
Effect on direct bilurubin: Thioacetamide significantly elevated direct bilurubin concentration in control animals by inducing hepatic damage compared to normal animals. But treatment with standard drug silymarin, EEAL and AEAL significantly reduced direct bilurubin level in respective groups while, there was no significant variation in direct bilurubin concentration in animals treated with PEAL compared to animals of positive control group.
Effect on total protein: In positive control group animals treated with thioacetamide, there was significant decrease in serum total protein concentration due to liver damage when compared to saline alone treated animals but administration of silymarin caused significant rise in total protein compared to control group. In animals administered with plant extracts EEAL and AEAL there was significant increase total protein level and it was dose dependent while, PEAL treated animals have shown similar values of total protein as positive control group.
Effect on albumin: Induction of liver damage by administration of thioacetamide significantly reduced serum albumin level in control animals when compared to normal animals (Figure 1). But the treatment with Silymarin has shown significant increase while PEAL and EEAL have shown dose dependent increase in serum albumin level compared to control group. But AEAL administration didn’t show any change in above parameter compared to control group (Figure 2).
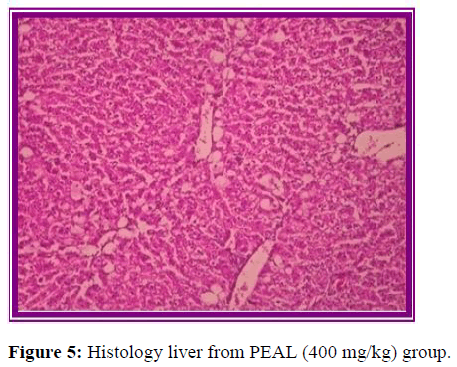
Effect on direct sodium and potassium: Thioacetamide significantly reduced serum sodium and potassium concentration in control animals due to liver necrosis compared to normal animals (Figure 3). But treatment with standard drug silymarin, EEAL and AEAL significantly increased concentration of serum sodium and potassium and effect of extracts on serum ions was dose dependent (Figure 4). But in the animals treated with PEAL above parameters were comparable to positive control group (Figure 5).
Effect on clotting time: There was prolongation in clotting time in control animals due to liver injury by administration thioacetamide (Table 2). Administration of silymarin caused significant reduction in clotting time in standard groups while EEAL, AEAL have shown dose dependent reduction in clotting time in respective animals as compared to control group. But in animals treated PEAL there was significant change in clotting time when compared to control animals (Table 3).
| S. no | Group | ALT IU/L | AST IU/L | ALP IU/L | Total bilurubin g/l | Direct bilurubin g/dl |
|---|---|---|---|---|---|---|
| 1 | Normal saline | 56.67 ± 1.6 | 109.7 ± 0.56 | 76.67 ± 1.6 | 0.32 ± 0.01 | 0.025 ± 0.014 |
| 2 | Positive control | 179.8 ± 35.7 | 212.8 ± 2.81 | 479.7 ± 4.62 | 0.86 ± 0.01 | 0.3 ± 0.02 |
| 3 | Sylimarine 25 mg/kg | 59.33 ± 1.4*** | 114.3 ± 1.6** | 138.8 ± 2.58*** | 0.35±0.001*** | 0.075 ± 0.02*** |
| 4 | PEAL 200 mg/kg | 222.1 ± 3.7ns | 193.4 ± 6.47ns | 471.5 ± 4.47ns | 0.84 ± 0.01ns | 0.32 ± 0.023ns |
| 5 | PEAL 400 mg/kg | 199.7 ± 9.2ns | 175.7 ± 20.33ns | 443.3 ± 6.5ns | 0.79 ± 0.01ns | 0.29 ± 0.016ns |
| 6 | EEAL 200 mg/kg | 58.75 ± 2.3*** | 142.4 ± 15.28*** | 191.6 ± 3.1*** | 0.39 ± 0.018*** | 0.099 ± 0.004*** |
| 7 | EEAL 400 mg/kg | 52.67 ± 1.9*** | 110.8 ± 5.245*** | 155.9 ± 4.1*** | 0.32 ± 0.029*** | 0.072 ± 0.016*** |
| 8 | AEAL 200 mg/kg | 74.43 ± 5.33*** | 151.1 ± 18.98*** | 201.2 ± 2.5*** | 0.3 ± 0.021*** | 0.12 ± 0.016*** |
| 9 | AEAL 400 mg/kg | 63.47 ± 9.46*** | 124.2 ± 12.66** | 165.9 ± 5.4*** | 0.27 ± 0.042*** | 0.08 ± 0.026*** |
Note: Values are mean ± S.E.M, n=6 symbols represent statistical significance.
nsp>0.05, *p<0.05, **p<0.01, ***p<0.001 vs. positive control.
Table 2: Effect of Albizia lebbeck extracts on serum parameters AST, ALT, ALP, direct bilirubin, total bilirubin.
| S. no | Group | Total protien g/dl | Albumin g/dl | Sodium mEq/L | Potassium mEq/L | Clotting time sec |
|---|---|---|---|---|---|---|
| 1 | Normal saline | 5.1 ± 0.2 | 4.6 ± 0.14 | 141.8 ± 0.8 | 5.1 ± 0.22 | 198.0 ± 1.9 |
| 2 | Positive control | 2.50 ± 0.17 | 2.1 ± 0.056 | 73.9 ± 1.3 | 2.08 ± 0.07 | 515.8 ± 4.8 |
| 3 | Sylimarine 25 mg/kg | 5.28 ± 0.09*** | 4.25 ± 0.06*** | 133.7 ± 1.7*** | 4.2 ± 0.15*** | 203.5 ± 2.1*** |
| 4 | PEAL 200 mg/kg | 2.35 ± 0.16ns | 1.86 ± 0.1ns | 74.12 ± 1.37ns | 2.13 ± 0.1ns | 510.0 ± 3.0ns |
| 5 | PEAL 400 mg/kg | 2.40 ± 0.15ns | 2.3 ± 0.2ns | 77.8 ± 0.68ns | 2.2 ± 0.2ns | 499.5 ± 4.1ns |
| 6 | EEAL 200 mg/kg | 5.5 ± 0.56*** | 3.56 ± 0.21*** | 127.9 ± 1.9*** | 4.51 ± 0.18*** | 340.0 ± 5.6*** |
| 7 | EEAL 400 mg/kg | 5.28 ± 0.8*** | 3.96 ± 0.2*** | 137.6 ± 1.9*** | 4.71 ± 0.13*** | 204.3 ± 3.7*** |
| 8 | AEAL 200 mg/kg | 5.47 ± 0.5*** | 3.66 ± 0.11*** | 130.4 ± 3.0*** | 4.7 ± 0.17*** | 344.0 ± 2.8*** |
| 9 | AEAL 400 mg/kg | 5.25 ± 0.4*** | 4.1 ± 0.15*** | 135.1 ± 2.7*** | 4.9 ± 0.15*** | 220.8 ± 1.3*** |
Note: Values are mean ± S.E.M, n=6 symbols represent statistical significance.
nsp>0.05, *p<0.05, **p<0.01, ***p<0.001 vs. positive control.
Table 3: Effect of Albizia lebbeck extracts on serum parameters total protein, albumin, clotting time and ions.
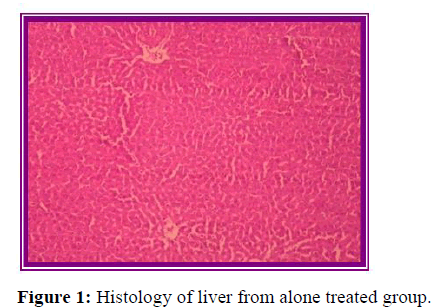
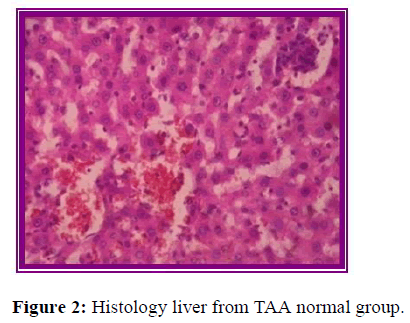
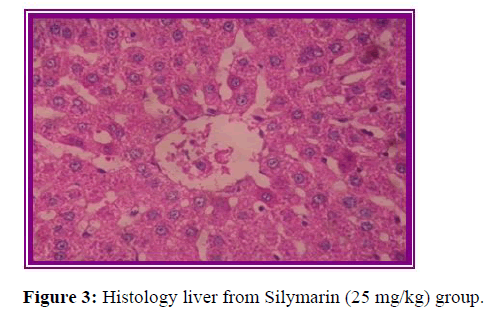
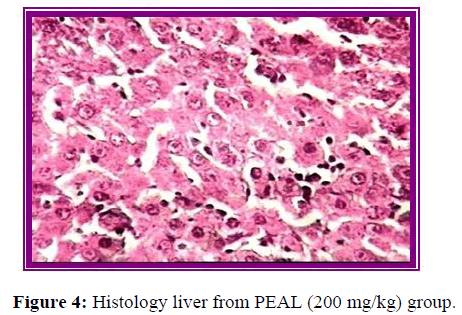
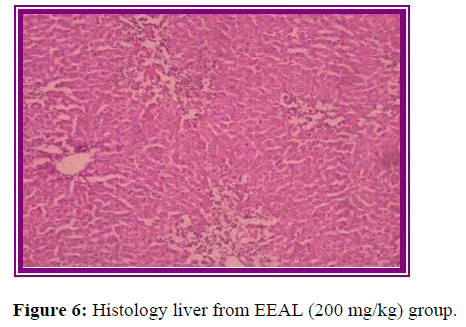
Histopathological evaluation
The results of histopathological evaluation for liver samples are as follows (Figure 6).
Conclusion
The results obtained from estimation of biochemical parameters suggesting that, ethanolic and aqueous extracts of Albizia lebbeck leaves possess significant hepatoprotective property in thioacetamide induced liver toxicity in rats model.
The histopathological studies supported the results of biochemical tests, showing less damage in the cytoarchitecture of the liver.
The results obtained in determination of antioxidant activity by ORAC, ABTS, and DPPH methods gives an idea about possible mechanism of action of the extracts required to protect liver against various hepatotoxins. However further investigation is required to perform separation and isolation of the phyto constituents from the extracts and to identifyspecific constituent responsible for the beneficial liver protecting property.
References
- Rajesh MG, Latha MS (2004) Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol 91:99-104.
- Setty SR, Quereshi AA, Swamy AV, Patil T, Prakash T, et al. (2007) Hepatoprotective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia 78:451-454.
- Achliya GS, Wadodkar SG, Dorle AK (2004) Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride-induced hepatic damage in rats. J Ethnopharmacol 90:229-232.
- Rao GM, Rao CV, Pushpangadan P, Shirwaikar A (2006) Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J Ethnopharmacol 20:484-490.
- Jain A, Soni M, Deb L, Jain A, Rout SP, et al.(2008) Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica roxb. leaves. J Ethnopharmacol. 11:561-566.
- Subramoniam A, Pushpangadan P (1999) Development of phytomedicines for liver disease. Ind J Pharmacol 31:166.
- Rajesh MG, Latha MS (2004) Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol 91:99-104.
- Cetkovic GS, Dilas SM, Canadanovic-Brunet JM, Tumbas VT (2003) Thin-layer chromatography analysis and scavenging activity of marigold (Calendula officinalis L) extracts. Acta Period Technol 93-102.
- Jose JK, Kuttan R (2002) Hepatoprotective activity of Emblica officinalis and chyavanaprash. J Ethnopharmacol 72:135-140.
- Ahmed B, Alam T, Khan SA (2001) Hepatoprotective activity of Luffa echinata fruits. J Ethnopharmacol 76:187-189.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi