Case Report, Clin Oncol Case Rep Vol: 3 Issue: 6
Hepatic Mass Potentially Representing Spontaneous Primary Tumor Involution
Nicholas Rawson1*, James Lee2 and Kevin Lowe3
1Department of General Surgery, Carle Foundation Hospital, Illinois, USA
2Carle Illinois College of Medicine, Illinois, USA
3Department of Surgical Oncology, Carle Foundation Hospital, Illinois, USA
*Corresponding Author: Nicholas Rawson
Department of General Surgery
Carle Foundation Hospital, Illinois, USA
E-mail: nrawson@illinois.edu
Received: July 07, 2020 Accepted: November 09, 2020 Published: November 20, 2020
Citation: Rawson N, Lee J, Lowe K (2020) Hepatic Mass Potentially Representing Spontaneous Primary Tumor Involution. Clin Oncol Case Rep 4:1
Abstract
Abstract
Incidental hepatic masses are not uncommon being found in up to 6% of those undergoing imaging. These lesions have the potential to represent metastatic disease or a primary Hepatocellular Carcinoma (HCC). Long-standing cirrhosis from chronic hepatitis, alcohol, or hepatosteatosis represent some of the well-documented risk factors for HCC. However, older men with a history of smoking are also pre-disposed to the disease. We report a case of a patient with multiple risk factors for HCC who was diagnosed with an incidental liver lesion. He then underwent a thorough workup to rule out any potential primary mass that could have metastasized. He eventually underwent resection of the suspected HCC. The final histology indicated a non-hepatic origin of the cancer, but no primary tumor was ever able to be identified. This may represent a case of spontaneous involution of the original malignancy.
Keywords: Hepatocellular; Carcinoma; Renal cell; Incidental; Liver; Hepatic; Cancer; Spontaneous involution
Keywords
Hepatocellular; Carcinoma; Renal cell; Incidental; Liver; Hepatic;
Cancer; Spontaneous involution
Introduction
Incidental hepatic masses are found in up to 6% of those studied [1]. Based on a patient’s individual risk factors such as cirrhosis or chronic hepatitis or a history of malignancy, and the lesion’s size, smooth versus irregular borders, enhancement pattern on imaging, and growth over time, a likely tissue of origin may be identified, or further work up and biopsy may be needed [2].
Primary hepatic malignancies are most commonly Hepatocellular Carcinoma (HCC). The majority of HCC is seen in Asia with only 5% of cases occurring in North America. This discrepancy is usually attributed to differences in the prevalence of hepatitis B as chronic hepatitis from both HBV and HCV are risk factors for developing HCC. Other risk factors are male sex, age, diabetes, alcohol use, tobacco use, and family history of HCC [3]. In the West, non-alcoholic fatty liver disease is a rising contributing factor to HCC development as well [4].
In this report, we discuss a patient 65 year-old former smoker with several risk factors for hepatocellular carcinoma but no history of malignancy who presented with an incidental hepatic mass. This mass was suspicious for HCC. Biopsy of the mass revealed atypical cells with diffuse necrosis raising suspicion for malignancy. A thorough work up was unable to identify a primary cancer. Surgical removal of the tumor was performed.
Clinical Presentation
A 65-year-old former smoker with a history of hyperproteinemia presented to the clinic with a complaint of unintentional weight loss of 40 lbs. over several months. This history prompted referral to Medical Oncology who found elevated IgG and kappa light chains without monoclonal proteins. The patient then underwent a bone marrow biopsy to rule out plasma cell dyscrasia, but this was unrevealing.
The patient developed dyspnea and lethargy that prompted an ED visit. CT scan identified a large segment 6, 7, and 8 cystic mass. The diagnosis remained unclear, but the differential included Castleman disease (Figure 1).
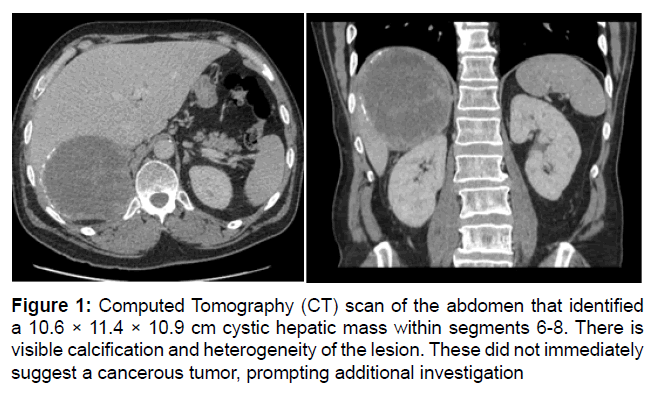
Figure 1: Computed Tomography (CT) scan of the abdomen that identified a 10.6 × 11.4 × 10.9 cm cystic hepatic mass within segments 6-8. There is visible calcification and heterogeneity of the lesion. These did not immediately suggest a cancerous tumor, prompting additional investigation
A PET scan was ordered to identify involved lymph nodes, but instead identified a hypermetabolic liver mass with positive perihepatic lymph nodes. Biopsies were taken of the mass. These eventually returned as poorly differentiated carcinoma. Bidirectional endoscopy failed to uncover a primary GI tumor (Figure 2).
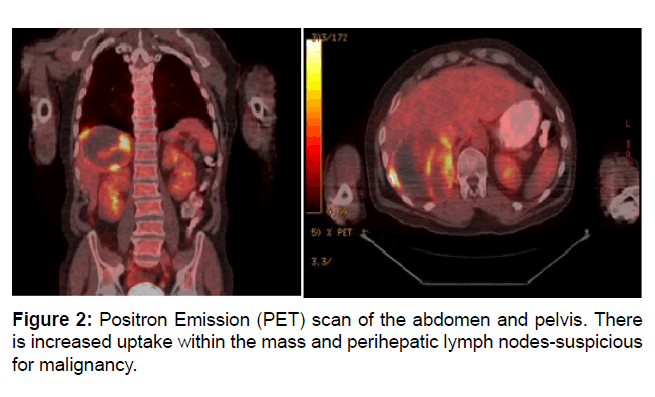
Figure 2: Positron Emission (PET) scan of the abdomen and pelvis. There is increased uptake within the mass and perihepatic lymph nodes-suspicious for malignancy.
At this point he was referred to Surgical Oncology where a right hepatectomy was recommended. Intraoperatively, the tumor was noted to be adherent to the retroperitoneum, but this did not preclude adequate gross margins. Gerota’s fascia was intact and was grossly normal and non-adherent. The patient followed a typical postoperative course and was discharged on postoperative day 11. Final pathology returned as likely metastatic cancer, but MRI failed to identify a primary site. The patient was scheduled for active surveillance with CT scan in 3 months (Figure 3).
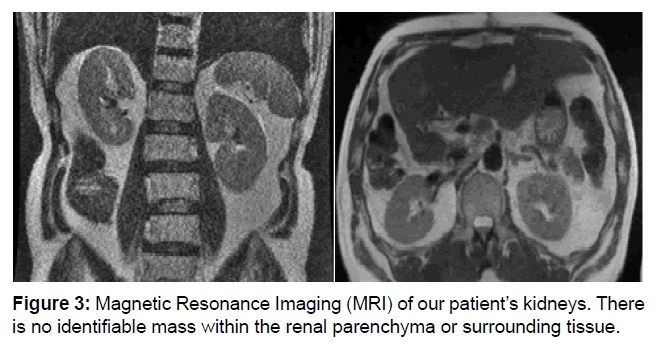
Figure 3: Magnetic Resonance Imaging (MRI) of our patient’s kidneys. There is no identifiable mass within the renal parenchyma or surrounding tissue.
Discussion
Cancers of the kidney and renal pelvis are commonly diagnosed in the United States and representing over 80% of these malignancies are Renal Cell Carcinomas (RCC) [5]. In fact, RCC is estimated to be responsible for nearly 15,000 deaths each year in the US. Men in their sixties are most likely to develop these neoplasms [6]. Other risk factors include tobacco use, hypertension, obesity, those on hemodialysis that develop cystic kidney disease, long-term NSAID use, and certain chemical exposures such as cadmium or petroleum products [7-10].
There are several histological variants of RCC such as clear cell, papillary, chromophobe, oncocytic, and collecting duct, and up to 2% remaining unclassified. Clear Cell RCC (CCRCC) is the predominate form of RCC representing 4 out of 5 cases of RCC [11]. CCRCC is unique for the patterns of clear cytoplasm seen on histology, an inactivation mutation in VHL, and carries the worst prognosis [11].
Renal Cell Carcinoma (RCC) is notoriously difficult to diagnose due to a lengthy, indolent growth; taking up to 20 years to become clinically relevant [12]. The classic triad of hematuria, palpable mass, and flank pain only presents in 10%-15% of patients [13]. Even when present, these symptoms usually represent invasive disease. Early diagnosis is crucial as metastatic RCC has a median survival of 1 year and a 10% 5-year survival [14]. Almost one third of patients will have metastases at diagnosis-most commonly the lungs, lymph nodes, liver, and bone [15,16]. However, the increasing prevalence of imaging has significantly increased incidental findings of small renal masses. Tumors less than 4 cm comprise 48-64% of all RCC diagnoses, of which up to 84% were asymptomatic [17].
Interestingly, RCC has been reported to spontaneously regress with some papers estimating the frequency to be about 1% [18]. Controversy remains regarding the mechanism, but immunologic, molecular, and mechanical insult are leading theories [19]. Most reports have identified metastatic involution with only the minority indicating that the primary tumor was obliterated.
While we offer no definitive hypothesis for regression, there was no biopsy, VEGF inhibition, or other identifiable insult as described in other papers. Thus immunologic destruction remains most likely, but why the liver lesion was unaffected remains unclear. There may be a novel mutation with the metastatic lesion driving its perseverance. Alternatively, a theoretical immune molecule could undergo processing within the liver that rendered it ineffectual against the RCC adjacent to the hepatocytes.
Further studies are needed to determine the prognosis and best practices for a patient who develops an isolated metastatic RCC with an R0 resection. Some may argue for adjuvant immunotherapy, but this remains controversial even with a more classic RCC presentation. Others would opt for close surveillance with CT and MRI as has been planned for this patient.
Conclusion
Incidental hepatic lesions are commonly encountered in clinical practice. In this case, despite rigorous screening for malignancy an isolated liver metastasis was resected. Further research is needed to determine how such patients should be managed post-operatively. Other studies could also investigate if more advanced renal imaging should be used pre-operatively to exclude RCC as a site of a potential primary malignancy in such suspicious liver lesions. This may not be cost effective as only 1% of RCC’s are suspected to spontaneously regress. The molecular mechanisms also deserve more attention as this may be a potential pathway to more targeted immunotherapeutic agents.
References
- Gore R, Pickhardt P, Mortele K, Fishman E, Horowitz J, et al. (2017) Management of incidental liver lesions on ct: A white paper of the acr incidental findings committee. J Am Coll Radiol 14: 1429-1437.
- WHO (2018) The Global Cancer Observatory. Liver Globocam WHO.
- Singal A, Lampertico P, Nahon P (2020) Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol 72: 250-261.
- Atkins M, Choueiri T (2020) Epidemiology, pathology, and pathogenesis of renal cell carcinoma. Wolter Kluwer uptodate 1-8.
- Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70: 7-30.
- Hidayat K, Du X, Zou SY, Shi BM (2017) Blood pressure and kidney cancer risk: Meta-analysis of prospective studies. J Hypertens 35: 1333-1344.
- Tsivian M, Moreira DM, Caso JR, Mouraviev V, Polascik TJ (2011) Cigarette smoking is associated with advanced renal cell carcinoma. J Clin Oncol 29: 2027-2031.
- Brennan JF, Stilmant MM, Babayan RK, Siroky MB (1991) Acquired renal cystic disease: Implications for the urologist. Br J Urol 67: 342-348.
- Brugarolas J (2019) Molecular genetics of clear-cell renal cell carcinoma. J Clin Oncol 32: 1968-1976.
- Holland JM (1975) Natural history and staging of renal cell carcinoma. CA A Can J Clin 25: 121-133.
- Murphy GP, Schirmer HK (1963) The diagnosis and treatment of hypernephroma. Geriatrics 18: 354-360.
- Siebels M, Schendel D (2005) New challenges and chances in the treatment of metastatic renal cell carcinoma. World J Uro 23: 153-154.
- Middleton RG (1967) Surgery for metastatic renal cell carcinoma. J Urol 97: 973-977.
- Chow WH, Devesa SS (2019) Contemporary epidemiology of renal cell cancer. Cancer J 14: 288-301.
- Sanchez-Martin FM, Millan-Rodriguez F, Urdaneta-Pignalosa G (2008) Small renal masses: Incidental diagnosis, clinical symptoms, and prognostic factors. Adv Urol 2008: 310694.
- Janiszewska AD, Poletajew S, Wasiuty√?¬?ski A (2019) Spontaneous regression of renal cell carcinoma. Contemp Oncol 17: 123-127.
- Fairlamb DJ (1981) Spontaneous regression of metastases of renal cancer: A report of two cases including the first recorded regression following irradiation of a dominant metastasis and review of the world literature. Cancer 47: 2102-2106.
- Cheville JC, Lohse CM (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Amer J Surg Pathol 27: 612-624.
- The International Agency (2020) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 