Research Article, Jrgm Vol: 13 Issue: 3
Genetic Diversity of Pluripotent Stem Cell Marker OCT4, NANOG and SOX9 Gene Expression with Functional Impact on Non-Obstructive Azoospermic Patients
Ajit Kumar Saxena1*, Shalini1, Meenakshi Tiwari1 and Mukta Agarwal2
1Department of Pathology / Laboratory Medicine, All India Institute of Medical Sciences, Patna, Bihar, India
2Department of Obstetrics / Gynaecology, All India Institute of Medical Sciences, Patna, Bihar, India
*Corresponding Author: Ajit Kumar Saxena
Professor and Head, Infertility Department of Pathology/Laboratory Medicine, All India Institute of Medical Sciences, Phulwarisharif, Patna,Bihar,India
E-mail: draksaxena1@rediffmail.com
Received: : 28-May-2024, Manuscript No. JRGM-24-137422,
Editor assigned: 29- May-2024, PreQC No. JRGM-24-137422 (PQ),
Reviewed: 12-Jun-2024, QC No. JRGM-24-137422
Revised: 14-Jun -2024, Manuscript No. JRGM-24-137422 (R)
Published: 21-Jun-2024, DOI:10.4172/2325-9620.1000305
Citation: Saxena AK, Shalini, Tiwari M,Agarwal M (2024) Genetic Diversity of Pluripotent Stem Cell Marker OCT4, NANOG and SOX9 Gene Expression with Functional Impact on Non-Obstructive Azoospermic Patients. J Regen Med 13:3.
Copyright: © 2024 Saxena AK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Stem cells, an early transcription factors play an important role in reproductive biology during differentiation of primary spermatocytes and further development of gonads. During fertilization, embryonic stem cells have ability to differentiate in three cell-lineages - ectoderm, endoderm, and mesoderm. Male gonads shows dual in origin i.e ectodermal and mesoderm, where NANOG, OCT4 and SOX9 are responsible for maintaining pluripotency during normal spermatogenesis. The differentiation of testes till normal functioning is purely a genetic event and are highly sensitive to environmental factors. During spermatogenesis, germ cells divides in synchronize fashion to maintain “genetic pool” till development of mature sperm. These stem cell markers NANOG or OCT4 or SOX9 are early transcription factors to maintain pluripotency during spermatogenesis. Present study has been designed with the aims to assess the functional role of NANOG, OCT4 and SOX9 genes in clinically diagnosed cases of Non-Obstructive Azoospermia (NOA). Genomic DNA was isolated from peripheral blood under sterile conditions, quantified and RT-PCR techniques were used for characterization of gene-expression (either over or under expression) using specific primers. The frequency of DNA Copy Number Variations (DNACNV) were also evaluated to assess the genomic instability. Interestingly, findings reveals that NANOG showing highest frequency (42.0 %) of over-expression with the values of odd ratio (0.07) with confidence intervals (C.I) at 95% were varies between 0.029 - 1.430. The over-expression of SOX9 observed in 33% cases with odd ratio (0.11) and calculated value of C.I at 95% varies between 0.040 - 0.070. The frequency of complete disappearance (mutation) band of OCT4 was observed in 9.0% and SOX9 (20.8%) cases, while, NANOG (28.8%) showing significant (p<0.01) differences between cases and controls. OCT4 showing higher frequency of DNACNVs (blue bar) as compare to controls (yellow bar) having calculated values of O.R. (0.12) and C.I. at 95% interval varies between 0.014 to 1.089. The present study concludes that 1) NANOG play an important role to regulate for germ- cells proliferation, 2) Over-expression of SOX9 inhibit the proliferation of differentiating cells, and 3) OCT4- gene-expression showing increase genetic susceptibility due to poor signalling. Hence, early detection of these stem cells may be use as “biomarker” and these stem cells in future becomes relevant for stem-cell therapy as a source of regenerative medicine.
Keywords: Stem Cell Marker, OCT4, NANOG and SOX9. Male Infertility
Introduction
Reproductive genetic is a frontier of medicine for diagnostic, and clinical relevance of stem cell may be use in therapeutics. More than 10-15% of young couples are suffering from reproductive dysfunction worldwide [1]. In human, genetic factors play a crucial role in male infertility, hence genetic screening becomes relevant to explore the etiopathology of the disease , weather these factors are familial (hereditary) or sporadic in nature. More than 15% of couples globally suffer from infertility and the primary reason is poor quality of semen with four category of clinically characteristics as azoospermia i.e. absence of sperm, oligozoospermia (insufficient number), asthenozoospermia (lack of mobility) and teratozoospermia (anatomical defect) during spermatogenesis. In case of Non-Obstructive Azoospermia (NOA), there is lack of sperm in semen either due to infectious disease or vasectomy or endocrine dysfunctions. The genetic factors including chromosome aberrations such as structural chromosome break-points, dicentrics, ring chromosome, D/G chromosome association and reciprocal translocation. Simultaneously, numerical karyotype variations such as 46, XY/47XXY or 46, XY/47XYY (mosaic) also interference in reproductive performance. The frequency of these Complex Chromosome Rearrangement (CCRs) varies in different population [2].
Stem cells are also known as early transcription factor and OCT, NANOG and SOX9 play an important role for proliferation of primordial germ cells (gonocytes) in the lumen of seminiferous tubules during spermatogenesis to maintain pluripotency. Early stem-cell are derived from the inner cell mass of the developing blastocyst and are capable of differentiate into specific cell- lineages i.e. ectoderm, mesoderm, and endoderm. The male infertility is exceedingly complex because of poor understanding of microenvironment regulating extrinsic or intrinsic factor during spermatogenesis. In human, OCT4 NANOG, and SOX2,4 & 9 expression play an imperative role in variety of disease like neural tube defect, infertility and cancer to maintain pluripotency during cellular- differentiation either alone or synergistic manner [3-5]. Hence, considerable interest has been generated in the present study to evaluate the frequency of stem cells genes regulation and their DNA copy number variations to assess the genomic susceptibility to explore the etiopathology of NOA cases. Another relevance of these stem cell study also helpful to assess “risk factor” and may use as “biomarker” for early detection and for stem cell therapy as a source of regenerative medicine.
Materials and Methods
Clinically diagnosed cases (n=70) of NOA form department of obstetrics and gynecology, All India Institute of Medical Sciences, Patna, Bihar, after written consent from patients with age matched fertile (n=90) controls having normal offspring. The peripheral blood samples (1.0 mL) were collected under sterile conditions for genomic study of stem cells. The study was approved by Institute Ethics Committee (IEC). Genomic DNA was isolated using kit (Promega, USA), protocol and samples were stored at -80°c, till farther analysis. The present case-control cohort study were carried out for the characterization of pluripotent stem cell marker - OCT4, NANOG, and SOX9 using forward & reverse amplicons (primers) with annealing temperature and details were documented in (Table 1).
| Types | Sequences (forward & reverse) | Length (bp) | Annealing Temp. |
|---|---|---|---|
| SOX9 | F: 5-AGAGGAAGCCGAGTGGT3’ R: 5-GGCGGGACGGAGATAG 3’ | 417 bp | 56º/30 s |
| NANOG | F:5’CTGTGATTTGTGGGCCTGA3’ R:5’TGTTTGCCTTTGGGACTGGT3’ | 171 bp | 54º/30s |
| OCT4 | F: 5′-AGCGAACCAGTATCGAGAAC-3′ R: 5′-TTACAGAACCACACTCGGAC-3′ | 577 bp | 55˚/30s |
Table 1: PCR strategy was used for specific forward and reverse primers in the cases of non-obstructive azoospermia with controls
The total volume of (25µl) PCR containing 50-100 ng DNA, 10 pmol of each primer, 200 µM of each dNTP with reaction buffer 5.0µl and 3 U Taq polymerase, cycling conditions were standardize 1 min for OCT4 (577bp ) , 4 min for NANOG and 2 min for SOX9 at 94°c for initial denaturation , 60°c/01 min. ,56°c/30 s and 60°c/30 s of annealing for OCT, NANOG and SOX9, respectively, after the final extension of 35 cycles at 72°c for 05 minutes. PCR products were characterization of on 1.5% agarose gel, stained with ethidium bromide. The individual bands density were visualized for DNA copy number variations using in build software in Gel Doc system (Bio Red, USA).
Statistical Analysis.
To evaluate the significance relationship between the frequency of OCT4, NANOG and SOX4 and DNA copy number variations, the x2- test was used to calculate “p” value between cases of NOA and controls. Further, Odd Ratio (O.R) and Confidence Interval (C.I) at 95% were calculated to determine the variations for developing “risk-factor” of the disease.
Results
The findings of the present study were based on amplifications of coding regions of exons using specific set of forward and reverse primers with individual constant annealing temperature used for PCR strategy to the characterization of pluripotent stem-cell markers such as NANOG, OCT4 and SOX9 as details are documented in (Table 1). In human, the genetic diversity of stem-cell gene NANOG, OCT4 and SOX9 is highly complex in nature, but essential to assess the mutational spectra based on either over or under-expression or complete disappearance (null) of base pair (bp) band on agarose gels consider as mutation (Table 2). showing the details findings of the frequency (%) variation of gene-expression of all the three pluripotent stem cell markers with convincing findings after statistical evaluation to calculate significance difference (p < 0.05) between cases (NOA) and controls. These findings were repeated three times and after validation to confirm the genetic diversity of stem cells becomes a crucial factor to regulate pluripotency during proliferation and differentiation of germ-cells in male gonads.
| S.N | Types of Stem Cell marker genes |
Frequency (%) | Odds ratio (O.R) | Confidence Interval (C.I) at 95% | p-Value | ||
|---|---|---|---|---|---|---|---|
| Cases | Control | Min. | Max | ||||
| 1. | Sox9 (n=28) Under expression Over expression Null/Absent |
20.0% 33.0% 20.8% |
00.0% 10.71% 3.5% |
0.14 0.11 0.22 |
0.0125 0.0408 0.0408 |
1.0071 0.7054 1.2309 |
0.05* 0.17 0.08 |
| 2. | Nanog (n=21) Under expression Over expression Null/Absent |
23.0% 42.0% 28.8% |
00.0% 7.4 % 3.7% |
0.20 0.07 0.14 |
0.0041 0.0297 0.0295 |
1.4305 1.4305 0.6877 |
0.45 0.04* 0.01* |
| 3. | Oct4 (n=21) Under expression Over expression Null/Absent |
4.7% 9.3% 9.1% |
0.00% 7.14% 0.00% |
0.50 0.75 0.33 |
0.0421 0.0691 0.0320 |
5.9440 9.0817 3.4696 |
0.58 0.82 0.35 |
Table-2: Statistical analysis showing the significant relationship of pluripotent stem-cell markers Oct4, Nanog, Sox9 gene in the cases of non-obstructive azoospermia and controls
1. OCT4 gene regulation in non-obstructive azoospermia and controls
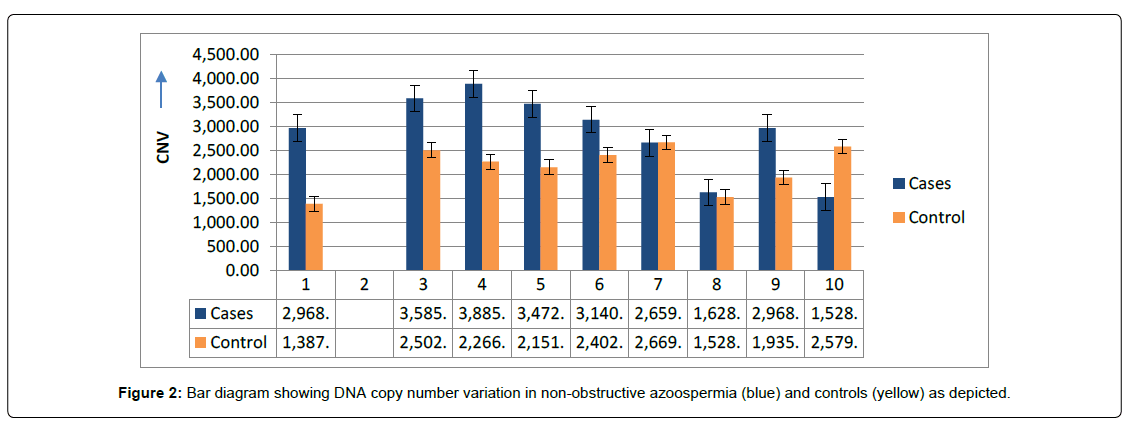
(Figure 1) showing PCR based analysis of differential frequency of OCT4 gene - expression between cases of NOA and controls. The highest frequency (9.3%) of over-expression were observed in NOA cases with calculated values of O.R (0.75) and C.I varying between 0.069-9.081 as compare to controls. The complete disappearance (null) of 577bp band were observed in 9.1% cases of NOA (arrow) consider as mutation. Apparently (Figure 2) bar diagram showing the DNACNVs also support the above findings that significant decreasing trend of OCT4 gene variations were observed with respect to controls, whereas case number two showing mutation (arrow) and case number three & four showing up-regulation (over-expression).
2. NANOG gene regulation in non-obstructive azoospermia and controls
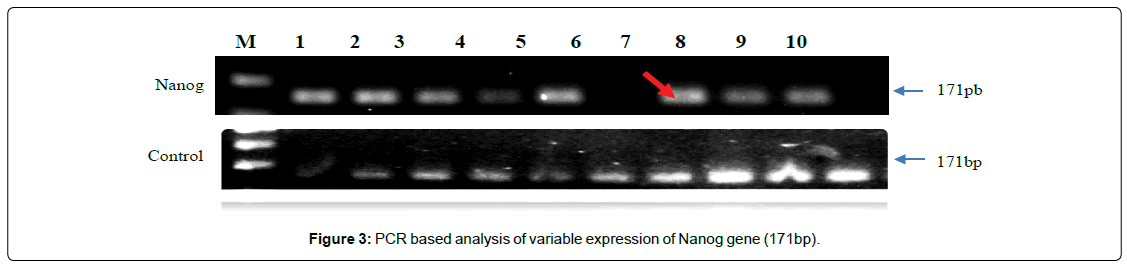
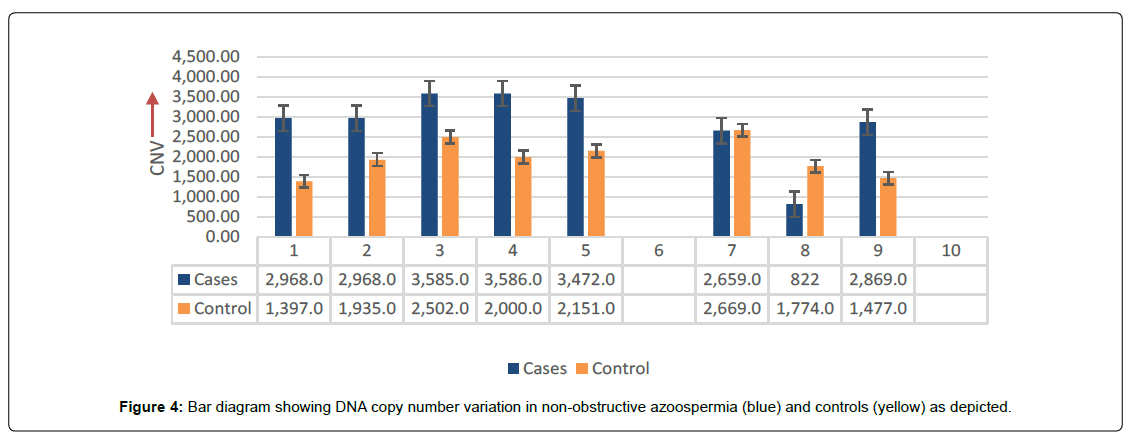
PCR based analysis of NANOG showing variable gene-expression between cases and controls. (Figure 3) showing the frequency (42%) of over-expression (up-regulation) with calculated values of O.R (0.07) and C.I varies at 95% interval between 0.029-1.43. The frequency (28.8%) of complete disappearance of band (151bp) was observed in the cases of NOA, consider as mutation and calculated values of O.R (0.14) with C.I varies from 0.029 to 0.687, showing highly significant differences (p<0.01) with respect to controls. The findings were further analysed and supported by DNACNVs variations, where the bar diagram showing systematic higher values in all the samples, except case number seven (bar), where, the frequency of controls observed more than NOA case as depicted in (Figure 4).
3. SOX9 gene regulation in non-obstructive azoospermia and controls
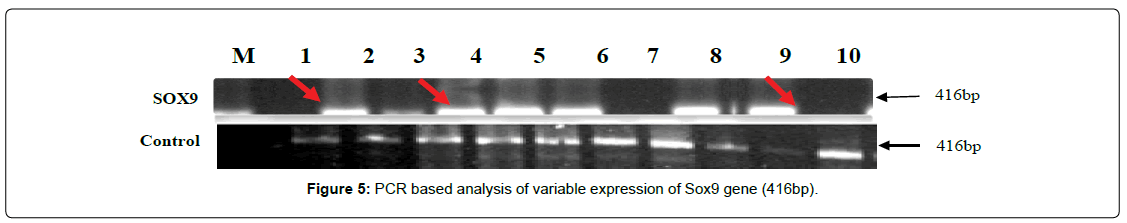
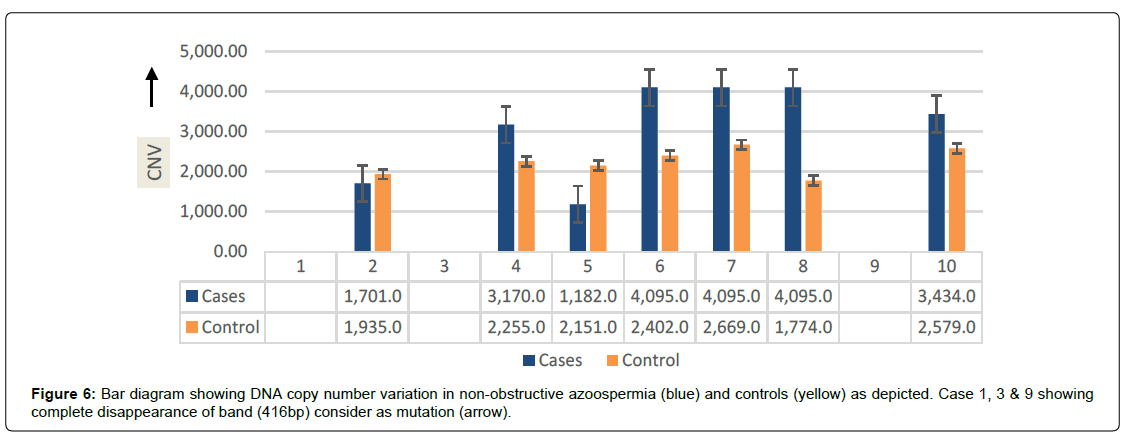
SOX9 gene (416bp) showing three different type of gene-regulation in term of either over-expression (up-regulation) or under-expression (down-regulation) and complete disappearance of band, consider as gene-mutation in the cases of non-obstructive azoospermia and findings were compare with controls as shown in (Figure 5).The SOX9 gene over-expression were observed in 30% of cases of NOA and calculated values of O.R (0.11) with C.I at 95% varies between 0.040 - 0.705, while under-expression was observed in 20% cases with calculated values of O.R (0.14) and C.I at 95% varies between 0.012-1.007. Interestingly, 20.8% cases showing complete disappearance (mutation) of band 416 bp in the NOA cases and calculated value of O.R (0.22) and C.I at 95% varies from 0.040 -1.230 as depicted in (Table 1). The data was further supported by DNACNVs, where, as a trends of over-expression was observed in cases (blue) when compared to controls (yellow) as shown in bar diagram (Figure 6).
Discussion
In human, the events of male reproductive system is highly complex because of interaction between genetic and epigenetics factors. The increasing mutational frequency of Complex Chromosomal Rearrangements (CCRs) are the leading cause of male infertility and becomes a serious problem in the World to the scientists as well as clinicians. The. These CCRs includes both structural such as chromosome or chromatid break points, rearrangement or change of position of gene in dicentrics or translocation or formation of ring of Y-chromosome, microdeletion of Y-chromosomes, and common numerical chromosomal involvement showing extra copy of Y- chromosomes in such male infertile cases like XXY, XYY and 46XY/47XYY (mosaic) were observed in the blood samples after GTG banded karyotypes. The fluorescence in-situ hybridization (FISH) analysis is highly sensitive technique used for further confirmation of extra copy of Y-chromosome in mosaic cases using Sry probe in infertile males [6]. In human, spermatogenesis is highly complex because of involvement of genetics or epigenetics factors such as DNA methylation to maintain intracellular folate-pool based on 5-10 Methylenetetrahydrofolate Reductase (MTHFR C677T) gene polymorphism and genetic heterogeneity unable maintain folate equilibrium results increase of “risk factor” for develop infertility [7, 8].
These stem-cells markers are also known as early transcription factor, is the reason to regulate variety of metabolic process in different disease other than infertility during organogenesis and play an important role to maintain pluripotency either through autocrine or paracrine gene regulation. SOX9 gene is assigned on chromosome -17, belongs to SOX stem cell family and variation in the their expression has been observed due to multiple copies during gonadal-ridge formation - a testicular determinant and in sex-reversal cases [9]. Earlier study of the same author again observed the down regulation of SOX9 gene-expression is in non-obstructive azoospermic cases due to frameshift mutation and removal of three amino acids glutamine, proline and alanine, suggesting increasing genetic susceptibility following by dysfunction of hypothalamic-pituitary gonadal axis resulting failure of spermatogenesis, The variation in SOX9 gene expression is essential for the normal development and function of Sertoli-cells [6,10].Present study showing lack of significant association (under-expression), suggesting poor cell-growth or proliferation of germ-cells inside the seminiferous tubules resulting may develop of “Sertoli cell only syndrome” leading cause of failure to maintain pluripotency. However, the contradictory findings were observed by Lane et al 2013, [11] , where the over-expression of SOX9 gene might have induce abnormal testicular differentiation due to interfering the function of OCT4 or NANOG stem cells gene resulting failure to maintain pluripotency. Biology of stem cell NANOG during organogenesis is quite interesting because of existing “isoform” has been reported other than infertility i.e., in neural tube defects patients. NANOG is homeodomain transcription factor regulates pluripotency either with OCT4 (OCTamer-binding transcription factor4) or SOX9 (SRY related HMG-box (SOX9) and act as activator or suppressor to the multiple targeted genes responsible for differentiation and self-renewal.NANOG3 play a key role to maintain pluripotency in differentiating either foetal or in adult cell type [12]. NANOG3 gene expression is superfluous, but essential for initial differentiation and reprogramming of germ cell (primary spermatocytes). Earlier study shows that NANOG3 express in embryonic stem cells (ESCs), embryonic germ cells (EGCs), and loss of NANOG and under- expression confirm interference in proliferating germ cells during spermatogenesis. Interestingly, our findings are similar to the earlier findings of Zaehres et al, 2005 [13], where these stem cells fail to maintain self-renewal during cell-differentiation. Present observation of NANOG shows highly variable in nature, suggesting poor-signalling and failure to maintain pluripotency in EGCs. NANOG, play a key role to regulate spermatogenesis in synchronize either through OCT4 or SOX9 stem cells in paracrine-fashion [14] .Earlier study of the same authors in human shows that all three stem cells marker gene OCT4, NANOG and SOX9 are required to regulate normal organogenesis for maintaining “central dogma” in infertility [15]. Significant over-expression of NANOG interfere embryonic ectoderm following instability in cascade of differentiating germ-cells [16].
Conclusion
Present study is small, but interesting to establish connecting link between stem-cell biology and genetic-susceptibility, suggesting significant variation of NANOG might have disturb the stem-cell functioning either due to extrinsic or intrinsic factors results dysregulation of the differentiating ,proliferating germ and non-germinal (Sertoli) cells during ontogenetically development of gonads. Hence, these comprehensive study of between early transcription factor based on OCT4, NANOG , SOX9 and DNA copy number variations becomes relevant to explain how fail to maintain pluripotency and suggesting either due to differential-expression or genetic susceptibility increase “risk” for infertility. Therefore, in future these makers may be use as early stem cell diagnostic “biomarker” and help to the clinicians for proper management .Furthermore, these stem-cells could also be used in therapeutics as a source of regenerative medicine.
Acknowledgement: Author is thankfully acknowledge to the Executive Director, All India Institute of Medical Sciences, Patna India for valuable suggestions and encouragement. Simultaneously also like to thanks to the patients.
Conflict of Interest: There is no conflict of interest between authors.
Authors Contribution. AKS is responsible for experimental design and writing , S responsible for data analysis and MT help finalizing and editing, while MA is helped for clinical diagnosis.
References
- Mouka A, Izard V, Tachdjian G, Brisset S, Yates F, et al (2017) Induced pluripotent stem cell generation from a man carrying a complex chromosomal rearrangement as a genetic model for infertility studies. Sci Rep; (7):1446-1458.
- Saxena AK, Kumar A (2019) Microdeletion of the AZFc locus with high frequency of mosaicism 46, XY/47XYY in cases of non-obstructive azoospermia in eastern population of India. Genet Mol Res; 18 (2):1-14.
- Pardal R, Clark MF, Morrison SJ (2003) Applying the principal of stem cell biology to cancer. Nat Rev Cancer; 3(12): 895-902.
- Levassesur DN, Wang J, Dorschner MO (2008) Oct4 dependence on chromatin structure within the extended NANOG locus in ES cells. Gene Dev; 22(5):575-580.
- Saxena AK, Pandey S, Pandey LK (2013) Genetic Diversity of stem cell and their functional impact on the development of neural tube defects in eastern population of India. Genet Mol Res; 12(3):2380-2390
- Saxena AK, Tiwari M, Kumar R, Aprajita, Kumar A, et al (2020) Impact of the Y-chromosome on Sox9 stem cell expression in non- obstructive azoospermic cases. Genet Mol Res; 19(1):1-9.
- Dell'Api M, Kelly TL, Chen ZT, Rozen R, Trasler JM (2001) Infertility and testicular defects in male mice deficient in methylenetetrahydrofolate reductase (MTHFR).Biol. Reprod.64:218-210.
- Kelly TL, Neaga OR, Schwahn BC, Rozen R, Trasler JM (2005) Infertility in 5, 10-methylenetetrahydrofolate reductase (MTHFR)-deficient male mice is partially alleviated by lifetime dietary betaine supplementation. Biol Reprod; 72:677-766.
- Alankarage D, Lavery R, Svingen T, Kelly S, Ludbrook L (2016) SOX9 regulates expression of the male fertility gene Ets variant factor 5 (ETV5) during mammalian sex development. Int J Biochem Cell Biol; 79: 41-51.
- Chaboissier MC, Kobayashi A, Vidal VI, Lützkendorf S, Van de Kant HJ, et al (2001) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development; 131(9):1891-1901.
- Lan KC, Chen YT, Chang C, Chang YC, Lin HJ et al (2013) Up regulation of Sox9 in Sertoli cells from testicular patients accounts for increasing anti mullerian hormone expression via impaired androgen receptor signalling. PLoS One; 8(10):e76303
- Pan G, Thomson JA (2007) NANOG and Transcriptional networks in embryonic stem cell pluripotency. Cell Res; 17(1): 42-49.
- Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz‐Eldor J, et al (2005) High efficiency of RNA interference in embryonic stem cells. Stem Cells; 23(3):299-305.
- Kuijk EW, de Gier J, Chuva de Sousa Lopes SM, Chambers I, van Pelt AM, et al (2010) A distinct expression pattern in mammalian testes indicates a conserved role for NANOG in spermatogenesis. PLoS One; 5(6):e10987.
- Saxena AK, Rastogi A (2014) Impairment of NANOG3 stem cell dysregulation associated with male infertility in Human. Hereditary Genet; 4 (2): 1-3.
- Darr H, Mayshar Y, Renvenisy N (2006) Overexpression of NANOG in ES cell enables feeder-free growth while inducing primitive ectoderm features. Development; 133(6):1193-1201.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi