Perspective, J Food Nutr Disor Vol: 12 Issue: 2
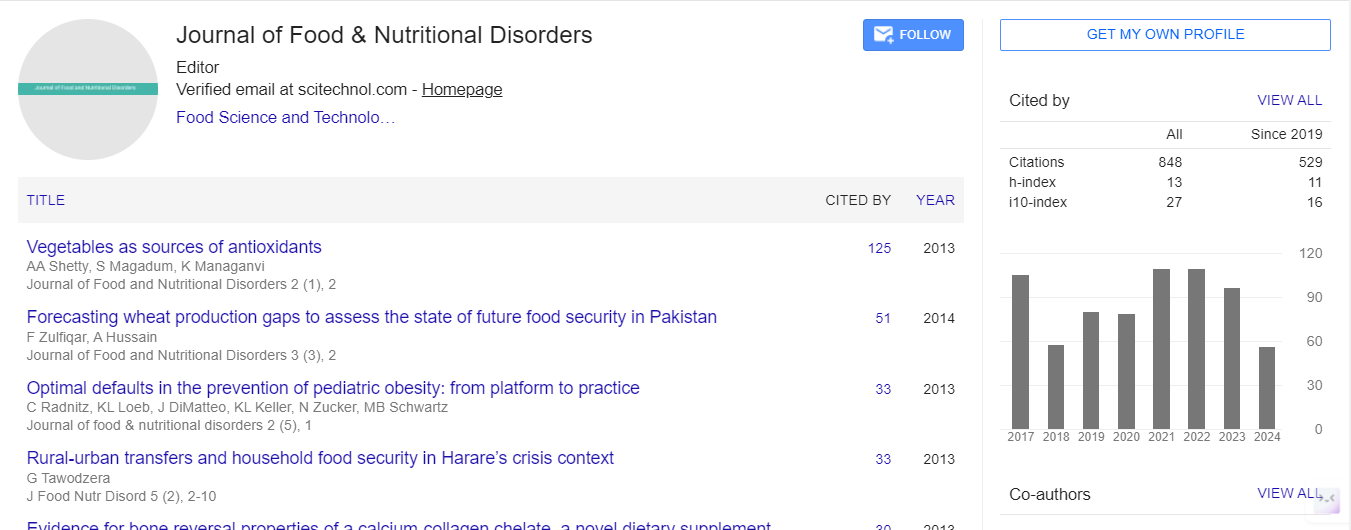
Genetic and Epigenetic Factors Contributing to Childhood Obesity
Krishna Murthy*
Department of Nutritional Science, Jaipur National University, Jaipur, Rajasthan, India
*Corresponding Author: Krishna Murthy
Department of Nutritional Science
Jaipur National University, Jaipur
Rajasthan, India
E-mail: krishnamurthy2@gmail.com
Received date: 30 March, 2023, Manuscript No. JFND-23-99023;
Editor assigned date: 02 April, 2023, Pre QC No. JFND-23-99023(PQ);
Reviewed date: 17 April, 2023, QC No. JFND-23-99023;
Revised date: 27 April, 2023, Manuscript No: JFND-23-99023(R);
Published date: 05 May, 2023, DOI: 10.35248/2324-9323.100351
Citation: Murthy K (2023) Genetic and Epigenetic Factors Contributing to Childhood Obesity. J Food Nutr Disor 12:2.
Description
Childhood obesity has become a global epidemic, posing significant health risks and long-term consequences for affected individuals. While lifestyle and environmental factors play a crucial role in the development of obesity, recent research has shed light on the contribution of genetic and epigenetic factors. This essay aims to explore the complex interplay between genetics, epigenetics, and childhood obesity, emphasizing the importance of understanding these factors for effective prevention and management strategies. Numerous studies have demonstrated that genetic factors influence an individual's susceptibility to obesity. Certain gene variants, such as FTO, MC4R, and TMEM18, have been consistently associated with increased Body Mass Index (BMI) and obesity risk in children. Genetic factors can interact with environmental factors, such as diet and physical activity, influencing an individual's response to these factors and ultimately contributing to obesity risk. Researchers have identified and studied specific genes involved in appetite regulation, metabolism, and fat storage, providing insights into the genetic mechanisms underlying childhood obesity.
Epigenetic mechanisms, including DNA methylation, histone modifications, and non-coding RNA molecules, can modulate gene expression without altering the DNA sequence. Altered epigenetic patterns have been observed in individuals with obesity, suggesting a potential role in its development. The DOHaD hypothesis proposes that early-life environmental factors can lead to long-lasting changes in epigenetic marks, influencing the risk of obesity and related metabolic disorders in childhood and adulthood. Emerging evidence suggests that epigenetic modifications can be inherited across generations, potentially explaining the increased risk of obesity observed in the offspring of obese parents. Genetic factors can influence epigenetic modifications, and conversely, epigenetic marks can modify gene expression patterns. This bidirectional interplay highlights the intricate relationship between genetics and epigenetics in the context of childhood obesity. Epigenetic modifications can directly impact genes involved in energy balance, adipogenesis, and appetite regulation, contributing to the development of childhood obesity. Genetic and epigenetic factors can interact with environmental influences, such as maternal nutrition during pregnancy or early-life exposure to obesogenic factors, amplifying the risk of childhood obesity.
Understanding the genetic and epigenetic factors contributing to childhood obesity can aid in the development of personalized prevention and management strategies, tailored to an individual's unique genetic and epigenetic profile. Identifying children at higher genetic or epigenetic risk for obesity can facilitate early intervention efforts, focusing on modifiable environmental factors to mitigate the genetic predisposition. Knowledge of specific genetic and epigenetic factors associated with childhood obesity may pave the way for novel therapeutic targets, such as gene-editing techniques or epigenetic modifiers, aiming to prevent or reverse obesity-related metabolic dysregulation. Numerous family studies have shown a strong hereditary component in childhood obesity. Identical twin studies have demonstrated a higher concordance rate for obesity compared to fraternal twins. The identification of specific genes associated with obesity, such as FTO and MC4R, further supports the genetic influence. Childhood obesity is a multifaceted condition influenced by a complex interplay of genetic and epigenetic factors. Genetic predisposition and epigenetic modifications can significantly contribute to obesity risk, interacting with environmental factors to shape an individual's susceptibility. Understanding the intricate relationship between genetics, epigenetics, and childhood obesity is important for developing effective prevention and management.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi