Research Article, J Pharm Drug Deliv Res Vol: 12 Issue: 3
Fabrication and Characterization of Drug Loaded Scaffolds for Corneal Tissue Engineering
Allan Joshua I, Asha M, Shanmuganathan S and Nagalakshmi S*
Department of Pharmaceutics, Sri Ramachandra Faculty of Pharmacy, Sri Ramachandra University, Chennai, India
*Corresponding Author: Nagalakshmi S
Department of Pharmaceutics, Sri
Ramachandra Faculty of Pharmacy, Sri Ramachandra University, Chennai, India
E-mail: nagalakshmi.s@sriramachandra.edu.in
Received date: 07 November, 2022, Manuscript No. JPDDR-22-79219;
Editor assigned date: 10 November, 2022, PreQC No. JPDDR-22-79219 (PQ);
Reviewed date: 24 November, 2022, QC No. JPDDR-22-79219;
Revised date: 03 February, 2023, Manuscript No. JPDDR-22-79219 (R);
Published date: 10 February, 2023, DOI: 10.4172/2325-9604.1000019
Citation: Joshua IA, Asha M, Shanmuganathan S, Nagalakshmi S (2023) Fabrication and Characterization of Drug Loaded Scaffolds for Corneal Tissue Engineering. J Pharm Drug Deliv Res 12:3.
Abstract
The present work was aimed towards synthesizing chitin from crab shell, followed by preparation of chitin and deacetylation of chitin to chitosan which was then fabricated into scaffold by using variety of polymers including (HPMC, SCMC, CMC, chitosan). ofloxacin possess high activity as a wound healing accelerator. The anti-infective ability and antibacterial activity of ofloxacin can be further enhanced by the polymer prepared from chitosan prepared from chitin.
Four formulations were developed (i.e., S1, S2, S3, S4,) using various usage of polymers such as (HPMC, SCMC, CMC, chitosan). The prepared scaffolds were studied for its characteristic properties such as weight loss, swelling ability, porosity measurement, X-ray diffraction, scanning electron microscopy, transmission electron microscopy, FT-IR, optical microscopy, zeta potential, In vitro release studies and In vitro antimicrobial studies.
Owing to the greater water uptake activity, sufficient porosity, improved antibacterial activity and extended drug release, the optimised formulation containing chitosan would be a promising biomaterial for corneal tissue engineering applications compared with other polymers.
From this research, it was concluded that the drug loaded scaffold is a viable alternative to existing conventional dosage forms which lead to improved bioactivity and a promising biomaterial for corneal tissue engineering applications in case of administration affords resulting in better patient compliance and cost effective therapy in the field of biomedical application.
Results indicated that chitosan scaffolds (S4) containing ofloxacin were well suited for the sustained release of ofloxacin and are promising carrier for the drug delivery in corneal tissue engineering due to their compliance with the corneal epithelial cells.
Keywords: Chitosan; Scaffold; Ofloxacin; Corneal tissue engineering
Introduction
The cornea is the outermost layer of the eye and together with the eyelids and sclera protects the inner part of the eye. Most of the focusing power of eye is due to the cornea. It is an elastic, non vascularised tissue, about 500 micro meters-600 micro meters thick, that conforms to the curvature of the eye and is composed of five distinct layers and three different cell types [1].
Tissue engineering is a multidisciplinary field that applies the principles of biology, chemistry, physics and engineering for the development of substitutes that replace, repair or enhance biological function of diseased and damaged human body parts, by manipulating cells via their extracellular microenvironment [2]. This three dimensional extracellular architecture ("scaffold") can be fabricated in the shape of the tissue we want to restore, with the help of either polymer hydrogel, self-assembly, nonwoven matrix, nano-fibrous electro spun matrices, 3D weaving, or any other textile technology based techniques, depending upon their structural and functional requirements [3].
A tissue engineering scaffolds can be 2D or 3D designed structure made of a preferably biodegradable material either of natural or synthetic that would provide a hospitable micro environment for cells to grow, differentiate and carry out their metabolic activity [4]. These materials are generally polymeric, but for hard tissue engineering some inorganic materials such as titanium oxides or phosphates are also being tested. There are certain requirements that should be fulfilled by these materials such as mechanical strength, controlled biodegradability, high porosity and optimum pore size, in order to allow cell infiltration and transfer of nutrients and wastes, as well as sufficient surface area and appropriate chemical and physical surface properties to promote cell attachment, proliferation and migration [5].
There have been several attempts to produce corneal equivalents via tissue engineering. For the epithelial layer, studies have focused on the production of surfaces that are conducive for proliferation of epithelial cells and cues that would lead to total coverage of the surface. To this end, fibrin, Epidermal Growth Factor (EGF) coated Polydimethylsiloxane (PDMS) films and crosslinked collagen gels have been used. PDMS films and collagen gels have performed well under in vitro conditions and the fibrin substrate has gone through clinical trials with considerable success [6]. Thus, the present work is an effort to develop drug loaded scaffolds using chitosan derived from crab shell for corneal tissue engineering using other polymers like HPMC, SCMC, CMC in the formulation.
Materials and Methods
Materials
Ofloxacin was supplied from carbanio.com, hydrogen peroxide, saturated calcium chloride, methanol was supplied from sigma aldrich, Chennai. 1% trisodium citrate, 0.001 M silver nitrate was supplied from nice chemicals Pvt. Ltd.
Methodology
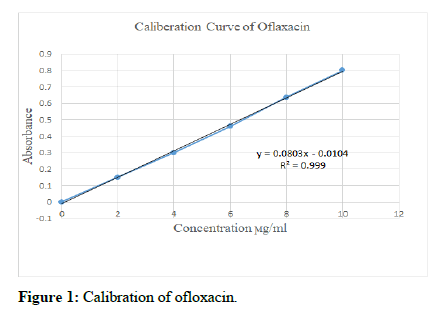
Calibration curve of ofloxacin: Stock solution of ofloxacin was prepared by dissolving 100 mg of ofloxacin in 100 ml of 0.1 N hydrochloric acid in a volumetric flask to get a concentration of 1000 μg/ml.10 ml of the stock solution was pipetted out and diluted to 100 ml with 0.1 N hydrochloric acid to get primary solution of concentration 100 μg/ml.10 ml of primary solution was pipetted out and further diluted to 100 ml with 0.1 N hydrochloric acid to get secondary solution of concentration 10 μg/ml. Secondary solution was diluted to get 2 μg/ml, 4 μg/ml, 6 μg/ml, 8 μg/ml and 10 μg/ml solutions [7]. The absorbance of each solution was measured at 294 nm using a UV visible spectrophotometer using 0.1 N hydrochloric acid as blank. The standard curve was plotted taking concentration in the x-axis and absorbance in the y-axis [8].
Preparation of chitin from crab shell: Crab shells were cleaned and washed thoroughly to remove any foreign materials, followed by grinding to get particle size 0.30 mm-0.35 mm [9]. In demineralization process, crab shell powder was added slowly to 7% Hydrochloric acid with continuous stirring to avoid effervescence and heated at 600°C for 2 hours-3 hours to remove carbonate and phosphate content from the crab shell powder.
In deproteinization step, the acid hydrolyzed sample was treated with 5% w/v sodium hydroxide to reduce nitrogen content of protein, followed by washing to remove any traces of sodium hydroxide. The sample was filtered, washed repeatedly with distilled water to remove any traces of chemicals and soluble impurities [10-13]. The filtered sample was then dried in an oven at 700°C for 3 hours. The dried demineralized, deproteinized and deodorized white sample of chitin was obtained. In bleaching step, the dried sample washed with hydrogen peroxide to reduce pigment of chitin, followed by drying and storing under airtight condition.
Deacetylation of chitin to chitosan: The process was then carried out by adding 50% sodium hydroxide to the obtained sample on a hot plate and boiling it for 2 hrs at 100°C. The sample was then allowed to cool at room temperature for 30 minutes. Then they were washed continuously with 50% sodium hydroxide, the sample obtained is filtered (chitosan is obtained) [14]. The sample was left uncovered and oven-dried for 6 hrs at 110°C.
Fabrication of scaffold: Weighed quantity of polymer Hydroxy Propyl Methyl Cellulose (HPMC) and 100 mg of ofloxacin drug were dissolved in water using a mechanical stirrer until a homogenous solution was formed. Secondly, chitosan was solubilized in 2% acetic acid, which was instilled dropwise into the HPMC mixture [15]. It was then mixed at 500 rpm. The stirring was kept for 24 hrs and, the gel formed was then transferred into the tissue culture dish and cooled at -24°C for 24 hrs and lyophilized to form scaffolds. These scaffolds were cross linked with CaCl2 solution for 30 minutes, followed by dripping in ethanol for 10 minutes. Finally, the scaffold was clarified with water and was lyophilized. The procedure was repeated with other polymers like SCMC/CMC/chitosan [16].
Characterization studies
Weight loss: The weight loss of the composites was carried out in vitro by incubating the composite in Simulated Body Fluid (SBF) at pH 7.4°C and 37°C for different periods (1,3,7,15,21 and 28 days).
At interval time the composites were taken from the medium and dried at 50°C over night. The weight loss was calculated by the following equation;
Weight loss (%) = ((Wo -Wt)/Wo) × 100
Where, Wo denotes the original weight of the composite, while Wt is the weight at time (t). Each experiment was carried out for three samples and the average value was taken to ensure the result [17].
Swelling ability: Swelling ability was determined by the percentage of water absorption. Dry weight of the scaffolds was denoted as Wi. Then, porous scaffolds were immersed in phosphate buffer solution (pH 7.4) at 37°C for 24 hours. Afterwards, the scaffolds were taken out from PBS solution and its wet weight was measured denoted as Wf. The ratio of the swelling was calculated using the equation;
Swelling ability (%) = ((Wf-Wi)/Wi) × 100
Porosity measurement: The porosity of the composite scaffolds can be determined by Archimedes’ principle. Ethanol was selected as the displacement liquid as it permeated the scaffolds without swelling or shrinking the matrix [18]. The dry weight of scaffolds was denoted as Wd, while Wi denoted the weight of the scaffolds after immersing in ethanol for 5 minutes. Then, the scaffolds were removed and the liquid on the surface removed by filter paper. Using equation;
Porosity (%) = (Ww–Wd)/(Ww–Wl) × 100
Fourier transform infrared analysis: The FT-IR is used to characterize and to know the chemical interactions between chitin and other excipients. The spectra of the chitin and drug loaded composite scaffolds were recorded using KBr pellet method in an FT-IR spectrophotometer (JASCO 4100 type A) with the range of 4000 cm-1 to 400 cm-1.
Surface analysis: The surface morphology of the bio composite scaffold was studied using optical microscopy, Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) [19].
Optical microscopy: In addition, optical microscopy was also performed with prepared scaffold using MOTIC digital microscope. The scaffold was viewed at 10X and 40X.
Scanning electron microscopy: SEM was used to identify the surface morphology of the formulated sample. The powdered sample was taken and mounted on a double side carbon tape, which was fixed to sample specimen stub. The SEM (QUANTA FEG) instrument was used for analysis.
Transmission electron microscopy: TEM analysis can be performed using the principle of different TEM techniques including bright field image, dark field image, electron diffraction and high resolution TEM. The principle employed to view the scaffolds was High Resolution Transmission Electron Microscopy (HRTEM).
The prepared formulation (20 μl) of solution was taken and dropped on the carbon coated side of the copper grid. The grid was dried at room temperature for few hours. The grid was then placed in the sample holder and mounted in the instrument. The instrument TECHNAI T20 was used for the analysis [20].
X-ray diffraction analysis: X-Ray Diffraction analysis (XRD) was performed with a PAN analytical Xpert pro x-ray diffractometer using Ni filtered Cu Kα radiation. The powdered sample for evaluation was taken on the glass slide and placed on the x-ray diffractometry [21]. The scanning rate was continued over a 20 range of 10°C to 90°C.
In vitro release studies: The drug release property of the prepared scaffolds was carried out as follows. 100 μg/ml solution of scaffold was prepared and transferred to a conical flask. To this, 3 ml of pH 7.4 phosphate buffer solution was added and kept in an orbital shaker. The amount of oflaxacin released from the scaffolds was estimated by collecting phosphate buffer solution from the test tubes and replacing by the addition of 3 ml fresh buffer at 30 minutes intervals for 8 hours [22]. The concentration of drug release was determined spectrophotometrically at 390 nm. The drug release concentration was calculated from the standard curve.
In vitro antibacterial activity
Agar disc diffusion method preparation of inoculum: Stock cultures were maintained at 4°C on slant of nutrient agar. Active cultures for experiments were prepared by transferring a loop full of cells from the stock cultures to test tubes of nutrient broth for bacteria that were incubated at 24 hrs at 37°C. The Assay was performed by agar disc diffusion method.
Antibacterial activity: Antibacterial activity of sample was determined by disc diffusion method on Muller Hinton Agar (MHA) medium. The Muller Hinton agar medium was weighed as 3.8 gms and dissolved in 100 ml of distilled water and to this add 1 gm of agar. Then the medium was kept for sterilization. After sterilization the media was poured in to sterile petriplates and were allowed to solidify for 1 hr. After the medium was solidified, the inoculums were spread on the solid plates with sterile swab moistened with the bacterial suspension. Discs were prepared with test specimen chitosan drug loaded composite scaffold 20 μl sample of respective concentrations (250 μg, 500 μg, 1000 μg,) negative control sterile distilled water and positive control 10 μl (10 μg) streptomycin were placed on MHA plates. These plates were incubated for 24 hrs at 37°C. Then the microbial growth was determined by measuring the diameter of zone of inhibition [23].
Results and Discussion
Calibration curve of ofloxacin
The stock solution of ofloxacin was prepared by dissolving 100 mg of ofloxacin in 100 ml of 0.1 N hydrochloric acid. 10 ml of the stock solution was pipetted out and diluted to 100 ml with 0.1 N hydrochloric acid to get solution 1, and further diluted. The absorbance of each solution was measured at 294 nm using a UVvisible spectrophotometer using 0.1 N hydrochloric acid as blank. The standard curve was plotted taking concentration in the x-axis and absorbance in the y-axis was shown in Table 1 and in Figure 1. This indicated that it obeys beer lambert law.
| Concentration (ϻg/ml) | Absorbance |
|---|---|
| 0 | 0 |
| 2 | 0.1499 |
| 4 | 0.2999 |
| 6 | 0.4597 |
| 8 | 0.6365 |
| 10 | 0.8085 |
Table 1: Calibration value.
Fabrication of drug loaded scaffold
The scaffolds were prepared as per the procedure described earlier Figure 2. The quantities of the ingredients in each scaffolds was given in Table 2. The scaffolds were characterized using numerous analytical techniques and physical parameters as mentioned.
| Formulation code | S1 (mg) | S2 (mg) | S3 (mg) | S4 (mg) |
|---|---|---|---|---|
| Chitosan | 30 | 30 | 30 | 30 |
| SCMC | - | 10 | - | - |
| CMC | - | - | 10 | - |
| HPMC | - | - | - | 10 |
| Ofloxacin | 100 | 100 | 100 | 100 |
| Dichloromethane | 50 ml | 50 ml | 50 ml | 50 ml |
| Water | 100 ml | 100 ml | 100 ml | 100 ml |
Table 2: Fabrication of drug loaded scaffold.
Weight loss
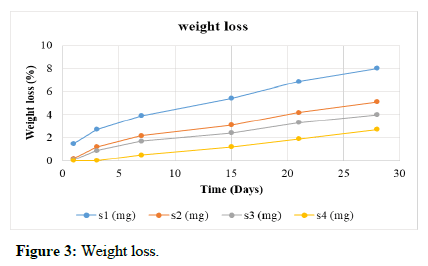
Weight loss is a measure of the degradation of the scaffolds with respect to time. The study was carried out in Simulated Body Fluid (SBF) at pH 7.4°C and 37°C for four weeks as described. Scaffold S1 has maximum weight loss of 7%. Scaffold S2 has weight loss of 6% during the period of study. The scaffold S4 showed less weight loss compared to S3. Scaffold S5 showed least loss of weight (2.7%) in four weeks and thus has the least degradation as shown in Table 3 and Figure 3.
| Time (days) | S1 (%) | S2 (%) | S3 (%) | S4 (%) |
|---|---|---|---|---|
| 1 | 1.5 | 0.2 | 0.1 | 0 |
| 3 | 2.7 | 1.2 | 0.9 | 0 |
| 7 | 3.9 | 2.2 | 1.7 | 0.5 |
| 15 | 5.4 | 3.1 | 2.4 | 1.2 |
| 21 | 6.9 | 4.2 | 3.3 | 1.9 |
| 28 | 8 | 5.1 | 4 | 2.7 |
Table 3: Weight loss.
Swelling ability
The swelling ability is a measure of the water uptake and retention properties of the scaffold. Swelling ability was calculated using the formula, the results of which are given in Table 4. The swelling ability was found to be increasing with increase in chitosan concentrations. The swelling ability is important when the scaffold is employed in a large area; the faster the better results obtained.
| Formulation code | Wi (g) | Wf (g) | Swelling ability (%) |
|---|---|---|---|
| S1 | 1 | 2.4 | 140 |
| S2 | 1 | 2.6 | 160 |
| S3 | 1 | 2.5 | 150 |
| S4 | 1 | 2.9 | 190 |
Table 4: Swelling ability.
Porosity measurement
Porosity is calculated using the given formula. The porosity of the scaffolds was similar to one another as shown in Table 5. The porosityis high when chitosan concentration is high. For wound healing applications, a porous scaffold should have ideal porosity of 90.5% to provide the optimal balance between a better surface area for cell attachment and its structural strength.
| Formulation code | Ww (g) | Wd (g) | Wl (g) | Porosity (%) |
|---|---|---|---|---|
| S1 | 0.58 | 0.25 | 1.15 | 57.89 |
| S2 | 0.59 | 0.25 | 1.19 | 56.66 |
| S3 | 0.62 | 0.25 | 1.2 | 63.79 |
| S4 | 0.65 | 0.25 | 1.24 | 67.79 |
Table 5: Porosity measurement.
Fourier transform infrared analysis
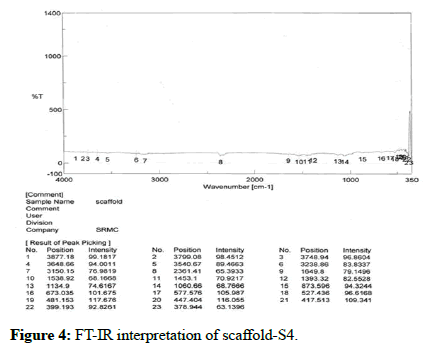
The presence of functional groups was confirmed by using FT-IR (instrument-SCO 4100 type A). The readings were obtained between 400 cm-1 to 4000 cm-1 using KBR pellet technique. FT-IR was carried out for chitosan and scaffold. The results of the analysis showed various stretching, bending and rocking vibrations based on the groups present. There is no interaction between active ingredient and other excipients as shown in Table 6 and Figure 4.
| S.No | Wave number (cm-1) | Vibrational frequency |
|---|---|---|
| 1 | 3748 | N-H stretching |
| 2 | 3540 | O-H stretching |
| 3 | 3150 | C-H stretching |
| 4 | 2361 | C=C stretching |
| 5 | 1649 | C=N stretching |
| 6 | 1538 | N-H bending |
| 7 | 1453 | C-H bending |
| 8 | 1393 | O-H bending |
| 9 | 1134 | C-N stretching |
| 10 | 1060 | C-C stretching |
| 11 | 873 | N-H rocking |
| 12 | 673 | C-H rocking |
Table 6: FT-IR Interpretation of optimised scaffold (S4).
Surface analysis
Optical microscopy: Optical microscopy was carried out for the surface morphology of scaffold using motic digital microscope. The scaffold was viewed at 10X and 40X as shown in Figure 5.
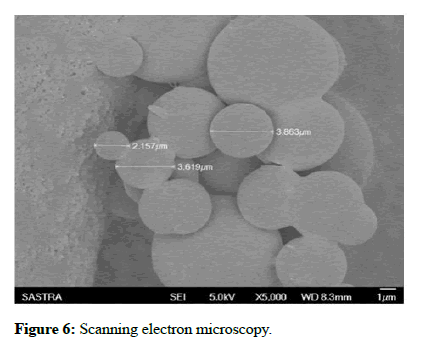
Scanning electron microscopy: Scanning electron microscopy was also carried out to identify the surface morphology of the scaffold. The scaffold was examined by SEM where SEM is a part of a dual beam instrument ‘QUANTA FEG’. The images shown in Figure 6 were taken with an acceleration voltage of 30 KV, with varying magnification of 24000X, 12000X, 6000X and 3000X. The images exhibited that the scaffold was found to have very porous structure with smooth surface morphology.
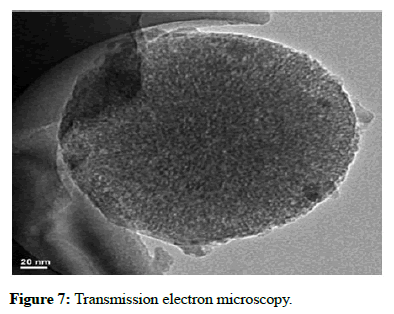
Transmission electron microscopy: TEM studies were useful in examining the morphological and crystalline arrangements of drug (ofloxacin) and chitosan, cellulose, drug interaction. TEM report analysis revealed the presence of internal morphology of chitin nanosilver composite scaffold with size of 20 nm and 50 nm. The internal morphology showed porous, spherical scaffold which were shown in Figure 7.
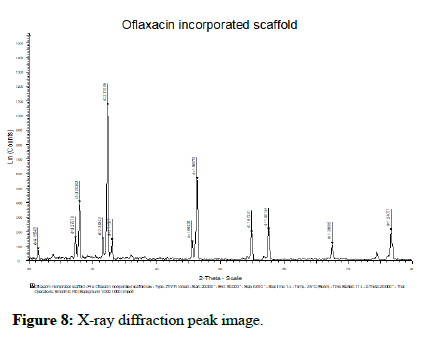
X-Ray diffraction analysis: XRD patterns of the chitosan drug loaded composite, scaffold was shown in Figure 8. In XRD analysis, the peaks were obtained at 20 level at positions 20.21, 21.24, 23.70, 27.22, 30.95, 31.37, 34.90, 43.14, 46.06, 57.80, 68.14, 79.71. Hence, the formulated scaffold was found to exhibit crystalline structure of scaffold.
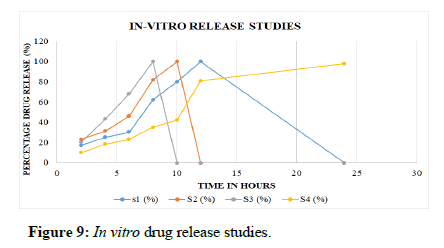
In vitro release studies: The scaffold S1 showed an initial burst release followed by a sustained release driven by diffusion of drug through the interior of the scaffolds and the release rate was found to be 100% at the end of 12 hours, whereas scaffolds S2 showed 100% release at the end of 10 hours and S3 showed release of 100% at the end of 8 hours of study. The S4 (incorporated with HPMC and chitosan) showed the release of 98% at the end of 24 hours. However, S4 showed sustained release profile over an extended period of study up to 24 hours. When compared with other polymers like SCMC/CMC, the S4 formulation has been optimized for further characterization studies. Table 7 and Figure 9 show the release profile of the formulated scaffolds.
| Time (hrs) | S1 (%) | S2 (%) | S3 (%) | S4 (%) |
|---|---|---|---|---|
| 2 | 17 | 23 | 20 | 10 |
| 4 | 25 | 31 | 43 | 18 |
| 6 | 30 | 46 | 68 | 23 |
| 8 | 62 | 82 | 100 | 35 |
| 10 | 80 | 100 | - | 42 |
| 12 | 100 | - | - | 81 |
| 24 | - | - | - | 98 |
Table 7: In vitro release studies.
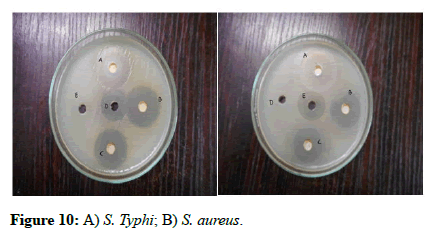
In vitro antibacterial activity: Table 8 and Figures 10A and 10B showed the results of antibacterial activity studies. Figure 10A showed bactericidal efficiency of drug loaded scaffold against gram negative S. typhi while Figure 10B showed activity against gram positive S. aureus. It was found that zone of inhibition is higher in S. typhi than S. aureus indicating higher susceptibility of gram-negative bacteria to drug loaded scaffold. Also as the concentration of drug loaded scaffolds was increased, the zone of inhibition was also found to be increased. These results showed that the antibacterial activity was due to the presence of oflaxacin and chitosan in scaffolds. Hence, the developed scaffolds exhibited good antibacterial activity.
| Microorganisms | Zone of Inhibition in mm | ||||
|---|---|---|---|---|---|
| 1000 µg | 500 µg | 250 µg | Sterile distilled water | Streptomycin 10 µg | |
| Oflaxacin | |||||
| S. typhi | 26 | 25 | 24 | - | 20 |
| S. aureus | 28 | 26 | 24 | - | 19 |
Table 8: In vitro antibacterial activity.
Conclusion
From this research, it was concluded that the drug loaded scaffold is a viable alternative to existing conventional dosage forms which lead to improved bioactivity and a promising biomaterial for corneal tissue engineering applications in case of administration affords resulting in better patient compliance and cost effective therapy in the field of biomedical application.
Acknowledgement
The authors would like to express their sincere thanks to department of pharmaceutics, SRIHER, Porur, Chennai, Tamil nadu, India. For providing the support to carry out this research work.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- DelMonte DW, Kim T (2011) Anatomy and physiology of the cornea. J Cataract Refract Surg 37:588-598.
[Crossref] [Google Scholar] [PubMed]
- Sridhar MS (2018) Anatomy of cornea and ocular surface. Indian J Ophthalmol 66:190.
[Crossref] [Google Scholar] [PubMed]
- Malhotra N, Kundabala M, Acharya S (2009) Current strategies and applications of tissue engineering in dentistry-A review part 1. Dent update 36:577-582.
[Crossref] [Google Scholar] [PubMed]
- Zhang F, King MW (2020) Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv Healthc Mater 9:1901358.
[Crossref] [Google Scholar] [PubMed]
- Sill TJ, Von Recum HA (2008) Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 29:1989-2006.
[Crossref] [Google Scholar] [PubMed]
- Klenkler BJ, Dwivedi D, West-Mays JA, Sheardown H (2010) Corneal epithelial cell adhesion and growth on EGF-modified aminated PDMS. J Biomed Mater Res A 93:1043-1049.
[Crossref] [Google Scholar] [PubMed]
- Lawrence BD, Marchant JK, Pindrus MA, Omenetto FG, Kaplan DL (2009) Silk film biomaterials for cornea tissue engineering. Biomaterials 30:1299-1308.
[Crossref] [Google Scholar] [PubMed]
- Min BM, Lee SW, Lim JN, You Y, Lee TS, et al. (2004) Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer 45:7137-7142.
[Crossref] [Google Scholar] [PubMed]
- Carletti E, Motta A, Migliaresi C (2011) Scaffolds for tissue engineering and 3D cell culture. Methods Mol Biol 695:17-39.
[Crossref] [Google Scholar] [PubMed]
- Henkel J, Hutmacher DW (2013) Design and fabrication of scaffold-based tissue engineering. Bio Nano Materials 14:171-193.
- Zhang J, Sisley AM, Anderson AJ, Taberner AJ, McGhee CN, et al. (2016) Characterization of a novel collagen scaffold for corneal tissue engineering. Tissue Eng Part C Methods 22:165-172.
[Crossref] [Google Scholar] [PubMed]
- Lu Q, Wang X, Lu S, Li M, Kaplan DL, et al. (2011) Nanofibrous architecture of silk fibroin scaffolds prepared with a mild self-assembly process. Biomaterials 32:1059-1067.
[Crossref] [Google Scholar] [PubMed]
- Fang D, Pan H, Cui M, Qiao S, Li X, et al. (2022) Fabrication of three dimensional-printed ofloxacin gastric floating sustained-release tablets with different structures. J Drug Deliv Sci Technol 67:102992.
- Jing X, Mi HY, Peng J, Peng XF, Turng LS (2015) Electrospun aligned poly (propylene carbonate) microfibers with chitosan nanofibers as tissue engineering scaffolds. Carbohydr Polym 117:941-949.
[Crossref] [Google Scholar] [PubMed]
- AbouSamra MM, El Hoffy NM, El-Wakil NA, Awad GE, Kamel R (2022) Computational investigation to design ofloxacin-loaded hybridized nanocellulose/lipid nanogels for accelerated skin repair. Gels 8:593.
- Wu Y, Jin M, Huang Y, Wang F (2022) Insights into the prospective aerogel scaffolds composed of chitosan/aramid nanofibers for tissue engineering. ACS Appl Polym Mater 4:4643-4652.
- Saudi A, Zebarjad SM, Alipour H, Katoueizadeh E, Alizadeh A, et al. (2022) A study on the role of multi-walled carbon nanotubes on the properties of electrospun poly (caprolactone)/poly (glycerol sebacate) scaffold for nerve tissue applications. Mater Chem Phys 282:125868.
- Ghaziof S, Shojaei S, Mehdikhani M, Khodaei M, Nodoushan MJ (2022) Electro-conductive 3D printed polycaprolactone/gold nanoparticles nanocomposite scaffolds for myocardial tissue engineering. J Mech Behav Biomed Mater 132:105271.
[Crossref] [Google Scholar] [PubMed]
- Murugan R, Ramakrishna S, Rao KP (2006) Nanoporous hydroxy-carbonate apatite scaffold made of natural bone. Materials Letters 60:2844-2847.
- Zhu N, Chapman D, Cooper D, Schreyer DJ, Chen X (2011) X-ray diffraction enhanced imaging as a novel method to visualize low-density scaffolds in soft tissue engineering. Tissue Eng Part C Methods 17:1071-1080.
[Crossref] [Google Scholar] [PubMed]
- Lammel AS, Hu X, Park SH, Kaplan DL, Scheibel TR (2010) Controlling silk fibroin particle features for drug delivery. Biomaterials 31:4583-45891.
[Crossref] [Google Scholar] [PubMed]
- Forouzideh N, Nadri S, Fattahi A, Abdolahinia ED, Habibizadeh M, et al. (2020) Epigallocatechin gallate loaded electrospun silk fibroin scaffold with anti-angiogenic properties for corneal tissue engineering. J Drug Deliv Sci Technol 56:101498.
- Ong SY, Wu J, Moochhala SM, Tan MH, Lu J (2008) Development of a chitosan based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 29:4323-4332.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi