Research Article, J Plant Physiol Patho Vol: 11 Issue: 6
Evaluation of Different Culture Media on the Mycelial Proliferation of Pleurotus ostreatus In Vitro
Sonu Poudel*, Prashamsa Bhusal and Sijan Poudel
Department of Bio Science, Institute of Agriculture and Animal Science, Tribhuvan University, Rupandehi, Nepal
*Corresponding Author: Sonu Poudel
Department of Bio Science, Institute of
Agriculture and Animal Science, Tribhuvan University, Rupandehi, Nepal;
E-mail: sonupoudel8@gmail.com
Received date: 14 February, 2023, Manuscript No. JPPP-23-89350;
Editor assigned date: 17 February, 2023, PreQC No. JPPP-23-89350 (PQ);
Reviewed date: 03 March, 2023, QC No. JPPP-23-89350;
Revised date: 21 April, 2023, Manuscript No. JPPP-23-89350 (R);
Published date: 28 April, 2023, DOI: 10.4172/2329-955X.1000365
Citation:Poudel S, Bhusal P, Poudel S (2023) Evaluation of Different Culture Media on the Mycelial Proliferation of Pleurotus ostreatus In Vitro. J Plant Physiol Patho 11:4.
Abstract
Pleurotus ostreatus, an edible species of mushroom, has a wide range of responses on mycelium growth to varying ranges of culture media. An experiment was conducted to investigate the effect of seven different culture media viz. corn meal agar, oat meal agar, WEA, synthetic potato dextrose agar, conventional potato dextrose agar, carrot root extract, and rice bran agar on the mycelial growth of P. ostreatus. They were evaluated under in vitro in completely randomized design with 5 replications at the plant pathology laboratory of institute of agriculture and animal science, Paklihawa, Nepal. The radial mycelium growth of P. ostreatus was recorded at 24 hours interval for eight consecutive days after inoculation. The findings of the experiment showed that after 2nd day of inoculation among different media, the growth of mycelium was highest on conventional potato dextrose agar (1.2875 cm) followed by synthetic potato dextrose agar (1.2300 cm) and lowest on corn meal agar (0.9625 cm). However, the trend of growth was not similar. Similarly, after 4th day of inoculation, the growth of the fungus was highest on oat meal agar (4.4700 cm) followed by rice bran agar (4.4275 cm), and least on corn meal agar (2.6700 cm). Likewise, after 6th day of inoculation, the growth of the fungus was highest on oat meal agar (7.8900 cm) followed by rice bran agar (7.7725 cm), and least on corn meal agar (5.8625 cm). After 8th day of inoculation, the growth of fungus was highest on oat meal agar, wheat extract agar, conventional potato dextrose agar, and rice bran agar i.e., 9.00 cm, and least on carrot root extract (8.4375 cm). The study indicated a significant effect of most of the media on growth of Pleurotus which suggests their application for culture maintenance.
Keywords: Aseptic condition, Growth, Inoculation, Mycelium, Oyster
Abbreviations:
ANOVA: Analysis of Variance; ℃: Degree Celsius; CRD: Completely Randomized Design; CV: Coefficient of Variance; DAI: Days After Inoculation; Et Al.: ET Alii (means and others, and everyone else); FIG: Figure; G: Gram; IAAS: Institute of Agriculture and Animal Science; LSD: Least Significant Difference; Ml: Milliliter; MIN.: Minute; PDA: Potato Dextrose Agar; WEA: Wheat Extract Agar
Introduction
Pleurotus ostreatus is the most common oyster mushroom found in Nepal. The P. ostreatus, one of 10,000 edible species of mushrooms, is ideal for climatic conditions and, in terms of consumption, comes in second place only behind the button mushroom in terms of popularity. According to Shrestha and Dhakal, 96.48% variation in gross cash income from P. ostreatus in Nepal. It can grow on wide range of agricultural wastes, having short cropping periods and ability to grow on wide range of temperature and humidity compared to another cultivated mushroom. P. ostreatus contains about 100 bioactive compounds and could be a source of dietary fiber [1]. Furthermore, they are high in protein, lipids, carbohydrates, vitamins and minerals, but low in calories and fat. P. ostreatus has the advantage of having a shorter growth time (when compared to other edible mushrooms), the substrate used requires pasteurization, it is highly profitable (converting a high percentage of the substrate to fruiting bodies), and it is less susceptible to diseases and pests.
A culture media is a specialized medium used in microbiological laboratories to cultivate different kinds of microorganisms and are composed of different nutrients that are essential for microbial growth. The growth medium is the most crucial component in the cultivation of mushrooms because it provides the nutrients required for the mushroom mycelia to grow.
For the oyster mushroom Pleurotus sp., the primary nutritional sources are cellulose, hemicellulose, and lignin, and the majority of these substrate materials require the addition of nitrogen sources like wheat and rice bran to achieve the ideal C/N ratio. Production of spawn (active mycelium) is one of the main obstacles to mushroom development worldwide [2-5]. For studying the life cycle and other cultivation aspects of medicinally important mushrooms, study of mycelial behaviour is important. Media and plant growth regulators can be crucial for in vitro mushroom mycelial colony proliferation. Too far, quite a few experiments have been conducted around the globe to determine the best type and media for spawn production. However, sufficient research has not been done in the nation on various mushroom cultivation varieties and media [6]. Study of mycelial growth pattern on different culture media can upgrade knowledge and culture practices regarding this problem [7]. This research would be conducted to investigate the mycelial colony proliferation of P. ostreatus on different media. Under this context, to access suitable media for mushroom cultivation [8].
Materials and Methods
The research was carried out at IAAS, Rupandehi, Nepal in aseptic condition for 21 days. The experiments were laid out in a CRD with Five replications in each treatment and number of treatments were 7 (corn meal agar, oat meal agar, WEA, synthetic PDA, conventional PDA, carrot root extract agar, rice bran agar) and synthetic PDA was used as standard check [9]. For preparation of pure culture, small portion of tissue was taken from collar region of fresh mushroom sample and these cut pieces were sterilized with 1% NaOCl for 30 sec followed by 3 serials washing in distilled water [10]. Samples were then transferred to pre-sterilized PDA culture medium (at 121℃ and 15 psi for 15 min), which were, incubated at 24℃ in the incubator for 1 week, fully grown white mycelium was obtained which was pure culture [11]. Before conducting experiments, maintained pure culture was transferred in PDA slants or PDA Petri plates to obtain subculture. Subculture was done until the pure fungal growth was observed without any contamination. From subculture, pure culture was obtained in 13 days [12].
These seven different media was prepared by standard methods. The various constituents of these media were integrated as corn meal agar (corn fine powdered 7.5 g, agar 5 g, distilled water 250 ml), oat meal agar (oat flour 7.5 g, agar 5 g, distilled water 250 ml), wheat extract agar (wheat grain 8 g, dextrose 5 g, agar-agar 5 g, distilled water 250 ml), synthetic PDA (PDA powder 11 g and distilled water 250 ml), conventional PDA (peeled potato 50 g, dextrose 5 g, agaragar 5 g, distilled water 250 ml), carrot root extract agar (carrot root 50 g, dextrose 5 g, agar-agar 5 g, distilled water 250 ml), rice bran agar (rice bran 50 g, dextrose 5 g, agar-agar 5 g, distilled water 250 ml) [13]. The different constituents of media were dissolved in water separately. In case of conventional potato dextrose agar, carrot root extract agar, rice bran agar, wheat extract agar, extraction was taken after boiling them on a water bath [14].
After preparation of different media, pouring was done in 35 different petri plate for each plate 20 ml with 7 different media in aseptic condition inside laminar air flow and was tagged and allowed to solidify. Control treatment was prepared by simply pouring the synthetic PDA [15]. Five mm circular disk mycelium mat from the edges of actively growing culture was transferred to the center of the amended PDA with the 5 mm cork borer. The plate was then sealed using para film and was well labelled. It was then incubated at 25℃ ± 1℃ at incubator for 8 days [16].
The mycelial growth was observed at 24 hours after the inoculation and the diameter of the growing mycelium was noted using plastic transparent geometry scale across the petri dish horizontally to obtained the 1st data. Afterwards, data was taken at the interval of 24 hours for consecutive 8 days [17]. All the data was imported in Ms. excel 2016 and analysis of variance was done using R-studio software (v2022.02.3), Duncan’s Multiple Range Test (DMRT).
Results
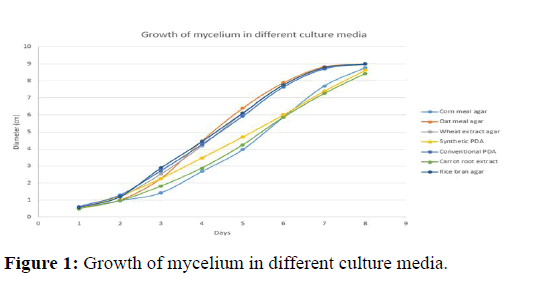
According to the experiment, after 2nd DAI among different media, growth of mycelium was highest on conventional PDA (1.2875 cm) followed by synthetic PDA (1.2300 cm) and rice bran agar (1.1950 cm) and lowest on corn meal agar (0.9625 cm) followed by carrot root extract (0.9650 cm). The trend of growth was not similar in all DAI. After 4th DAI, the growth of fungus was highest on oat meal agar (4.4700 cm) followed by rice bran agar (4.4275 cm) and conventional PDA (4.2550 cm) and least on corn meal agar (2.6700 cm) followed by carrot root extract (2.8775 cm). After 6th DAI, the growth of fungus was highest on oat meal agar (7.8900 cm) followed by rice bran agar (7.7725 cm) and WEA (7.7650 cm) and least on corn meal agar (5.8625 cm) followed by carrot root extract (5.8650 cm). After 8th day DAI, the growth of fungus was highest on oat meal agar, WEA, Conventional PDA and rice bran agar i.e., 9.00 cm followed by corn meal agar (8.7750 cm) and least on carrot root (8.4375 cm) followed by synthetic PDA (8.6300 cm) (Table 1).
| Culture media | Day 2 | Day 4 | Day 6 | Day 8 |
|---|---|---|---|---|
| Corn meal agar | 0.9625c | 2.6700c | 5.8625b | 8.7750b |
| Oat meal agar | 1.0125bc | 4.4700a | 7.8900a | 9.0000a |
| Wheat extract agar | 1.1925ab | 4.1900a | 7.7650a | 9.0000a |
| Synthetic PDA | 1.2300a | 3.4775b | 6.0050b | 8.6300b |
| Conventional PDA | 1.2875a | 4.2550a | 7.6475a | 9.0000a |
| Carrot root extract | 0.9650c | 2.8775c | 5.8650b | 8.4375c |
| Rice bran agar | 1.1950ab | 4.4275a | 7.7725a | 9.0000a |
| Mean | 1.120714 | 3.766786 | 6.9725 | 8.834643 |
| CV% | 0.16468 | 0.200487 | 0.139595 | 0.027339 |
| LSD | 0.1898036** | 0.3681235*** | 0.3800215*** | 0.1588223*** |
Note: CV: Coefficient of Variation, LSD means Least Significant Difference followed by the same letter in a column are not significantly different by DMRT at 5% level of significance. Values are mean of five replications.
The Figure 1 depicts sigmoid growth of mycelium of P. ostreatus in different culture media. Up to second DAI, there was slight significant difference among various media used. However, significant effect of different media on mycelial proliferation increased after fourth DAI. Besides, there was highly significant differences among those media that showed highest mycelial growth (oat meal agar, WEA, conventional PDA and rice bran agar) and those media that showed least mycelial growth (carrot root extract, synthetic PDA and corn meal agar) after seventh DAI.
Discussion
Microorganisms require nutrients, an energy source, and specific environmental conditions to grow and reproduce. Growth medium is the most important factor in mushroom production because it supplies necessary nutrients for the growth of mushroom mycelia.
Among different growing agar media used in this investigation, oat meal agar, WEA, conventional PDA and rice bran agar was proved to be the best while carrot root extract found to be inappropriate media for the mycelial proliferation of P. ostreatus. The media which possess higher growth potential might exhibit higher carbon sources and other nutrients like protein, fat, vitamin E that increase the bioactivity of fungus. Carrot root tissue have been shown to contain a number of metabolites (6-methoxymellein and falcarindiol) which acts as antifungal properties. The difference of mycelial growth on different agar media may be due to availability of different carbon sources and other required nutrients.
Our results are in accordance with the finding of Grandgirard, et al., observed mycelial growth of 7 strains of edible fungi on different culture media. Mycelial growth rates were found higher on WDA (Wheat/Dextrose/Agar) medium which is also the most economic, as the wheat grain after extraction is used for spawn production than on PDA media in all strains investigated.
This result is also supported by other investigators such as Naik, et al., which revealed that growth of Pleurotus ostreatus was significantly rapid on wheat extract agar medium as compared to rest of other media used. Similarly, Maurya, et al., reported that P. ostreatus showed highest growth of mycelium on conventional PDA among different media used.
Unlikely, P. ostreatus showed highest growth of mycelium on synthetic PDA containing more amount of glucose and sucrose than other sources among different media used Kumar, et al.; Hoa, et al.; Hussain, et al. According to Ishaq, et al., maximum mycelial growth of Pleurotus spp. was on corn meal agar that constitute additional dietary fibre and growth factors which is in contrast with our result obtained.
Conclusion
Pleurotus ostreatus is a very popular and commonly cultivated mushroom which can be grown on various substrates and media. Mushroom mycelium is affected by various factors including culture media. Generally, synthetic PDA is the most widely used culture media for the mycelial growth but from the research conducted it was found that the oat meal agar, conventional PDA, WEA and rice bran agar were found to be suitable for the mycelial growth. Therefore, among these media which are economically feasible and locally available can be alternative of synthetic PDA in mycelial culture preparation which helps in spawn preparation and enhance mushroom productivity.
Acknowledgement
Our sincere gratitude to Institute of Agriculture and Animal Science (IAAS), Bhairahawa, Nepal for the financial support offered to carry out the research work.
References
- Perez SR, Oduardo NG, Savon RCB, Boizan MF, Augur C (2008) Decolourisation of mushroom farm wastewater by Pleurotus ostreatus. Biodegradation 19:519-526.
[Crossref] [Google Scholar] [PubMed]
- Bhatt P, Singh RP, Sati SC (2010) Evaluation of different Pleurotus hybrids for their growth requirements in vitro. Indian Phytopathol 63:424-426.
- Papaspyridi LM, Katapodis P, Gonou-Zagou Z, Kapsanaki-Gotsi E, Christakopoulos P (2010) Optimization of biomass production with enhanced glucan and dietary fibres content by Pleurotus ostreatus ATHUM 4438 under submerged culture. Biochem Eng J 50:131-138.
- Grandgirard J, Poinsot D, Krespi L, Nenon JP, Cortesero AM (2002) Costs of secondary parasitism in the facultative hyperparasitoid Pachycrepoideus dubius: Does host size matter? Entomol Exp Appl 103:239-248.
- Hoa HT, Wang CL (2015) The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiol 43:14-23.
[Crossref] [Google Scholar] [PubMed]
- Yehia RS, Al-Sheikh H (2014) Biosynthesis and characterization of silver nanoparticles produced by Pleurotus ostreatus and their anticandidal and anticancer activities. World J Microbiol Biotechnol 30:2797-2803.
[Crossref] [Google Scholar] [PubMed]
- Patel H, Gupte A, Gupte S (2009) Biodegradation of fluoranthene by basidiomycetes fungal isolate Pleurotus ostreatus HP-1. Appl Biochem Biotechnol 157:367-376.
[Crossref] [Google Scholar] [PubMed]
- Devi KS, Behera B, Mishra D, Maiti TK (2015) Immune augmentation and Dalton's Lymphoma tumor inhibition by glucans/glycans isolated from the mycelia and fruit body of Pleurotus ostreatus. Int Immunopharmacol 25:207-217.
[Crossref] [Google Scholar] [PubMed]
- Sun Y, Liu J (2009) Purification, structure and immunobiological activity of a water-soluble polysaccharide from the fruiting body of Pleurotus ostreatus. Bioresour Technol 100:983-986.
[Crossref] [Google Scholar] [PubMed]
- Wolff ER, Wisbeck E, Silveira ML, Gern RM, Pinho MS, et al. (2008) Antimicrobial and antineoplasic activity of Pleurotus ostreatus. Appl Biochem Biotechnol 151:402.
[Crossref] [Google Scholar] [PubMed]
- Mihai RA, Melo Heras EJ, Florescu LI, Catana RD (2022) The edible gray oyster fungi Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm a potent waste consumer, a biofriendly species with antioxidant activity depending on the growth substrate. J Fungi 8:274.
[Crossref] [Google Scholar] [PubMed]
- Souilem F, Fernandes A, Calhelha RC, Barreira JC, Barros L, et al. (2017) Wild mushrooms and their mycelia as sources of bioactive compounds: Antioxidant, anti-inflammatory and cytotoxic properties. Food Chem 230:40-48.
[Crossref] [Google Scholar] [PubMed]
- Nguyen TM, Ranamukhaarachchi SL (2020) Effect of different culture media, grain sources and alternate substrates on the mycelial growth of Pleurotus eryngii and Pleurotus ostreatus. Pak J Biol Sci 23:223-230.
[Crossref] [Google Scholar] [PubMed]
- Devi KS, Roy B, Patra P, Sahoo B, Islam SS, et al. (2013) Characterization and lectin microarray of an immunomodulatory heteroglucan from Pleurotus ostreatus mycelia. Carbohydr Polym 94:857-865.
[Crossref] [Google Scholar] [PubMed]
- Shrestha LK, Dhakal SC (2014) Comparative resource productivity of oyster (Pleurotus ostreatus p.) and button mushrooms (Agaricus bisporus J.) in Kathmandu, Nepal. J Agric Environ 15:30-40.
- Sterna V, Zute S, Brunava L (2016) Oat grain composition and its nutrition benefice. Agric Agric Sci Procedia 8:252-256.
- Tesfay T, Godifey T, Mesfin R, Kalayu G (2020) Evaluation of waste paper for cultivation of oyster mushroom (Pleurotus ostreatus) with some added supplementary materials. AMB Express 10:15.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi