Research Article, J Clin Nutr Metab Vol: 7 Issue: 1
Efficacy of VIQUA® in Alleviating PMS Symptoms
Yuki Ikeda1*, Mizuho Nasu1 and Jean-Yves Bruxer2
1Innovation Labo research center, Tokyo, Japan
2Axialys Innovations research center, Annecy, France
*Corresponding Author: Yuki Ikeda,
Innovation Labo research center, Tokyo, Japan
E-mail: development@innovationlabo.com
Received date: 04 January, 2023, Manuscript No. JCNM-23-85577;
Editor assigned date: 6 January, 2023, Pre QC No. JCNM-23-85577 (PQ);
Reviewed date: 27 January, 2023, QC No. JCNM-23-85577;
Revised date: 03 February, 2023, Manuscript No. JCNM-23-85577 (R);
Published date: 10 February, 2023, DOI: 10.35841/jcnm.1000106
Citation: Ikeda Y, Nasu M, Bruxer JY (2023) Efficacy of VIQUA® in Alleviating PMS Symptoms. J Clin Nutr Metab 7:1.
Abstract
Introduction: Pre-Menstrual Syndrome (PMS) is considered as a disorder with neuro-hormonal and gynecological dimensions. As a high prevalent condition that affects the quality of life for women, there is an increasing demand in using nutritional supplements to alleviate the symptoms of PMS. Pomegranate is a fruit containing different bioactive molecules that are previously shown to have anti-inflammatory and anti-depression properties suggesting its possible efficacy in physiological as well as psychological symptoms seen associated with PMS. This study attempted to evaluate the efficacy and safety profiles of VIQUA®, a pomegranate based polyphenol complex in subjects with PMS.
Materials and methods: This was a single center, prospective, randomized, double blind, two arm, parallel, placebo controlled; clinical study adhered to the good clinical practice guidelines and was approved by the ethical review board. Forty female adult subjects aged between 25-35 years participated in the study and all subjects completed the study without any adverse events. PMS symptoms were assessed using Premenstrual Symptoms Screening Tools Questionnaire (PSST) for 3 consecutive menstrual cycles. Plasma Brain Derived Neurotrophic Factor (BDNF) levels and serum 14-Dihydro-15- Keto Prostaglandin F2-Alpha (PGFM) were evaluated to understand the impact of test product supplementation in neurotransmitter and prostaglandin levels.
Results: At the end of 3rd menstrual cycle, mean PSST score in VIQUA® group showed a statistically significant reduction compared to baseline (P value>0.0001). In the subgroup analysis of PSST score, VIQUA® group showed significant reduction in physical symptoms score, psychological symptoms score and interfering symptoms scores. Compared to baseline, VIQUA® group showed a significant improvement in BDNF levels (P value>0.05) and significant reduction in 14- Dihydro-15-Keto Prostaglandin F2-Alpha (PGFM) levels (P value>0.05). It was also noted that VIQUA® group had a considerable increase in the fecal Short Chain Fatty Acids (SCFA) levels.
Conclusion: Considering the significant improvement in different aspects of PMS including inflammation, pain and psychological and psycho-somatic factors, VIQUA® shows great prospects as an effective supplement in treating PMS symptoms.
Keywords: Pomegranate; Clinical trial; Premenstrual syndrome; PMS; VIQUA®; Women health.
Introduction
A certain degree of discomfort is associated with menstruation in most women of reproductive age. But many of them report physical or psychological symptoms that impair their daily life and mental wellbeing, during the luteal phase of their menstrual cycle [1]. It is known as premenstrual syndrome and is considered as a neuro-hormonal gynecological disorder [2].
The changes in hormonal levels especially that of estrogen and progesterone during the luteal phase can impact the brain and nervous system by altering the levels of neurotransmitters. The fall in these hormonal levels also lead to a rise in the prostaglandin levels. Studies have shown that progesterone reduces serotonin-based neurotransmission by decreasing the serotonin receptors and increasing serotonin degradation. It has also been reported that women with PMS shows altered functional sensitivity of the GABA receptor. Previously published data suggest the possible involvement of prostaglandins and neurotransmitters in the pathophysiology of PMS. Most studies indicate that prostaglandins are more responsible for the physical symptoms of PMS whereas neurotransmitters are mostly linked to the psychological symptoms [3-9].
As a high prevalent condition that affects the quality of life for women, various treatment modalities are tried and tested to treat PMS [10]. But most drugs used for treating PMS can have other side effects and hence there is an increasing demand in using nutritional supplements, herbs and other natural ingredients to alleviate the symptoms of PMS [11].
Pomegranate is a fruit containing different bioactive molecules that are previously shown to have anti-inflammatory and anti-depression properties suggesting its possible efficacy in prostaglandin led (inflammatory/physiological) symptoms as well as neurotransmitter led (depression/psychological) symptoms seen associated with PMS [12-18]. Pomegranate is a rich source of different micro and macro nutrients including vitamins, minerals and polyphenols. The current study product, VIQUA® is a proprietary polyphenol complex produced from naturally fermented pomegranate developed conjointly by Axialys Innovations in Annecy, France and Innovation Labo Sciences Co. Ltd. (Tokyo, Japan) and it is produced using the unique Royal rubis pomegranate variety cultivated in an organic, micro biome rich, biodynamic, sea water-irrigated plantation in Spain.
The main bioactive compounds present in VIQUA® are polyphenols such as punicalagin, granatin B, pedunculagine, resveratrol, punicalin and ellagic acid; vitamins such as vitamin D; metabolites, such as urolithin A, and minerals such as zinc.
Brain Derived Neurotrophic Factor (BDNF) is previously studied in association with the pathophysiology of PMS and studies have reported that serum BDNF levels were significantly lower in women with PMS compared to women without PMS [19]. And studies in post-menopausal women and depression patients have shown that BDNF levels increase post treatment with anti-depressive medication [20]. Hence serum BDNF can be a useful peripheral marker to assess the severity of mood swings and depression in women suffering from PMS.
As discussed above, the role of prostaglandins in premenstrual syndrome is widely studied and well-established. Many of the PMS symptoms can be attributed to the increased prostaglandins activity during this time. Direct impacts of prostaglandins are not only limited to inflammation but also expands to temperature regulation, cerebral blood flow, neurotransmitter release etc [21-25]. The release of endometrial prostaglandin F2-alpha occurs in the luteal phase of menstrual cycle but since it is inactivated into 13, 14-Dihydro-15-Keto Prostaglandin F2-Alpha (PGFM) during the first passage in lungs, plasma PGFM levels are usually used to assess the release of PGF2α secretion in the endometrium [26].
Different studies have shown that the anti-inflammatory action of pomegranate ingredients is exerted via inhibition of COX-2 expression, prevention of I κ B α degradation, regulation of NF- κ B and Nrf2 signaling etc [27]. Inhibition of COX-2 expression also leads to reduced production of prostaglandins which helps to alleviate the pain and associated symptoms in PMS [28,29].
The anti-inflammatory action of pomegranate ingredients mainly involves the activation of the Estrogen Receptor β (ERβ) and the serotonergic system [30]. Apart from this, many pomegranate ingredients show direct anti-depressant like activity that involves modification of BDNF. The neuroprotective action and anti-depressant like action of quercetin is connected to its ability to modulate BDNF because the mechanism of action involves the activation of BDNFTrkB- PI3K/Akt signaling pathway [31]. Animal studies have shown that the anti-depressant action of another important pomegranate polyphenol, ellagic acid, is mediated by increased BDNF level [32]. Resveratrol which is another important pomegranate polyphenol is known to activate Extracellular Signal Regulated Kinase 1/2 (ERK1/2) and Camp Responsive Element-Binding protein (CREB) and thereby upregulate astroglia-derived neurotrophic factors [33].
The role of gut microbiota in health and diseases is long known. A large number of studies have identified and established the involvement of gut microbiota dysbiosis in various diseases such as autoimmune diseases, obesity and diabetes, heart diseases, liver diseases and neurological/psychological diseases etc. Recent studies have revealed the characteristic gut microbiota dysbiosis in PMS as well. Previous animal studied have shown that BDNF levels are increased with butyrate treatment and recent micro biome studies have clearly demonstrated that decreased levels of butyrate producing gut bacteria such as Butyricicoccus and Megasphaera are a characteristic feature of gut microbiota in PMS patients and are negatively correlated with the severity of PMS symptoms [34-47]. Epigenetic modifications are also implicated in the pathophysiology of depressive symptoms and butyrate, which is a histone deacetylase inhibitor, is known to exert an anti-depressant like activity via altering the gene expressions in the hippocampus [48]. Previous studies have shown that pomegranate extracts and its major components such as gallic acid and ellagic acid can increase the production of Short Chain Fatty Acids (SCFAs), especially butyrate [49]. These data points to another mechanism of action in the efficacy of pomegranate ingredients in alleviating PMS symptoms via gut microbiota modulation and increased butyrate production.
This current clinical trial is designed to evaluate the efficacy of VIQUA® in alleviating PMS symptoms by assessing the Premenstrual Symptoms Screening Tools questionnaire (PSST) score and direct markers of inflammation and depression (Serum PGFM and Plasma BDNF)
Methodology
This was a single center, prospective, randomized, double blind, two arm, parallel, placebo controlled; clinical study adhered to the good clinical practice guidelines and was approved by the ethical review board (registration number ILNP 212018-S088).
Subjects
Forty female adult subjects aged between 25-35 years were enrolled after signing the written informed consent. Subjects with regular menstrual cycle (21-35 days) and diagnosed PMS who were not pregnant, lactating, or participating in other clinical studies were included. Subjects were excluded if they: have any known allergy to any of the ingredients in the test product, have consumed any nutritional supplements within 1 month from start of study, have any serious mental or physical illnesses that might interfere with the outcome of the study. Study product, VIQUA® is a complex formula of polyphenols and their metabolites from pomegranate.
Study procedure: Subjects were randomly assigned in a 1:1 ratio to receive either VIQUA® or placebo once daily (daily dosage of 300 mg) for 12 weeks. Both the test product and placebo were packed in identical containers, labeled with the trial number, and stored at room temperature.
Efficacy assessments: PMS symptoms were assessed using Premenstrual Symptoms Screening Tools (PSST) questionnaire (Annex 1) administered at baseline (retrospective based on previous cycle) and end of menstrual cycle for the next three cycles during the study period. Scoring was done based on the severity of symptoms on a scale of 0-3 (0-not at all, 1-mild, 2-moderate, 3-severe) [50].
Blood samples were collected at mid luteal phase for assessment of plasma BDNF levels and serum PGFM. BDNF and PGFM levels were analyzed using Enzyme-Linked Immunosorbent Assay (ELISA) kits according to manufacturer’s instructions. For butyrate and other SCFA analysis, fecal samples were collected at mid luteal phase around the same time as blood sample collection. SCFAs were measured using a high-performance liquid chromatography organic acid analysis system and sample concentrations were normalized to the weight of fecal material.
Safety parameters: Patients underwent two physical examinations and laboratory evaluation at baseline and the end of the treatment period (hemoglobin, hematocrit, differential blood count, leucocytes, erythrocytes, thrombocytes, LDH, SGOT, SGPT, gamma-GT, bilirubin, creatinine, sodium, potassium, calcium, magnesium, phosphate, total protein, albumin, prolactin, iron, amylase, cholinesterase, alkaline phosphatase, triglycerides, cholesterol etc were measured). Adverse events were monitored throughout the study period.
Statistical analysis: Quantitative data were represented as mean ± SD and analyzed by independent student t-test. P value of <0.05 was considered statistically significant. To perform statistical analyses, statistical package for social science version 16 (SPSS Inc., Tokyo, Japan) was used.
Results
All 40 subjects completed the study without any discrepancies and all their data is used in the final result analysis. Subjects menstrual period ranged from 3-7 days and interval between the periods ranged from 24-32 days. Baseline assessment showed that the groups did not show any statistically significant difference in any of the parameters tested (P value>0.05) (Table 1).
Table 1: Baseline characteristics of VIQUA® and placebo group (data is presented as mean ± SD).
VIQUA® reduced severity of PMS symptoms
At the end of 3rd menstrual cycle, mean PSST score in VIQUA® group showed a statistically significant reduction compared to baseline. Retrospective PSST score recorded at the baseline did not show a significant difference between VIQUA® and placebo group (VIQUA® group PSST score=34.95 ± 4.26 and placebo group PSST score=35.00 ± 4.70, P value 0.972). At the 12th week analysis (3rd menstrual cycle after intervention), VIQUA® group showed a statistically significant reduction in total PSST score compared to the placebo group (VIQUA® group PSST score=25.70 ± 3.44 and placebo group PSST score=32.60 ± 4.54, P value<0.0001) (Figures 1(a)-1(d)).
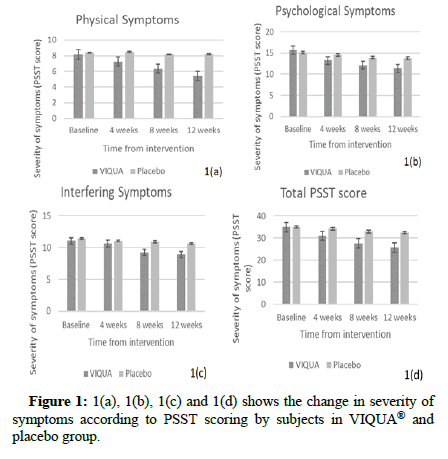
Figure 1: 1(a), 1(b), 1(c) and 1(d) shows the change in severity of symptoms according to PSST scoring by subjects in VIQUA® and placebo group.
VIQUA® improved plasma BDNF levels
Anti-depressant like effect of VIQUA® supplementation is assessed via Plasma BDNF measurement at mid luteal phase. Compared to baseline, VIQUA® group showed a significant improvement (p-value <0.05) in BDNF levels whereas the BDNF levels in placebo group did not show any improvement (Figure 2).
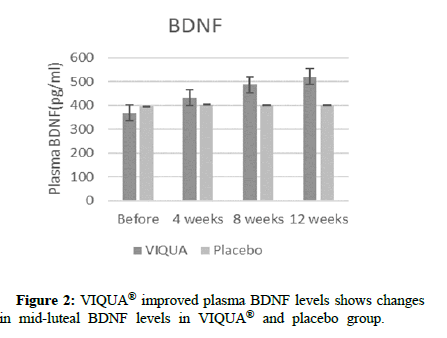
Figure 2: VIQUA® improved plasma BDNF levels shows changes in mid-luteal BDNF levels in VIQUA® and placebo group.
VIQUA® reduced PGFM levels
VIQUA® group recorded a significant reduction in the PGFM levels indicating a reduction of prostaglandin secretion in the uterus during luteal phase. It shows the changes in PGFM levels from baseline to end of study period in both VIQUA® and placebo groups (Figure 3).
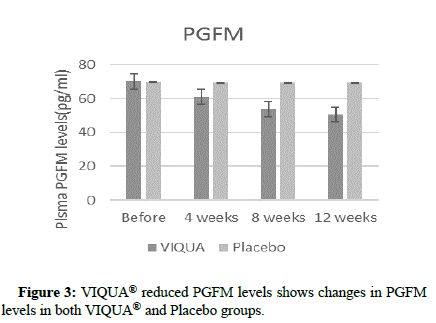
Figure 3:VIQUA® reduced PGFM levels shows changes in PGFM
levels in both VIQUA® and Placebo groups.
In the subgroup analysis of PSST score, VIQUA® group showed significant reduction in physical symptoms score, psychological symptoms score and interfering symptoms score. Table 2 summarizes the results of PSST score for different symptoms from baseline to end of study period for both VIQUA® and placebo group. Data is presented as mean ± SD. P value<0.05 is considered statistically significant.
Table 2: Average PSST scores in VIQUA® and placebo group from baseline to end of 12 weeks. VIQUA® significantly reduced PMS symptoms from baseline to end of study period. (a) Paired t-test, (b)Unpaired t-test.
VIQUA® improved butyrate production
VIQUA® supplementation increased the SCFA production by the gut microbiota (Figure 4).
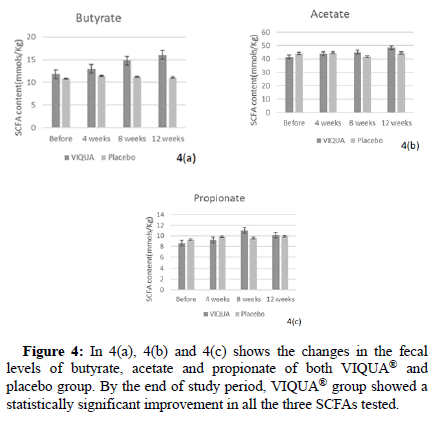
Figure 4: In 4(a), 4(b) and 4(c) shows the changes in the fecal levels of butyrate, acetate and propionate of both VIQUA® and placebo group. By the end of study period, VIQUA® group showed a statistically significant improvement in all the three SCFAs tested.
Discussion
The results of this clinical trial indicated that 12 weeks of VIQUA® supplementation resulted in significant reduction in PMS symptoms. Consistent with previous studies, the baseline analysis showed that subjects in both VIQUA® and placebo group had lower BDNF levels compared to women without PMS. Pomegranate bio actives are previously shown to have an anti-depressant like effect and the fact that the reduction in psychological symptoms score in VIQUA® group is also supported by an increase in BDNF levels during the mid-luteal phase indicates the efficacy of the study product in reducing psychological symptoms in PMS by promoting the BDNF levels [16-18].
This increased production of BDNF is further corroborated by an increase in the fecal Butyrate levels in the VIQUA® group. It has been shown that butyrate treatment could alleviate depression symptoms and elevate BDNF levels [47,49]. From this, we can safely assume that the efficacy of VIQUA® in gut micro biota modulation and the resultant increase in butyrate production could be the possible mechanism of action behind the significant reduction of psychological symptoms showed by the subjects in VIQUA® group.
Another important finding in this trial is the significant reduction in the pain and other physical symptoms associated with PMS recorded by the subjects in the VIQUA® group. This is further supported by a reduction in the prostaglandin levels in these subjects.
Conclusion
From data presented above, we can safely say that daily supplementation of VIQUA® is safe and effective in alleviating PMS symptoms. This study serves as the basis of future studies to explore more practical and side effect-free natural supplementations to effectively improve the quality of life for women suffering from PMS. Considering the significant improvement in different aspects of PMS including Inflammation, pain and psychological and psycho-somatic factors, it can be assumed that the study product shows great prospects as an effective supplement in treating PMS symptoms. Further multicentered clinical trials with larger ethnically diverse populations are recommended to generalize and establish the current results.
References
- Yonkers KA, O'Brien PS, Eriksson E (2008) Premenstrual syndrome. The Lancet 371:1200-1210.
- Nappi RE, Cucinella L, Bosoni D, Righi A, Battista F, et al (2022) Premenstrual syndrome and premenstrual dysphoric disorder as centrally based disorders. Endocrines 3:127-138.
- Horrobin DF (1983) The role of essential fatty acids and prostaglandins in the premenstrual syndrome. J Reprod Med 28:465-468.
- Budoff PW (1983) The use of prostaglandin inhibitors for thepremenstrual syndrome. J Reprod Med 28:469-478.
- Koshikawa N, Tatsunuma T, Furuya K, Seki K (1992) Prostaglandins and premenstrual syndrome. Prostaglandins Leukot Essent Fatty Acids 45:33-36.
- Girish C, Raj V, Arya J, Balakrishnan S (2013) Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur J Pharmacol 710:49-58.
- Ryu A, Kim TH (2015) Premenstrual syndrome: A mini review. Maturitas 82:436-440.
- Rapkin AJ, Kuo J (2007) Neurotransmitter physiology: The basics for understanding premenstrual syndrome (1st edn). In The Premenstrual Syndromes. CRC Press 81-94.
- Rapkin AJ (1992) The role of serotonin in premenstrual syndrome. Clin Obstet Gynecol 35:629-636.
- Wichianpitaya J, Taneepanichskul S (2013) A comparative efficacy of low-dose combined oral contraceptives containing desogestrel and drospirenone in premenstrual symptoms. Obstetrics 2013:487143.
- Whelan AM, Jurgens TM, Naylor H (2009) Herbs, vitamins and minerals in the treatment of premenstrual syndrome: A systematic review. Can J Clin Pharmacol 16:e407-29.
- Al-Dujaili E, Smail N (2012) Pomegranate juice intake enhances salivary testosterone levels and improves mood and well being in healthy men and women. Endocrine Abstracts 28:P313.
- Zhu X, Sun-Waterhouse D, Cui C (2021) A red pomegranate fruit extract-based formula amelioratesanxiety/depression-like behaviors via enhancing serotonin (5-HT) synthesis in C57BL/6 male mice. Food Science and Human Wellness 10:289-296.
- Valdés-Sustaita B, Estrada-Camarena E, González-Trujano ME, López-Rubalcava C (2021) Estrogen receptors-β and serotonin mediate the antidepressant-like effect of an aqueous extract of pomegranate in ovariectomized rats. Neurochem Int 142:104904.
- Ridzwan N, Jumli MN, Baig AA, Rohin MAK (2020) Pomegranate-derived anthocyanin regulates MORs-cAMP/CREB-BDNF pathways in opioid-dependent models and improves cognitive impairments. J Ayurveda Integr Med 11:478-488.
- Lansky EP, Newman RA (2007) Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol 109:177-206.
- Wang P, Zhang Q, Hou H, Liu Z, Wang L, et al (2020) The effects of pomegranate supplementation on biomarkers of inflammation and endothelialdysfunction: A meta-analysis and systematic review. Complement Ther Med 49:102358.
- Akter R, Ahn JC, Nahar J, Awais M, Ramadhania ZM, et al (2022) Pomegranate juice fermented by tannin acyl hydrolase and Lactobacillus vespulae DCY75 enhance estrogen receptor expression and anti-inflammatory effect. Front Pharmacol 13:1010103.
- Cubeddu A, Bucci F, Giannini A, Russo M, Daino D, et al (2011) Brain-derived neurotrophic factor plasma variation during the different phases of the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology. 36(4):523-530.
- Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, et al (2006) Brain-Derived Neurotrophic Factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry 30:1256-1260.
- Willman EA, Collins WP, Clayton SG (1976) Studies in the involvement of prostaglandins in uterine symptomatology and pathology. Br J Obstet Gynaecol 83:337-341.
- Pickles VR, Hall WJ, Best FA, Smith GN (1965) Prostaglandins in endometrium and menstrual fluid from normal and dysmenorrhoeic subjects. J Obstet Gynaecol Br Commonw 72:185-192.
- Downie J, Poyser NL, Wunderlich M (1974) Levels of prostaglandins in human endometrium during the normal menstrual cycle. J Physiol 236:465-472..
- Craig GM (1975) Prostaglandins in reproductive physiology. Postgrad Med J 51:74-84.
- Clark KE, Farley DB, van Orden DE, Brody MJ (1977) Role of endogenous prostaglandins in regulation of uterine blood flow and adrenergic neurotransmission. Am J Obstet Gynecol 127:455-461.
- Mishra DP, Meyer HH, Prakash BS (2003) Validation of a sensitive enzymeimmunoassay for 13, 14-dihydro-15-keto-PGF2α in buffalo plasma and its application for reproductive health status monitoring. Anim Reprod Sci 78:33-46.
- Mandal A, Bhatia D, Bishayee A (2017) Anti-inflammatory mechanism involved in pomegranate-mediated prevention of breast cancer: the role of NF-κB and Nrf2 signaling pathways. Nutrients 9:436.
- Harel Z (2004) Cyclooxygenase-2 specific inhibitors in the treatment of dysmenorrhea. J Pediatr Adolesc Gynecol 17:75-79.
- Tjandrawinata RR, Nofiarny D, Susanto LW, Hendri P, Clarissa A (2011) Symptomatic treatment of premenstrual syndrome and/or primary dysmenorrhea with DLBS1442, a bioactive extract of Phaleria macrocarpa. Int J Gen Med 4:465.
- Valdés-Sustaita B, Estrada-Camarena E, González-Trujano ME, López-Rubalcava C (2021) Estrogen receptors-β and serotonin mediate the antidepressant-like effect of an aqueous extract of pomegranate in ovariectomized rats. Neurochem Int 142:104904.
- Pathak L, Agrawal Y, Dhir A (2013) Natural polyphenols in the management of major depression. Expert Opin Investig Drugs 22:863-880.
- Bedel HA, Kencebay Manas C, Özbey G, Usta C (2018) The antidepressant-like activity of ellagic acid and its effect on hippocampal brain derived neurotrophic factor levels in mouse depression models. Nat Prod Res 32:2932-2935.
- Zhang F, Lu YF, Wu Q, Liu J, Shi JS (2012) Resveratrol promotes neurotrophic factor release from astroglia. Exp Biol Med 237:943-948.
- De Luca F, Shoenfeld Y (2019) The microbiome in autoimmune diseases. Clin Exp Immunol 195:74-85.
- Xu H, Liu M, Cao J, Li X, Fan D, et al (2019) The dynamic interplay between the gut microbiota and autoimmune diseases. J Immunol Res 2019:7546047.
- Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, et al (2016) Gut microbiota, obesity and diabetes. Postgrad Med J 92:286-300.
- Gérard P (2016) Gut microbiota and obesity. Cell Mol Life Sci 73:147-162.
- Gurung M, Li Z, You H, Rodrigues R, Jump DB, et al ( 2020) Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51:102590.
- Liu H, Zhuang J, Tang P, Li J, Xiong X, et al (2020) The role of the gut microbiota in coronary heart disease. Curr Atheroscler Rep 22:1-2.
- Tang WW, Hazen SL (2014) The contributory role of gut microbiota in cardiovascular disease. J Clin Invest 124:4204-4211.
- Minemura M, Shimizu Y (2015) Gut microbiota and liver diseases. World journal of gastroenterology. World J Gastroenterol 21:1691-1702.
- Compare D, Coccoli P, Rocco A, Nardone OM, de Maria S, et al (2012) Gut–liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 22:471-476.
- Cenit MC, Sanz Y, Codoñer-Franch P (2017) Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol 23:5486.
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, et al (2014) Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26:1155-1162.
- Okuma K, Kono K, Otaka M, Ebara A, Odachi A, et al (2022) Characteristics of the gut microbiota in japanese patients with premenstrual syndrome. Int J Womens Health 14:1435-1445.
- Takeda T, Yoshimi K, Kai S, Ozawa G, Yamada K, et al (2022) Characteristics of the gut microbiota in women with premenstrual symptoms: A cross-sectional study. PLoS One 17:e0268466.
- Schroeder FA, Lin CL, Crusio WE, Akbarian S (2007) Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 62:55-64.
- Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, et al (2018) Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res 1680:13-38.
- Bialonska D, Ramnani P, Kasimsetty SG, Muntha KR, Gibson GR, et al (2010) The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int J Food Microbiol 140:175-182.
- Steiner M, Macdougall M, Brown E (2003) The Premenstrual Symptoms Screening Tool (PSST) for clinicians. Arch Womens Ment Health 6:203-209.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 