Research Article, J Biochem Physiol Vol: 7 Issue: 2
Efficacy of the Anti-Tubulin Drug Paclitaxel as a Single Treatment and its Combination with the Commercially used Drug Metronidazole for the Treatment of Giardiasis in Experimentally Infected Mice
Ashraf Ahmed Elgendy1*, Ashraf Rizk Ramadan2, Alyaa Farid1, Ibraheem Rabee3, Azza Molhamed El Amir2
1Department of Immunology, New Kaser Al-Aini Teaching Hospital, Cairo, Egypt
2Department of Zoology, Cairo University, Giza, Egypt
3Department of Parasitology, Theodore Bilharz Research Institute, Giza, Egypt
*Corresponding Author: Elgendy AA,
Department of Immunology, New Kaser Al-Aini
Teaching Hospital, Cairo, Egypt
E-mail: Ashrafelgendy20.ae@gmail.com
Received date: 06 June, 2024, Manuscript No. JBPY-24-138416;
Editor assigned date: 10 June, 2024, PreQc No. JBPY-24-138416 (PQ);
Reviewed date: 24 June, 2024, Qc No. JBPY-24-138416;
Revised date: 01 July, 2024, Manuscript No. JBPY-24-138416 (R);
Published date: 08 July, 2024, DOI: 10. 4172/jbpy.24.7.1000158
Citation: Elgendy AA et al. (2024) Efficacy of the Anti-Tubulin Drug Paclitaxel as a Single Treatment and its Combination with the Commercially used Drug Metronidazole for the Treatment of Giardiasis in Experimentally Infected Mice. J Biochem Physiol 7:2
Abstract
Giardia infection is still a medical problem worldwide, it is the leading cause of intestinal diarrhea and its consequences. Despite its side effects, the reliance on metronidazole as a basic treatment persists, especially when metronidazole is used for longer periods due to recurring infections. Therefore, research is ongoing to identify participants in the treatment of Giardia and at the same time working to limit its side effects. Cancer patients were shown to be 1.24 fold more susceptible and were at a higher risk of infection, which should alert physicians to the possible consequences. This study represents a step toward decreasing the burden on cancer patients who are more exposed to Giardia infection. The importance of the participation of paclitaxel in therapeutic protocols is to develop an anti-Giardial compound that targets the cell division machinery of the parasite and reduces the genotoxicity effects of metronidazole. The dual treatment (metronidazole with paclitaxel combination) yielded a significant reduction in the percentage of IgA and IgG antibodies, indicating an increase in the evanescence of the antigens that stimulate the production of antibodies. It is true that the dual treatment removed fewer eggs in the stool and the trophozoite than metronidazole alone did, but it also led to better elimination of the pathogens, which stimulates the production of antibodies; this is the most important thing that is safer to use, especially if it is necessary to prolong the treatment period This is confirmed by histopathological examination of liver and small intestine tissue samples collected from all the mice within the different groups.
Keywords: Giardia intestinalis,Metronidazole, Paclitaxel,Treatment
Introduction
Giardia lamblia enteritis is a type of small intestine gastroenteritis caused by the pathogenic protozoan Giardia lamblia, it is otherwise known as Giardia duodenalis or Giardia intestinalis [1]. Giardia duodenalis assemblages A and B are the main human-infectious assemblages [2]. There was a similar distribution of Giardia assemblages between children with and without diarrhea. Increasing age is a risk factor for Giardia infection [3]. It has a wide range of clinical manifestations, from asymptomatic carriage to acute or chronic diarrhea accompanied by dehydration, abdominal pain, nausea, vomiting, excessive flatulence, and weight loss [4,5]. Furthermore, chronic infection can result in stunting and reduced psychomotor development [6,7].
There are 280 million Giardia infections annually [8-10]. The prevalence of Giardiasis ranges from 3% to 7% in developed countries and from 20% to 30% in developing countries [11-13]. Despite being considered a predominant disease in low-income and developing countries with low sanitation and socioeconomic conditions, current migratory flows have caused an increase in Giardiasis cases in high-income countries [14,15]. Giardia cysts are highly infectious; infection may occur after the ingestion of just one to ten cysts from contaminated food or water [16]. Currently, there are a wide variety of chemotherapeutic treatments available to combat this parasitic infection, most of which have potentially serious side effects, such as genotoxic, carcinogenic, and teratogenic effects. The need to create novel treatments and discover new therapeutic targets to fight this illness is evident [15,10]. Cancer accounted for approximately 13% of all deaths in 2008, globally, with an estimated eleven million deaths in 2030. Viral, bacterial, and protozoan infections are the most common causes of death due to cancer and are preventable [17]. The results of demonstrated that the immunodeficiency status of cancer patients is a possible risk factor for acquiring Giardia infection, which requires strict preventive measures.
Chen et al., investigated the prevalence of Giardia lamblia infections among colorectal cancer patients and detected an 8.1% prevalence of G. lamblia infections among the study subjects by PCR [18]. Hurník et al., Described few cases of coincidental Giardiasis and pancreatic tumors. Among these patients, three described Giardiasis cases coincided with confirmed pancreatic [19]. Claimed that these results are the first systematic review and meta-analysis showing a general overview of G. duodenalis infection incidence and associated risk factors among cancer patients globally, and the estimated weighted incidence of G. duodenalis infection in cancer patients was computed to be 6.9% globally based on data from 32 studies. Disease in people with a healthy immune status is self-limiting and without a clinical course, whereas immunocompromised patients may experience harsh clinical outcomes [20,21]. The application of chemotherapeutics in cancer patients may provide an immunosuppressive, favourable environment for parasitic infections [13].
However, several promising drug candidates that can overcome antibiotic resistance in Giardia, including derivatives of 5-nitroimidazoles and benzimidazoles, as well as hybrid compounds created from combinations of different anti giardial drugs, have recently been identified, and a growing number of refractory cases are being reported [22,23]. Such infections are also overlooked in industrialized nations due to their low prevalence and because they do not have pathognomonic signs [24]. Thus, they are a silent threat, particularly in immunocompromised individuals receiving chemotherapy, leading to hyperinfection by parasitic agents as well as other infectious agents [25]. Drug resistance to common anti-giardial agents and the incidence of treatment failure have increased in recent years. Therefore, the search for new molecular targets for drugs against Giardia infection is essential [12,26]. The persistence of infection is linked to several factors, including drug resistance, suboptimal drug concentrations, reinfection, an immunocompromised state and IgA deficiency [8,27]. There are no randomized controlled trials determining the optimal treatment for nitroimidazole-refractory Giardiasis. Refractory Giardiasis has traditionally been treated with prolonged courses and/or higher doses of nitroimidazoles [28]. However, these compounds have side effects associated with residual toxicity in the host. Dose-dependent side effects include leukopenia, headache, vertigo, nausea, insomnia, irritability, metallic taste, and CNS toxicity [29].
Combination therapy, based on the mechanisms of action of antigiardial drugs, is another approach that may also be useful in patients with Giardiasis refractory to first line drugs. Clinicians are increasingly combining drugs with different mechanisms of action to treat refractory disease. Drug combinations may exert synergistic or additive effects and prevent potential cross-resistance [28,30]. MTZ is a synthetic antibiotic derived from azomycin that has been found to be effective against Giardia lamblia and a variety of infections due to its high efficacy compared with other drugs; however, its side effects must be considered, such as nausea, abdominal pain, and diarrhea [31,32]. Nevertheless, serious neurotoxicity, optic neuropathy, peripheral neuropathy, and encephalopathy have been reported in rare cases, and neurotoxicity is not dose dependent and is fully reversible with discontinuation of the drug [33]. Few studies have been performed on the genotoxicity of MTZ in humans, and the genotoxic effects of MTZ observed in animal models are controversial. The genotoxicity of MTZ induces single and double DNA strand breaks, and it can induce effects in human cells both in vitro and in vivo; however, MTZ has been shown to play a carcinogenic role in mice and rats. According to the International Agency for Research on Cancer (IARC), there is evidence that MTZ is an animal carcinogen, but there is insufficient evidence in humans [32,34]. Paclitaxel (Pacl) (trade name Taxol) is a tricyclic diterpenoid compound naturally produced in the bark and needles of Taxus brevifolia. Its molecular formula is C47H51NO14 [35]. Pacl is a well-known anticancer agent with a unique mechanism of action. It is considered to be one of the most successful natural anticancer drugs available [36]. It is also used in treating coronary heart disease, skin disorders, renal and hepatic fibrosis, inflammation, and axonal regeneration, and clinical trials are being conducted for treating degenerative brain diseases [37].
Moreover, Pacl can affect the outcome of immunotherapy through various mechanisms of action on immune cells, and it also functions as an immunomodulator [36]. Many studies have shown that Pacl directly kills tumor cells and regulates various immune cells, such as effector T cells, dendritic cells, natural killer cells, regulatory T cells, and macrophages [38]. Recently, Pacl was shown to inhibit the growth of Toxoplasma gondii [39], Trypanosomatid protozoa, such as Leishmania and Trypanosoma [40,41] and moreover, Plasmodium spp. [42]. The present work aimed to study the efficacy of the antitubulin drug (Pacl) as a single treatment and of combination with the commercially available drug Metronidazole (MTZ) for the treatment of Giardiasis in experimentally infected mice.
Materials and Methods
Experimental animals
A laboratory-bred male Swiss albino mouse strain was used (n=60 and weighing 18 g-20 g). Experimental animals were maintained for 8 weeks at 21°C ± 2°C and fed dry food (containing 24% protein). The experiments were carried out according to internationally valid guidelines and at the institution responsible for animal ethics (Schistosome Bilogical Supply Program Unit at Theodor Bilharz Research Institute (SBSP/TBRI) [43]. The protocol of this study was approved by the ethical committee of the Cairo University Institutional Animal Care and Use Committee (CU-IACUC), Basic Science Sector and TBRI. Approval number (CU-I-F-66-19)
Cysts and infection
Giardia cysts were obtained from Theodor Bilharz Research Institute (SBSP/TBRI). A 0.2 ml aliquot of the cyst suspension was placed on a glass plate. With the aid of a dissecting microscope, the number of cysts was determined. Generally, counts were made, and the average number per 0.1 ml was used to calculate the total number of cysts per 1 ml of suspension [44]. The presence of Giardia cysts was confirmed by microscopic wet examination.
Determination of viability
The excystation method of was used [45]. The percentage of exocystant cells was determined by counting the number of Intact Cysts (IC), Partially Excysted Trophozoites (PET), and Total Excysted Trophozoites (TET) via the following formula:
% excystation = (TET/2+PET)/(TET/2+PET+IC) × 100
The number of total excysting trophozoites was divided by 2 in the formula because every cyst in which complete excystation occurred promptly yielded a pair of trophozoites [46].
Animal infection and infection determination
Mice were orally infected with isolated Giardia cysts via oralgastric gavage. Each mouse was infected with Giardia cysts at a dose of approximately 10.000 ± 1 cyst/mouse [47]. Two weeks after infection, the fecal pellets were collected and subjected to parasitological examination using Working Lugol’s iodine to detect Giardia cysts and to ensure that the mice had been infected.
Drug administration
In this study, drugs were administered after 7 days of infection by cysts.
MTZ (Flagyl) administration
MTZ (flagyl) (125 mg) manufactured and provided by Sanofi Aventis Pharma (Egypt) was used. Flagyl was administered orally to Groups III and V in suspension at a dose of 120 mg/kg body weight (0.04 mg/mouse/day) for five consecutive days post infection [48,49].
Paclitaxel
Pacl (Bristol-Myers Squibb Company (BMS) supplemented with 6 mg/ml Pacl was administered orally to Groups IV and V in suspension at a dose of 120 mg/kg body weight (0.04 mg/mouse/day) for five consecutive days. The doses were calculated by extrapolation of human therapeutic doses to animal doses according to previous methods [50].
Animal grouping
The animals were divided into five main groups (each n=12 mice):
Group I: The negative control group (noninfected).
Group II: The positive control group (infected nontreated group).
Group III: Infected group treated with MTZ.
Group IV: Infected group treated with Pacl.
Group V: Infected group treated with Pacl combined with MTZ.
Parasitological examination
After the administration of drugs, fecal pellets were collected from infected mice on the 7th, 10th, 14th and 21st days post infection and examined using direct smear and the Moleciolateiodine-Formaldhyde concentration technique (MIFc). The number of parasites was expressed per gram of feces [51].
Animal scarification
All the mice were scarified at the end of the experiment, 4 weeks post infection. The animals were anesthetized with xylene ketamine 100 mg/kg. Blood was collected individually from the jugular vein. Blood was allowed to stand for 1 hour at 37°C, then overnight at 4°C and centrifuged at 2,500 rpm for 15 min (80-1 Electric Centrifuge). The serum was obtained and kept in aliquots at -20°C for the immunoglobulin assay [49]. The small intestine and liver were removed and subjected to histopathological examination.
Immunological parameters
Measurements of serum polyclonal antigiardial IgG and IgA antibodies by Enzyme-Linked Immunosorbent Assay (ELISA). Assays were performed in accordance with the manufacturer’s instructions (Sigma Chemical Co., USA).
Histopathological studies
Immediately after blood collection, the animals were dissected. Liver and small intestine tissue samples were collected from all mice within the different groups. The preparation of the slides was performed at the Pathology Department at TBRI. Histopathological sections 4 μm in thickness were stained with Hematoxylin and Eosin (H and E). The slides were examined microscopically for the detection of pathological changes and assessment of cure rates after drug administration [52]. The grade of necroinflammation in the liver was evaluated according to the Ishak scoring system, which assigns numbers to the severity of necroinflammatory features (interface hepatitis, confluent necrosis, parenchymal injury and portal inflammation and adds the numbers to arrive at a grade that can range from 0 to 18. 6) [53].
Statistical Analysis
The present data were analyzed by IBM SPSS version 22 (SPSS, Inc., Chicago, IL, USA). One-way Analysis of Variance (ANOVA) was used to analyze the effect of treatment on the studied parameters. Duncan’s test of homogeneity was used to test the similarities between the experimental groups. Correlation coefficient and regression analyses were used to evaluate the relationships between the studied variables. All the results are expressed as the mean ± Standard Error of the Mean (SE).
Results
Parasitological results
MTZ: In the MTZ group, the mean number of Giardia cysts shed in one gram of stool on the 14th day post infection (p.i.) and before treatment was 20416.33 ± 1304.81, while after treatment on the 21st day (p.i.), it was 591.50 ± 89.88 (Table 1).
| No. of cysts | Infected untreated | Treatments | ||
|---|---|---|---|---|
| FL+Px | Px | FL | ||
| In intestine | 29666.7 ± 3513 | 3424.83 ± 353.6 | 12691.67 ± 417 | 1559.00 ± 89 |
| In Stool | 20416.33 ± 130 | 1141.67 ± 144 | 7564.17 ± 190 | 591.50 ± 89 |
Table 1: The cyst count in the intestine and stool of the mice. Each value represents the mean ± SEM.
Paclitaxel: In the Pacl group, the mean number of Giardia cysts shed in one gram of stool on the 14th day post infection (p.i.) and before treatment was 20416.33 ± 1304.81, while after treatment on the 21st day (p.i.), it was 7564.17 ± 19 (Table 1).
MTZ combined with Pacl: In the MTZ combined with Pacl group, the mean number of Giardia cysts shedding in one gram of stool on the 14th day p.i. and before treatment was 20416.33 ± 1304.81, while after treatment on the 21st day (p.i.), it was 1141.67 ± 14 (Table 1).
Immunological results
Immunological assessment of the levels of IgG and IgA in the five groups
MTZ: In the MTZ group, the serum IgA and IgG levels on the 21st day p.i. were 2.87 ± 0.08 and 1.90 ± 0.07, respectively, while after treatment on the 21st day (p.i), they were 2.17 ± 0.05and 0.90 ± 0.09, respectively (Table 2).
| Condition | Control | Infected untreated | Treatments | ||
|---|---|---|---|---|---|
| FL | Px | FL+Px | |||
| IgA | 0.40 ± 0.06a | 2.87 ± 0.08d | 2.17 ± 0.05b | 2.50 ± 0.05c | 2.00 ± 0.06b |
| (% change)P | - | - | (-24.40%) | (-12.84%) | (-30.27%) |
| IgG | 0.25 ± 0.01a | 1.90 ± 0.07d | 0.90 ± 0.09c | 0.90 ± 0.06c | 0.67 ± 0.05b |
| (% change)P | - | - | (-52.63%) | (-52.63%) | (-64.83%) |
| No. of cysts in intestine | 0.00 ± 0.00a | 29666.7 ± 3513.47c | 1559.00 ± 89.94a | 12691.67 ± 417.54b | 3424.83 ± 353.67a |
| (% change)P | - | - | (-94.74%) | (-57.22%) | (-88.46%) |
| No. of cysts in Stool | 0.00 ± 0.00a | 20416.33 ± 1304.81c | 591.50 ± 89.88a | 7564.17 ± 190.15b | 1141.67 ± 144.41a |
| (% change)P | - | - | (-97.10%) | (-62.95%) | (-94.41%) |
Table 2: The levels of serum IgA and IgG as well as the cyst count in the intestine and stool of the mice. Each value represents the mean ± SEM. Note: In each row, the mean values marked with the same superscript letter are similar (nonsignificant; P>0.05), whereas those with different ones are significantly different (P<0.05); P: Percent change in relation to the corresponding infected untreated group.
Paclitaxel: In the Pacl group, the serum IgA and IgG levels on the 21st day p.i. were 2.87 ± 0.08 and 1.90 ± 0.07, respectively, while after treatment on the 21st day p.i., they were 2.50 ± 0.05 and 0.90 ± 0.06, respectively (Table 2).
MTZ combined with Pacl: In the MTZ combined with Pacl group, the levels of serum IgA and IgG on the 21st day p.i. were 2.87 ± 0.08 and 1.90 ± 0.07, respectively, while after treatment on the 21st day p.i., they were 2.00 ± 0.06 and 0.67 ± 0.05, respectively (Table 2).
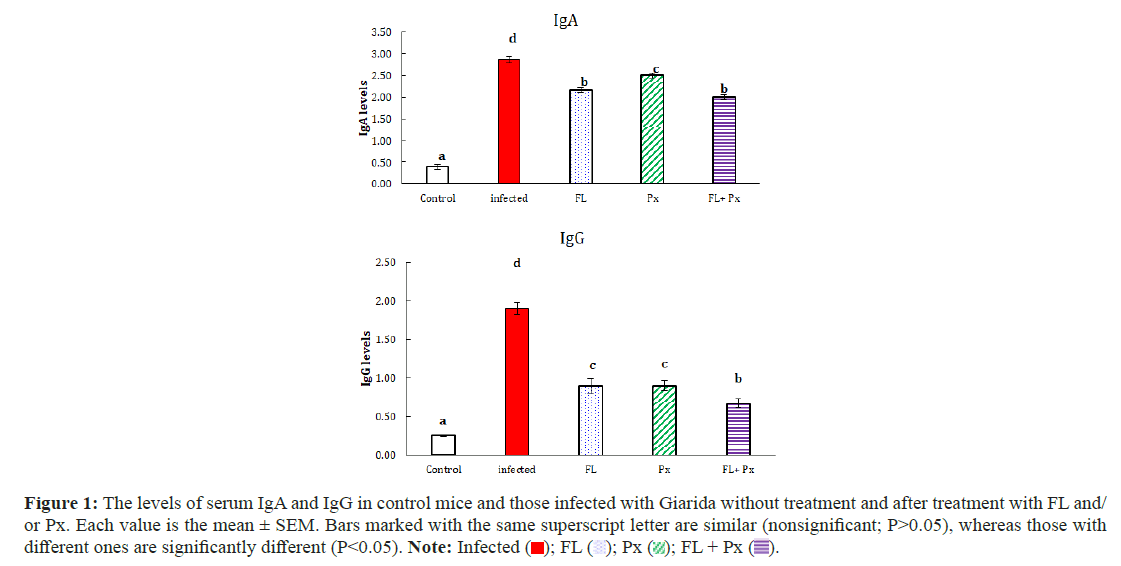
Comparison of Giardia Serum IgA and IgG levels before and after treatment among the groups; immunological assessment of the IgA and IgG groups revealed low levels of IgG in all treated groups compared with high levels of IgA in all treated groups. While in the other hand all. However, in all the treated groups, the immunological assessment of IgA and IgG was lower than that in the infected untreated group (Table 3) (Figure 1).
| Infected untreated | Condition | Treatments | ||
|---|---|---|---|---|
| FL+Px | Px | FL | ||
| 2.87 ± 0.08 | IgA | 2.00 ± 0.06 | 2.50 ± 0.05 | 2.17 ± 0.05 |
| 1.90 ± 0.07 | IgG | 0.67 ± 0.05 | 0.90 ± 0.06 | 0.90 ± 0.09 |
Table 3: The levels of serum IgA and IgG, each value represents the mean ± SEM.
Figure 1: The levels of serum IgA and IgG in control mice and those infected with Giarida without treatment and after treatment with FL and/
or Px. Each value is the mean ± SEM. Bars marked with the same superscript letter are similar (nonsignificant; P>0.05), whereas those with
different ones are significantly different (P<0.05). Note: Infected ( ); FL (
); FL ( ); Px (
); Px ( ); FL + Px (
); FL + Px ( ).
).
Histopathological results
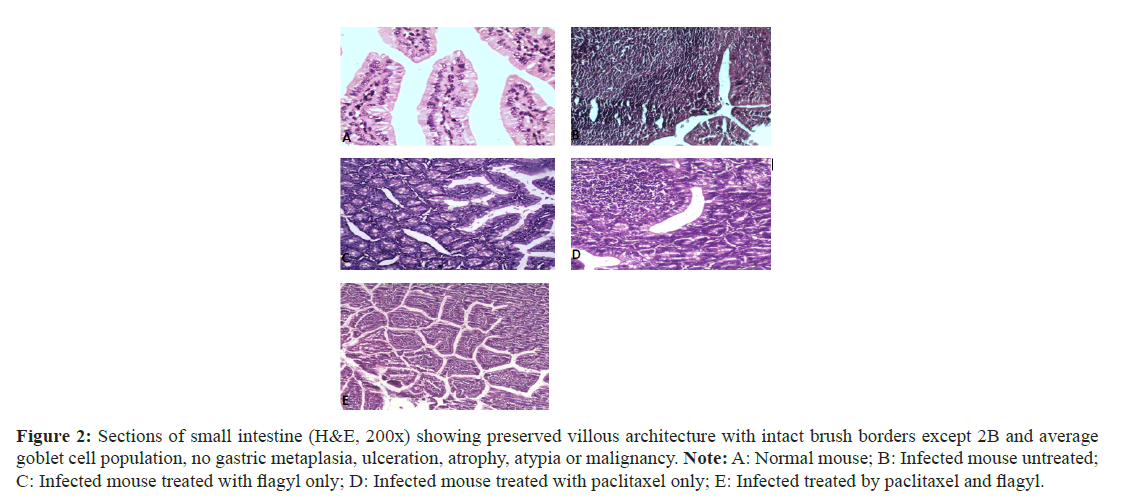
Duodenal biopsies: Biopsies were assessed for the four widely accepted criteria for duodenitis: Neutrophil infiltration, mononuclear (lymphocytes and plasma cells) infiltration, gastric metaplasia and villous atrophy. Inflammatory cells were assessed in the lamina propria and epithelial layer for gastric metaplasia and villous atrophy (Figure 2).
Figure 2: Sections of small intestine (H&E, 200x) showing preserved villous architecture with intact brush borders except 2B and average goblet cell population, no gastric metaplasia, ulceration, atrophy, atypia or malignancy. Note: A: Normal mouse; B: Infected mouse untreated; C: Infected mouse treated with flagyl only; D: Infected mouse treated with paclitaxel only; E: Infected treated by paclitaxel and flagyl.
In addition to the general characteristics, the lamina propria showed average lymphoplasmacytic cellular infiltration (Figures 2A and 2B), and showed shortening of the epithelial brush border microvilli, the lamina propria showed moderate lymphoplasmacytic cellular infiltration, and mild edema. Few trophozoites were observed (Figure 2C).
The lamina propria showed mild lymphoplasmacytic cellular infiltration, scattered neutrophils, and mild edema. Scattered Giardia lamblia trophozoites were observed related to brush borders (Figure 2D). The lamina propria showed mild lymphoplasmacytic cellular infiltration (black arrow), mild neutrophilic infiltration, and mild edema. Numerous G. lamblia trophozoites were observed related to brush borders, and in (Figure 2E), the lamina propria showed mild lymphoplasmacytic cellular infiltration and mild edema.
Concerning the duodenal biopsies, biopsies were assessed for the four widely accepted criteria for duodenitis: Neutrophil infiltration, mononuclear (lymphocyte and plasma cell) infiltration, gastric metaplasia and villous atrophy.
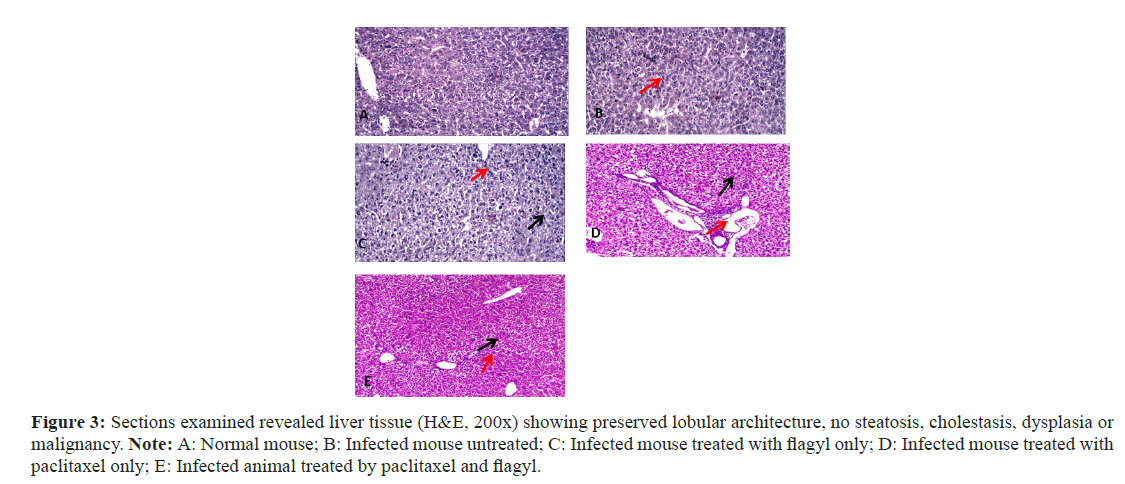
Liver biopsies
The severity of the necroinflammatory features (interface hepatitis, confluent necrosis, parenchymal injury and portal inflammation) was assessed (Figure 3). In addition to the general characteristics, (Figure 3A); the hepatocytes are within normal range. No spotty necrosis was observed. The portal tract showed no inflammatory cellular infiltrates (Figure 3B). The hepatocytes were within normal ranges. Focal spotty necrosis was observed (red arrow). No portal tracts were observed (Figure 3C). The hepatocytes showed mild hydropic degeneration (black arrow). No spotty necrosis was observed. The portal tract showed minimal inflammation (red arrow). No interface hepatitis was observed (Figure 3D). The hepatocytes showed mild hydropic degeneration (black arrow). The portal tract showed mild lymphoplasmacytic cellular infiltration (red arrow). The central veins are patent (Figure 3E). The hepatocytes showed mild hydropic degeneration (black arrow). The portal tract showed no evidence of portal inflammation (red arrow). The central veins are patent.
Figure 3: Sections examined revealed liver tissue (H&E, 200x) showing preserved lobular architecture, no steatosis, cholestasis, dysplasia or malignancy. Note: A: Normal mouse; B: Infected mouse untreated; C: Infected mouse treated with flagyl only; D: Infected mouse treated with paclitaxel only; E: Infected animal treated by paclitaxel and flagyl.
Discussion
Giardia lamblia is the most common intestinal parasite and the etiological agent of diarrhea worldwide. MTZ is an antibiotic widely used to treat Giardiasis and a variety of infections due to its high efficacy compared with other drugs; however, its side effects must be considered, and its use has been associated with toxicity and other adverse effects [32]. A combination of two or more drugs may be a viable approach. Selecting drugs from different classes and with different mechanisms of action is an excellent way to improve the treatment efficacy and avoid potential cross-resistance and dose-limiting toxicities [54]. A combination of drugs has also been recommended when monotherapy fails for the treatment of Giardiasis. There is evidence from other infectious diseases (i.e., malaria and HIV/AIDS) that resistance is less likely to occur when two drugs acting on distinct targets are used simultaneously [30,55].
In this study, treatment with 120 mg/kg MTZ twice daily for 5 successive days yielded a very high percentage of reduction in both trophozoite and cyst counts (94.74 and 97.10%, respectively). Our result is better than the results recorded with [48], which were 94.2, 93.9% and 92.15, 93.23%, respectively, and agree with [49], which were recorded (93.44 and 98.67%, respectively). Treatment with pacl at nearly 2 mg/kg daily for 5 successive days yielded a percentage of reduction in both the trophozoite and cyst counts (57.22 and 62.95, respectively). The decreases in both the trophozoite and cyst counts may be attributed to the low dose of Pacl used because chemotherapyinduced peripheral neuropathy is a major adverse effect of Pacl. Thus, assessing the drug concentration in target tissues is important for determining the dose‒response relationships for efficacy and toxicity [56]. We selected our dose on the basis of our previous animal study, which emphasized that no correlation was found between the Taxol dose (0.5-2 mg/kg) and the extent of neuropathy [57]. Biruk and colleagues reported that Pacl-treated Schistosoma-exposed mice treated with a single dose of 25 mg/kg could clear Schistosoma eggs faster [58]. However, they found that Pacl at this dose suppressed type 2 immunity, which subsequently downregulated the activation of TGF-β and decreased the severity of PH following Schistosoma exposure.
The combination of MTZ with Pacl also yielded a very high percentage of reduction in both trophozoite and cyst counts (88.46% and 94.41%, respectively); however, no previous study has compared our results. Moreover, the results of this study nearly agree with the results recorded by [48] when the combination of Giardia with MTZ and artemether caused a reduction in both trophozoite and cyst counts (98.3% and 95.5%, respectively). By observing our study results, we expect that the dose and the schedule of administration of the Pacl used in cancer patients will provide better results at limiting Giardia infection. We administered Pcl orally in suspension at a dose of 120 mg/kg body weight for five consecutive days, while the treatment schedule may reach 175 mg of paclitaxel per square meter by intravenous infusion for 3 hours every 3 weeks for 4 doses and 80 mg of paclitaxel per square meter by intravenous infusion for 1 hour weekly for 12 doses [59].
Histopathological examination revealed that treatment with FL, Px or FL+Px led to mild hydropic degeneration of the hepatocytes, but no focal spotty necrosis was observed in the case of infection. The portal tract showed mild lymphoplasmacytic cellular infiltration in infected mice treated with flagyl alone and infected mice treated with paclitaxel only, but there was no evidence of portal inflammation in infected animals treated with paclitaxel and flagyl. The central veins are patent in mice treated with paclitaxel only or with paclitaxel and flagyl. Thus, combination treatment comprising paclitaxel and a flagyl agent is more effective and safer. Concerning the duodenal biopsies, the four widely accepted criteria for duodenitis, neutrophil infiltration, mononuclear (lymphocytes and plasma cells) infiltration, gastric metaplasia and villous atrophy, and elimination of Giardia trophozoites, confirmed that treatment with a combination of paclitaxel and flagyl was the best result.
Secretory antibodies are attractive candidates for immune defense against Giardia because they are secreted in large quantities into the intestinal lumen, and their actions are antigen specific. AntiGiardial IgA is important for controlling and eliminating Giardia infection, and Giardia infection leads to a prolonged increase in the specific anti-Giardia IgG response [60]. Through this study, after analysis by ELISA, we found a significant increase in IgA and IgG levels in the period of infection compared with the control, which did not show almost any registration of antibodies. Such elevations in G. intestinalis-specific serum IgA and IgG levels after infection have been reported in several previous studies [61,49]. After treatment with MZT, a reduction in the OD values of IgA was recorded by (24.4%), as was that of IgG (52.63%). However, in the case of Pacl treatment, the percentage of reduction in IgA was less than that in Mzt (12.84%), although the percentage of reduction in IgG was the same as that in MZT (52.63%). When dual treatment was used (MTZ combined with pacl), the percentage of IgA and IgG antibodies significantly decreased, and the best results were obtained during this study, especially for IgG, for which decreases of 30.27% and 64.83%, respectively, were recorded. These findings indicate an increase in the evanescence of the antigens that stimulate the production of antibodies. Previous studies have shown a decrease in specific antibody levels after treatment of patients infected with Giardia species [62,63]. After treatment with secnidazole, Jiménez and his workers reported that all patients were cured with a reduction in IgA antibody levels in 26 of 34 children and a reduction in IgG-specific antibody levels in 18 of 34 children [64].
Our results demonstrated that the serologic IgG level is more strongly correlated with Giardia infection than is the IgA level, which conflicts with the findings of [49], who showed that the reduction in IgA levels is more significant than that in IgG levels. This result was likely caused by a reduction in antigen load as a consequence of parasite elimination. At the end of the treatment course, the antibodies did not disappear completely because of the failure to completely eliminate the infection; therefore, there was still an immune response to the residual antigens, which required extension of the treatment period. Here, the importance of the participation of Pacl in therapeutic protocols has two paths: the first involves the development of an anti-giardial compound that targets cell division machineries, where Giardia has a complex microtubule cytoskeleton that is utilized for parasite attachment and facilitation of rapid mitosis and cytokinesis [65], where Pacl inhibit microtubule depolymerization, delay of anaphase A and dramatic defects in chromosome segregation and spindle morphology in both nuclei [66].
The second involves reduces of the adverse effects of MTZ. Elizondo et al., (1996) reported that MTZ can induce an increase in chromosomal aberrations and chromatid and isochromatic breaks in the cells of patients treated with therapeutic doses of MTZ. Although MTZ is widely used, it has been associated with neurotoxicity and genotoxicity [32]. The mechanisms of Pacl action associated with the inhibition of tumor growth can act on different levels, initiating a cascade of signaling pathways resulting in programmed cell death [67,68]. Modulation of epigenetic markers regulates the expression of certain microRNAs associated with cancer progression. Furthermore, a variety of immune responses are modulated via the regulation of chemokines, cytokines, or immune cells [69].
Conclusion
This study and its results, it appears to provide oncologists with important information that is useful in the treatment of cancer patients infected with the Giardia parasite, especially since cancer patients were shown to be 1.24-fold more susceptible and at a greater risk of Giardia parasite infection. The prevalence of G. lamblia is high among colorectal cancer patients, detected at 8.1% by PCR and the prevalence of abdominal pain is significantly greater in colorectal cancer patients with G. lamblia infections than in those without infections. The estimated weighted prevalence of G. duodenalis infection in cancer patients was computed to be 6.9%. However, further research is needed to establish the safety of this combination and how it compares to other combination strategies.
References
- Rumsey P, Waseem M (2018) Giardia lamblia enteritis. StatPearls. 30285390.
- Egan S, Barbosa A, Feng Y, Xiao L, Ryan U (2024) Critters and contamination: Zoonotic protozoans in urban rodents and water quality. Water Res. 17:121165.
- Alemu Y, Abdissa A, Mekonnen Z, Sharew B, Johansen ØH, et al. (2024) Prevalence and assemblage of Giardia duodenalis in a case-control study of children under 5 years from Jimma, Southwest Ethiopia. Parasitol Res. 123(1):38.
- Quezada-Lázaro R, Vázquez-Cobix Y, Fonseca-Liñán R, Nava P, Hernández-Cueto DD, et al. (2022) The cysteine protease giardipain-1 from giardia duodenalis contributes to a disruption of intestinal homeostasis. Int J Mol Sci. 23(21):13649.
- Adam RD (2021) Giardia duodenalis: Biology and pathogenesis. Clin Microbiol Rev. 34(4):e00024-19.
- Rogawski ET, Bartelt LA, Platts-Mills JA, Seidman JC, Samie A, et al. (2017) Determinants and impact of Giardia infection in the first 2 years of life in the MAL-ED birth cohort. J Pediatric Infect Dis Soc. 6(2):153-160.
- Kabir F, Iqbal J, Jamil Z, Iqbal NT, Mallawaarachchi I, et al. (2023) Impact of enteropathogens on faltering growth in a resource-limited setting. Front Nutr. 19:1081833.
- Escobedo AA, Hanevik K, Almirall P, Cimerman S, Alfonso M (2014) Management of chronic Giardia infection. Expert Rev Anti Infect Ther. 12(9):1143-1157.
- Svärd EE (2016) SG An up-date on Giardia and giardiasis. Curr Opin Microbiol. 34:47-52.
- Lagunas-Rangel FA (2024) Giardia telomeres and telomerase. Parasitol Res. 123(4):179.
- Leung AK, Leung AA, Wong AH, Sergi CM, Kam JK (2019) Giardiasis: An overview. Recent Pat Inflamm Allergy Drug Discov. 13(2):134-143.
- Palomo-Ligas L, Gutiérrez-Gutiérrez F, Ochoa-Maganda VY, Cortés-Zárate R, Charles-Niño CL, et al. (2019) Identification of a novel potassium channel (GiK) as a potential drug target in Giardia lamblia: Computational descriptions of binding sites. PeerJ. 27:7:e6430.
- Mahdavi F, Sadrebazzaz A, Chahardehi AM, Badali R, Omidian M, rt al. (2022) Global epidemiology of Giardia duodenalis infection in cancer patients: A systematic review and meta-analysis. Int Health. 14(1):5-17.
- Meena S, Meena JK, Kumar D, Mathur P (2023) Spectrum and trends of intestinal parasitic infections at a tertiary care hospital during pandemic times: A laboratory-based retrospective study. J Lab Physicians. 15(04):503-509.
- Gaona-López C, Martínez-Vázquez AV, Villalobos-Rocha JC, Juárez-Rendón KJ, Rivera G (2023) Analysis of giardia lamblia nucleolus as drug target: A review. Pharmaceuticals (Basel). 16(8):1168.
- Gardner TB, Hill DR (2001) Treatment of giardiasis. Clin Microbiol Rev. 14(1):114-128.
- Cong W, Liu GH, Meng QF, Dong W, Qin SY, et al. (2015) Toxoplasma gondii infection in cancer patients: prevalence, risk factors, genotypes and association with clinical diagnosis. Cancer Lett. 359(2):307-313.
- Hui CH (2022) Prevalence and risk factors of Giardia lamblia infections among colorectal cancer patients in Henan Province. Chin. J. Schistosomiasis Control. 34(4):370.
- Hurník P, Žiak D, Dluhošová J, Židlík V, Šustíková J, et al. (2019) Another case of coincidental Giardia infection and pancreatic cancer. Parasitol Int. 71:160-162.
- Krones E, Högenauer C (2012) Diarrhea in the immunocompromised patient. Gastroenterol Clin North Am. 41(3):677-701.
- Halliez MC, Buret AG (2013) Extra-intestinal and long term consequences of Giardia duodenalis infections. World J Gastroenterol. 12;19(47):8974.
[Crossref] [Google Scholar] [PubMed]
- Tejman-Yarden N, Eckmann L (2011) New approaches to the treatment of giardiasis. Curr Opin Infect Dis. 24(5):451-456.
- Watkins RR, Eckmann L (2014) Treatment of giardiasis: Current status and future directions. Curr Infect Dis Rep. 16:1-8.
- Plata JD, Castañeda X (2020) Parasites in cancer patients. Onco Crit Care. 1441-1450.
- Van Tong H, Brindley PJ, Meyer CG, Velavan TP (2017) Parasite infection, carcinogenesis and human malignancy. EBioMedicine. 1;15:12-23.
- Carter ER, Nabarro LE, Hedley L, Chiodini PL (2018) Nitroimidazole-refractory giardiasis: A growing problem requiring rational solutions. Clin Microbiol Infect. 1;24(1):37-42.
- Nash TE (2001) Treatment of Giardia lamblia infections. Pediatr Infect Dis J. 1;20(2):193-195.
- Escobedo AA, Lalle M, Hrastnik NI, Rodríguez-Morales AJ, Castro-Sánchez E, et al. (2016) Combination therapy in the management of giardiasis: What laboratory and clinical studies tell us, so far. Acta Trop. 1;162:196-205.
- Ansell BR, McConville MJ, Ma'ayeh SY, Dagley MJ, Gasser RB et al. (2015) Drug resistance in Giardia duodenalis. Biotechnol Adv. 1;33(6):888-901.
- Nabarro LE, Lever RA, Armstrong M, Chiodini PL (2015) Increased incidence of nitroimidazole-refractory giardiasis at the Hospital for Tropical Diseases, London: 2008-2013. Clin Microbiol Infect. 1;21(8):791-796.
- Samuelson J (1999) Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother. 1;43(7):1533-1541.
- Ceruelos AH, Romero-Quezada LC, Ledezma JR, Contreras LL (2019) Therapeutic uses of metronidazole and its side effects: An update. Eur Rev Med Pharmacol Sci. 1;23(1):397-401.
- Arora N, Wasti KP, Babbar N, Saroch A, Pannu AK, et al. (2020) Neurological complications during treatment of liver abscess: Think of metronidazole toxicity. Trop Doct. 50(2):165-166.
- Bendesky A, Menéndez D, Ostrosky-Wegman P (2002) Is metronidazole carcinogenic?. Mutat Res. 511(2):133-44.
- MC W (1972) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 19:2325-2326.
- Zhu L, Chen L (2019) Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 24(1):40.
- Zhang D, Yang R, Wang S, Dong Z (2014) Paclitaxel: New uses for an old drug. Drug Des Devel Ther. 20:279-284.
- Vassileva V, Allen CJ, Piquette-Miller M (2008) Effects of sustained and intermittent paclitaxel therapy on tumor repopulation in ovarian cancer. Mol Cancer Ther. 7(3):630-637.
- Estes R, Vogel N, Mack D, McLeod R (1998) Paclitaxel arrests growth of intracellular Toxoplasma gondii. Antimicrob Agents Chemother. 42(8):2036-2040.
- Dostál V, Libusová L (2014) Microtubule drugs: Action, selectivity, and resistance across the kingdoms of life. Protoplasma. 251:991-1005.
- Santiago VS, Moraes JD, Sobral Alves ES, Santos MA, Lima CG, et al. (2016) The effectiveness of natural diarylheptanoids against trypanosoma cruzi: Cytotoxicity, ultrastructural alterations and molecular modeling studies. PLoS One. 11(9):e0162926.
- Chakrabarti R, Patankar S (2016) Combination assays and molecular docking can identify binding sites of anti-microtubule drugs on plasmodium falciparum tubulin. Infect Disord Drug Targets. 16(3):204-216.
- Nessim NG, Demerdash Z (2000) Correlation between infection intensity, serum immunoglobulin profile, cellular immunity and the efficacy of treatment with praziquantel in murine schistosomiasis mansoni. Arzneimittelforschung. 50(02):173-177.
- Moore DV, Yolles TK, Meleney HE (1949) A comparison of common laboratory animals as experimental hosts for Schistosoma mansoni. J Parasitol. 35(2):156-170.
- Bingham AK, Meyer EA (1979) Giardia excystation can be induced in vitro in acidic solutions. Nature. 277(5694):301-302.
- Bingham AK, Jarroll Jr EL, Meyer EA, Radulescu S. Giardia sp.: Physical factors of excystation in vitro, and excystation vs eosin exclusion as determinants of viability. Exp Parasitol. 47(2):284-291.
- Solaymani-Mohammadi S, Genkinger JM, Loffredo CA, Singer SM (2010) A meta-analysis of the effectiveness of albendazole compared with metronidazole as treatments for infections with Giardia duodenalis. PLoS Negl Trop Dis. 4(5):e682.
- Aly E, Sabry H, Fahmy Z, Zalat R (2014) Efficacy of combination therapy (metronidazole and/or artemether) in experimental giardiasis and its impact on nonenzymatic oxidative stress biomarkers. Parasitol United J. 7(1):68-72.
- Madbouly NA, Nashee H, Elgendy AA, Rabee I, El Amir A (2020) Encapsulation of low metronidazole dose in poly (D, L-lactide-co-glycolide) (PLGA) nanoparticles improves giardia intestinalis treatment. Infect Chemother. 52(4):550.
- Paget GE (1964) Evaluation of drug activities. Pharmacometrics. 135-166
- Blagg W, Schloegel EL, Mansour NS, Khalaf GI (1955) A new concentration technic for the demonstration of protozoa and helminth eggs in feces. Am J Trop Med Hyg. 4(1):23-28.
- Drury RA, Wallington EA (1980) Carleton’s histological technique 5th ed. New York: Churchill Livingstone. 135-166.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, et al. (1995) Histological grading and staging of chronic hepatitis. J Hepatol. 22(6):696-699.
[Crossref] [Google Scholar] [PubMed]
- Escobedo AA, Almirall P, Chirino E, Pacheco F, Duque A, et al. (2018) Treatment of refractory paediatric giardiasis using secnidazole plus albendazole: A case series. Infez Med. 26(4):379-384.
- Choi JH, Han DS, Kim J, Yi K, Oh YH, et al. (2017) Diffuse nodular lymphoid hyperplasia of the intestine caused by common variable immunodeficiency and refractory giardiasis. Intern Med. 56(3):283-287.
- Zang X, Lee JB, Deshpande K, Garbuzenko OB, Minko T, et al. (2019) Prevention of paclitaxel-induced neuropathy by formulation approach. J Control Release. 10;303:109-116.
- Polomano RC, Mannes AJ, Clark US, Bennett GJ (2001) A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 94(3):293-304.
- Kassa B, Mickael C, Kumar R, Sanders L, Koyanagi D, et al. (2018) Paclitaxel blocks Th2-mediated TGF-β activation in Schistosoma mansoni-induced pulmonary hypertension. Pulm Circ. 9(1):2045894018820813.
- Sparano JA, Wang M, Martino S, Jones V, Perez EA, et al. (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 358(16):1663-1671.
[Crossref] [Google Scholar] [PubMed]
- Sullivan PB, Neale G, Cevallos AM, Farthing MJ (1991) Evaluation of specific serum anti-Giardia IgM antibody response in diagnosis of giardiasis in children. Trans R Soc Trop Med Hyg. 85(6):748-749.
- Shukla G, Kaur T, Sehgal R, Rishi P, Prabha V (2010) Protective potential of L. acidophilus in murine giardiasis. Open Med. 5(4):456-463.
- Heyworth MF, Vergara JA (1994) Giardia muris trophozoite antigenic targets for mouse intestinal IgA antibody. J Infect Dis. 169(2):395-398.
- Cervetto JL, Ramonet M, Nahmod LH, Gallardo F (1987) Giardiasis Functional, immunological and histological study of the small bowel. Therapeutic trial with a single dose of tinidazole. Arq Gastroenterol. 24(2):102-112.
- Jiménez JC, Pinon A, Dive D, Capron M, Dei-Cas E, et al. (2009) Antibody response in children infected with Giardia intestinalis before and after treatment with Secnidazole. Am J Trop Med Hyg. 80(1):11.
- Hennessey KM, Alas GC, Rogiers I, Li R, Merritt EA, et al. (2020) Nek8445, a protein kinase required for microtubule regulation and cytokinesis in Giardia lamblia. Mol Biol Cell. 31(15):1611-1622.
- Sagolla MS, Dawson SC, Mancuso JJ, Cande WZ (2006) Three-dimensional analysis of mitosis and cytokinesis in the binucleate parasite Giardia intestinalis. J Cell Sci. 119(23):4889-4900.
- Matsuyoshi S, Shimada K, Nakamura M, Ishida E, Konishi N (2006) Bcl-2 phosphorylation has pathological significance in human breast cancer. Pathobiology. 73(4):205-212.
- Pan Z, Avila A, Gollahon L (2014) Paclitaxel induces apoptosis in breast cancer cells through different calcium-Regulating mechanisms depending on external calcium conditions. Int J Mol Sci. 15(2):2672-2694.
- Wanderley CW, Colon DF, Luiz JP, Oliveira FF, Viacava PR, et al. (2018) Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res. 78(20):5891-5900.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi