Research Article, J Nanomater Mol Nanotechnol Vol: 12 Issue: 2
Eco Friendly Synthesis of Zinc-Metal Organic Framework Luminescent Nano Particles (Zn-Mofnp), Characterization and their Extended Applications to Antimicrobial Studies
Jayanthi SS1*, Arunkumar P1, Vijayalakshmi R1, Shanmuga Priya N1, Thejaswini V1, Ezhilarasi RM1 and Ramamurthy K2,3
1Department of Chemistry, Guru Nanak College, Chennai, Tamil Nadu, India
2Department of Chemistry, National center for Ultrafast Process, Chennai, Tamil Nadu, India
3Department of Chemistry, Vels institute of science technology and Advanced studies (VISTAS), Chennai, Tamil Nadu, India
*Corresponding Author: Jayanthi SS
Department of Chemistry, Guru Nanak College, Velachery,Chennai, India
E-mail: jayanthi.ss@gurunanakcollege.edu.in
Received date: 10 April, 2023, Manuscript No. JNMN-23-95021;
Editor assigned date: 12 April, 2023, Pre QC No. JNMN-23-95021 (PQ);
Reviewed date: 26 April, 2023, QC No. JNMN-23-95021;
Revised date: 03 May, 2023, Manuscript No. JNMN-23-95021 (R);
Published date: 10 May, 2023, DOI: 10.4172/2324-83414.1000352
Citation: Jayanthi SS, Arunkumar P, Vijayalakshmi R, Shanmuga PN, Thejaswini V, et al. (2023) Eco Friendly Synthesis of Zinc-Metal Organic Framework Luminescent Nano Particles (Zn-Mofnp), Characterization and their Extended Applications to Antimicrobial Studies. J Nanomater Mol Nanotechnol 12:2.
Abstract
Zinc metal oxygen framework nano particle (Zn-MOFNP) can be synthesized using a simple solvent free environmentally benign combustion method. The structural characterization of synthesized nano particles are carried out using XRD, FT-IR and SEM. The XRD results show that Zn-MOFNP is in single phase. The SEM results show that Zn-MOFNP is highly porous with nano sheet appearance. The optical characterization was carried out using UV and Photoluminescence spectrofluorimeter. The life time experiments were carried out using single photon counting instrument and it was about 3.8 ns. The FT-IR studies clearly indicate the formation of the compound. The compound shows antibacterial and antimicrobial properties in par with the standard. Further substitution can enhance the antimicrobial properties
Keywords: Zinc-MOF; Fluorescence; Lifetime measurement; Antimicrobial; Antibacterial
Introduction
Metal-Organic Frameworks (MOFs) are emerging new class of functional crystalline solid-state materials. MOFs with its vast structural modularity, exceptional controlled porosity, make it as the most promising candidate to address many of the overbearing societal challenges pertaining to energy and environmental sustainability [1]. Initially, MOFs were solely based on the coordination of monodentate N-donor polytopic pyridine-based ligands with single metal ions [2]. The great variety of linear dicarboxylic ligands were used for making MOF and the great information gathered from obtained structures, is what made this platform highly sought after by researchers to identify the impact of functional and structural modifications on material properties [3].
This high degree of customizability of MOFs properties has attracted the interest of many researchers. To date, there are more than 20,000 different structures of MOFs being reported and studied [4]. Depending on the final structures and properties, MOFs may be prepared using several distinct synthetic methods such as: slow diffusion, hydrothermal (solvothermal), electrochemical, mechanochemical, microwave assisted heating and ultrasound [5-10]. These synthetic methods and formation mechanism of MOF have been comprehensively reviewed by Seoane and coworkers recently [11].
In the last two decades, research interest in Metal–Organic Frameworks (MOFs) has grown dramatically, driven by the MOFs most attractive applications in gas storage and separation, Photosensors, chemical sensor, and heterogeneous catalyst and also in antimicrobial fields [12-16]. Due to the higher application of MOF in various fields, we would like to introduce simple, ecofriendly, solvent free method for the synthesis of Zn-MOF material.
Materials and Methods
The following analytical grade materials were used without further purification: Zinc acetate hexa hydrate Zn (CH3COO) 2.6H2O. A.C.S. reagent (Sigma-Aldrich, 99% purity by wt) and the glycerol (anhydrous) were obtained from Merck (99%). Benzene-1,4-Di- Carboxylic acid (AR) grade (BDC) was obtained from sigma Aldrich. Ortho phosphoric acid obtained from sigma Aldrich was also used as dispersant. Time-resolved fluorescence decays were obtained by the Time Correlated Single-Photon Counting (TCSPC) method and the experimental setup is carried as reported [17]. Antimicrobial studies are carried out using well diffusion method.
Synthesis of zinc MOF
About 0.2 g of the zinc aceate hexa hydrate was weighed and made in to a paste with 2 drops of glycerol in a 50 ml silica crucible. The amount of glycerol used was optimized after several trials. The excess glycerol leads to charring of substances. The initial temperature was set to 50°C and the temperature was slowly raised to 300°C in the muffle furnace. The substance is calcinated at 300°C for 4 hrs. The brown crystalline Zinc–MOF powder obtained was characterized. Zinc-oxide and cerium oxide nanoparticles were already synthesized by authors and reported inthe earlier literature (Figures 1 and 2) [18].
The similar experiment was carried out using same amount of zinc acetate and Benzene-1,4-Di-Carboxylic acid (BDC) with few drops of ortho phosphoric acid as initiator. Since phosphoric acid is a good initiator, we expected to get a good product [19]. The product gets charred and huge amount of fumes are released.
Characterization
The surface morphology of the synthesized Zinc–MOF was characterized using SEM analysis. The scanning electron microscope used for this purpose is a Jeol-JSM-3.5 CF-Japan. The powder X-ray diffraction was performed using Scifert X-ray diffractometer with a CuKα radiation. The diffracted intensities were recorded from 10 to 70° angle. The absorption and emission spectra were recorded using Perkin Elmer LS 25 spectrophotometer and Perkin Elmer LS 45 spectro fluorimeter respectively. For the spectroscopic analysis, Zinc MOF sample was dispersed in HPLC methanol with the help of the sonicator for 10 minutes. The spectrum was recorded under room temperature. The life time of Zn-MOF was determined using time-correlated single-photon-counting spectrometer. Antibacterial and antifungal studies are carried out using well diffusion agar–agar method.
Results and Discussion
The structural characterization was carried out using powder XRD. By application of XRD technique, substantial information is acquired about crystal structure, phase type and product purity. Figure 3 shows typical XRD pattern of the as-obtained Zn-MOF particles. As you can see, all peaks are distinct. Sharp peaks in this spectrum corroborate the crystalline structure of Zn-MOF nanoparticles.
The analysis of powder XRD pattern at room temperature was compared with the literature report [15]. The spectrum observed is similar to the literature and the substance formed is single phase with the hexagonal symmetry [20]. The absence of extra peak claims the purity of the substance and also the complete conversion of Zinc acetate.
FT-IR
FT-IR is used to identify the organic compounds and functional groups in different materials. Figure 4 shows FT-IR spectrum of Zn- MOF nanoparticles after calcination at 300 C for 3 h in KBr matrix. As depicted, there is no peak due to impurities and preliminary reagents and it demonstrates the completion of the reaction. Also, various peaks were presented such as 400–1000 cm1 for Zn–O bonds, 3000 cm-1 for OH of carboxylic acid, 1700 cm-1 for C stretching bond and around 1400 cm-1 for confirming the aromaticity of structure. The FT-IR spectrum also matches with the literature report [21].
The reduction in peak around 3000 cm-1 indicates the formation of link between zinc and benzene di-carboxylic acid. The absence of extra peak claims the purity of the substance and also the complete conversion of zinc acetate.
The surface morphology of the resulting powder was examined using scanning electron microscope. The SEM micrographs of the Zn- MOF powder shown in Figure 5 represent the formation in single phase and the constituents are Nano sheets which shows curled petal in a flower like appearance.
The Nano structures are of 15 nm to 25 nm in size. The advantages in the above reaction are no solvent, no solid by-products, economic and ecofriendly. The role of the glycerol is to act as an organic dispersant to overcome the agglomeration.
Optical characterization
The brown crystalline Zn MOF powder was not soluble in water and almost in all organic solvents. Hence UV–Visible spectra were recorded for the Zinc-MOF dispersed in methanol solution and is represented in Figure 6. The absorption band observed at 271 nm is the characteristic peak of Zn-MOF nanomaterial.
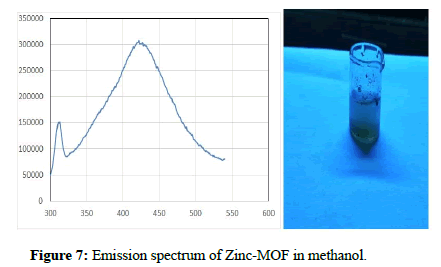
The compound was excited at 251 nm and the compound shows emission at 420 nm. The Zn-MOF compound shows blue emission. The emission spectrum was recorded at the excitation wavelength of 251 nm using Perkin Elmer spectro fluorimeter and is represented in Figure 7. The strong violet emission around 420 nm is the result of recombination of electron between Zinc interstitial and hole in the valence band. It is known that the transition metal ions without any free electron are known to possess linker based high emissive charge. Thus the Zn-MOF is expected to have luminescent property apparently due to linker-based emission. Zn-MOF shows emission maxima at 430 nm when excited at 372 nm.
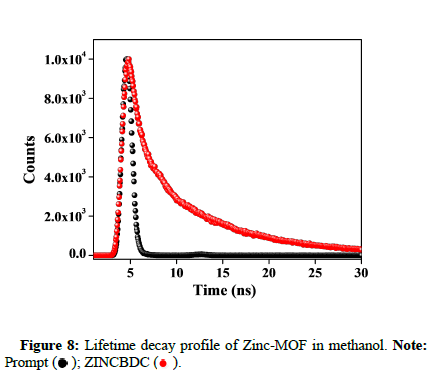
The fluorescence decay of ZINCBDC was recorded in water after sonication using the time correlated single photon counting spectrometer with a PMT (R3237 PMT) as a detector. The time resolved fluorescence decay of ZINCBDC in the water was monitored at 410 nm by exciting at 295 nm. The fluorescence decay of ZINCBDC in water was given in Figure 8. The observed decay lifetime was compiled in Table 1.
| S.No | τ1(ns) | τ2(ns) | τ3(ns) | A1% | A2% | A3% | χ2 |
|---|---|---|---|---|---|---|---|
| 1 | 0.196 | 1.89 | 8.98 | 9.61 | 16.6 | 73.78 | 1.211 |
Table 1: The fluorescence lifetime data of ZINCBDC in water.
Antibacterial studies
Antibacterial activity of sample was determined by well diffusion method on Muller Hinton Agar (MHA) medium. The Muller Hinton agar medium was weighed as 3.8 gms and dissolved in 100 ml of distilled water and added 1 gm of agar. Then the medium is kept for sterilization. After sterilization the media was poured into sterile petriplates and were allowed to solidify for 1hr. After the medium was solidified, the inoculums were spread on the solid plates with sterile swab moistened with the bacterial suspension. Wells were made using cork borer. Sample (Zn-MOF) was loaded in four different concentrations (25,50,75 and 100) and Streptomycin (1 mg/ml-20 μl) loaded in respective wells. These plates were incubated for 24 hrs at 37°C. Then the microbial growth was determined by measuring the diameter of zone of inhibition (Table 2 and 3) (Figure 9).
| Sample/Microorganisms | Zone of Inhibition in mm | |||||
|---|---|---|---|---|---|---|
| Concentrations (µ) | S (20 µl) | |||||
| 25 | 50 | 75 | 100 | |||
| Zn-MOF | ||||||
| Escherichia coli. | - | - | 11 | 12 | 21 | |
| Bacillus cereus | - | 11 | 13 | 15 | 30 | |
| Salmonella typhi | - | 11 | 12 | 16 | 25 | |
Table 2: 1-25 µl, 2-50 µl, 3-75 µl, 4-100 and 5-Streptomycin (Control).
| Sample/Microorganisms | Zone of Inhibition in mm | ||||
|---|---|---|---|---|---|
| Concentrations (µl) | K (20µl) | ||||
| 25 | 50 | 75 | 100 | ||
| Zn-MOF | |||||
| Candida albicans | - | - | - | - | 18 |
| Aspergillus niger | - | - | - | 16 | 16 |
Table 3: 1-25 µl, 2-50 µl, 3-75 µl, 4-100 and 5-ketocanzole (Control). The Zinc-MOF shows both antibacterial and antifungal activity. The compound shows activity on both Gram positive and Gram negative bacteria.
Antifungal activity/Agar well diffusion method/Preparation of inoculum
Stock cultures were maintained at 4°C on slant of potato dextrose agar. Active cultures for experiments were prepared by transferring a loop full of cells from the stock cultures to test tubes of nutrient broth for bacteria that were incubated at 24 hrs at 37ºC. The Assay was performed by agar disc diffusion method.
Antifungal activity
Antifungal activity of sample was determined by well diffusion method on Potato Dextrose Agar (PDA) medium. The potato dextrose agar medium was weighed as 4.4 gms and dissolved in 100 ml of distilled water and add 1 gm of agar. Then the medium is kept for sterilization. After sterilization the media was poured in to sterile petriplates and were allowed to solidify for 1 hr. After the medium was solidified, the inoculums were spread on the solid plates with sterile swab moistened with the fungal suspension. Wells were made using cork borer. Sample (Zn-MOF) was loaded in four different concentrations (25,50,75 and 100) and Positive control (ketocanzole 1 mg/ml-20 μl) was loaded in respective wells. These plates were incubated for 24 hrs at 37ºC. Then the microbial growth was determined by measuring the diameter of zone of inhibition (Table 3).
Conclusion
The present study illustrates a simple, solvent free and ecofriendly method for the synthesis of Zinc–MOF Nano particles through the direct combustion of Zinc acetate in the presence of minimum amount of glycerol as organic dispersant. The XRD analysis reveals that the Zn-MOF formed is of single phase. The SEM analysis tells us about the morphology of the particle which shows Nano petals likes structure. The porous structure depicts that Zn-MOF is a suitable candidate for catalysis. The characteristic peaks in the absorption and emission spectrum and the lifetime analysis of the compound confirms that the compound is a promising candidate for sensor. Zn-MOF also shows antibacterial and antifungal properties. Hence this is a benign method for the synthesis of Zn-MOF.
Acknowledgement
The authors are thankful for the support and seed money provided by the secretary, and Principal, guru Nanak College, Chennai-42. Tamil Nadu, India. The authors are thankful to Prof. P Ramamurthy and Dr. C Selvaraju of national centre for ultrafast processes, University of Madras, Chennai-600 113, and India for permitting us to avail the fluorescence emission and life time measurements.
References
- Kuppler RJ, Timmons DJ, Fang QR, Li JR, Makal TA, et al. (2009) Potential applications of metal-organic frameworks. Coord Chem Rev 253: 3042-3066.
- Li MX, Miao ZX, Shao M, Liang SW, Zhu SR (2008) Metal-Organic frameworks constructed from 2,4,6-Tris(4-Pyridyl)-1,3,5-Triazine. Inorg Chem 47: 4481-4489.
[Crossref] [Google Scholar] [Indexed]
- Luning C, Xibo Z, Xiqing C, Zhaoxiong X, Qin K et al. (2020) The function of metal–organic frameworks in the application of MOF-based composites. Nanoscale Adv 2: 2628-2647.
[Crossref] [Google Scholar] [Indexed]
- Bin W, Lin-Hua X, Xiaoqing W, Xiao-Min L, Jinping Li (2018) Applications of metal–organic frameworks for green energy and environment: New advances in adsorptive gas separation, storage and removal. Green Ener Environ 3: 191-228.
- Min HY, Kam LF, George ZC (2017) Synthesis and applications of MOF-derived porous nanostructures. Green Ener Environ 2: 218-245
- Mazen AG, Razan I, Mohamad H (2017) Synthesis, size and structural evolution of metal–organic framework-199 via a reaction–diffusion process at room temperature. Cryst Eng Comm 19: 608-612
- Denisov GL, Primakov PV, Korlyukov AA, Novikov VV, Nelyubina YV (2019) Solvothermal synthesis of the Metal-Organic Framework MOF-5 in autoclaves prepared by 3D printing. Russ J Coord Chem 45: 836-842.
- Hanan AK, Jorge G, Ernst JRS, Liza R (2015) Electrosynthesis of metal-organic frameworks: Challenges and opportunities. Chem Electro Chem 2: 462-474
- Maria K, Peter K, Andreas FT, Klaus R, Franziska E (2010) Mechanochemical synthesis of metal−organic frameworks: A fast and facile approach toward quantitative yields and high specific surface areas. Chem Mater 22: 5216-5221.
- Melvin SS, Jayanta B, Parthiban C, Gayathri V, Pradeep SND (2018) Ultrasound-assisted synthesis of metal organic framework for the photocatalytic reduction of 4-nitrophenol under direct sunlight. Ultrason Sonochem 49: 215-221.
- Dolgopolova EA, Rice AM, Martin CR, Shustova NB (2018) Photochemistry and photophysics of MOFs: Steps towards MOF-Based sensing enhancements. Chem Soc Rev 47: 4710-4728.
[Crossref] [Google Scholar] [Indexed]
- Fei-Yan Y, Dongxiao C, Meng-Ke W, Lei H, Hai-Long J (2016) Chemical sensors based on metal–organic frameworks. Chem Plus Chem 81: 1-17
[Crossref] [Google Scholar] [Indexed]
- Dhakshinamoorthy A, Asiri AM, Garcia H (2016) Mixed-Metal or Mixed-Linker metal organic frameworks as heterogeneous catalysts. Catal Sci Technol 6: 5238-5261.
- Neha B, Satish KP, Jyotsana M, Sanjeev KB, Ki-Hyun K, et al. (2018) Bioactive nano-metal-organic frameworks as antimicrobials against Gram-positive and Gram-negative bacteria. Toxicol Res 7: 931-941.
[Crossref] [Google Scholar] [Indexed]
- Jimmy R, Zakarias S, Javier S, Sonia A, Pilar GS, et al. (2017) An antibacterial Zn–MOF with hydrazinebenzoate linkers. Eur J Inorg Chem 2017: 574-580.
- Saravanan M, Dhivakar S, Jayanthi SS (2012) An ecofriendly and solvent free method for the synthesis of zinc oxide nano particles using glycerol as organic dispersant. Materials Letters 67: 128-130.
- Kaviyarasan R, Ramanan V, Senthil KT, Perumal R (2019) Environmentally benign, facile and selective recovery of gold from aqueous media: Synergic role of carbon dots as green reductant and sensor towards Au3+ions. RSC Adv 9: 39689-39698
[Crossref] [Google Scholar] [Indexed]
- Fatemeh A, Mehdi M, Narendra PSC, Ghasem S (2020) A novel synthesis of new antibacterial nanostructures based on Zn-MOF compound: Design, characterization and a high performance application. Heliyon 6: e03231
[Crossref] [Google Scholar] [Indexed]
- Zahra N, Mohammad AT, Hamid F (2017) A Zn based metal organic framework nanocomposite: Synthesis, characterization and application for preconcentration of cadmium prior to its determination by FAAS. RSC Adv 7: 44890-44895.
- Minmini R, Sanay N, Sivan V (2017) New zinc functionalized metal organic framework for selective sensing of chromate ion. Sens Actuators B Chem 251: 644-649.
- Christina AB, Tatiana VT, Thomas BS, Brian DP, Vincent HL, et al. (2007) Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks. J Am Chem Soc 129: 7136-7144.
[Crossref] [Google Scholar] [Indexed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 


 ).
).
 ); Sample DPT: (
); Sample DPT: ( ).
).

 ).
).
 ); ZINCBDC (
); ZINCBDC ( ).
).




