Research Article, J Spine Neurosurg Vol: 8 Issue: 2
Early Clinical Outcomes Comparing Porous PEEK, Smooth PEEK, and Structural Allograft Interbody Devices for Anterior Cervical Discectomy and Fusion
Clint P. Hill1,2 and K. Brandon Strenge1,3
1The Orthopaedic Institute of Western Kentucky, Paducah, KY, 4787 Alben Barkley Dr, Paducah, KY 42001
2Baptist Health Hospital, Paducah, KY
3Mercy-Lourdes Hospital, Paducah, KY
*Corresponding Author : Clint P. Hill, MD
The Orthopaedic Institute of Western Kentucky, Paducah, KY, 4787 Alben Barkley Dr, Paducah, KY 42001, USA
Tel: +270-442-9461
E-mail: hillcp@me.com
Received: January 19, 2019 Accepted: January 30, 2019 Published: February 07, 2019
Citation: Hill CP, Strenge KB (2019) Early Clinical Outcomes Comparing Porous PEEK, Smooth PEEK, and Structural Allograft Interbody Devices for Anterior Cervical Discectomy and Fusion. J Spine Neurosurg 8:1. doi: 10.4172/2325-9701.1000318
Abstract
Background: Recently, material surface advancements have been promoted to improve spinal implant osseointegration. While rough and porous titanium implants have gained traction due to their osteoconductive properties, polyetheretherketone (PEEK) devices have remained popular due to their radiographic properties and similar modulus of elasticity to bone. However, traditional smooth PEEK devices elicit fibrous tissue responses leading to poor implant osseointegration. Recently, PEEK implants have been developed with surface porosity allowing for direct bone in-growth. Despite preclinical data suggesting implant osseointegration with porous PEEK implants, comparative clinical results between implants with and without porous surface architecture are heretofore reported. The objective of this single-site retrospective study was to comparatively evaluate early clinical efficacy in patients undergoing ACDF treated with porous PEEK interbody implants to patients treated with structural allograft or traditional smooth PEEK implants. Methods: 167 consecutive patients presenting with cervical degenerative disc disease and radiculopathy underwent ACDF using one of three implants (porous PEEK (Cohere®, NuVasive Inc., San Diego, CA), structural allograft, or smooth PEEK). After preop evaluation and surgery, patients were followed per standard of care 0.5, 1.5, 3, 6, and 12 months post-op. At each time, patient outcomes were measured by disability (Neck Disability Index) and pain (visual analogue score (VAS) neck/arm pain). Results: Patients treated with porous PEEK implants achieved significantly greater NDI and neck pain improvement by 6 weeks post-op when compared to patients receiving structural allograft or smooth PEEK devices. These significantly greater NDI and neck pain improvements for patients treated with porous PEEK devices compared to structural allograft and smooth PEEK were sustained through 12 months post-op. Conclusion: 12 month follow-up data in patients with degenerative disc disease and radiculopathy demonstrates a clear benefit of porous PEEK in promoting improved early outcomes over structural allograft and smooth PEEK in ACDF procedures.
Keywords: Porosity; Porous PEEK; Structural allograft; Smooth PEEK; ACDF; Fusion; In-growth; Radiolucent
Introduction
Cervical degenerative disc disease (DDD) can lead to debilitating pain and motor weakness. The primary route of care involves physical therapy and pain management [1]; however, if these approaches fail to provide symptomatic relief, surgery can deliver cervical decompression to alleviate these symptoms [2]. Successful anterior cervical discectomy and fusion (ACDF) procedures require anterior cervical decompression, removal of diseased or degenerated disc, removal of radial osteophytes and bone spurs, and enlargement of the central foramen, which can lead to effective decompression, restoration of cervical lordosis, and restoration of intervertebral height. In order to increase stability after removal of the diseased disc, a rigid graft (interbody spacer/implant/cage/device) is placed in the intervertebral space to maintain decompression and disc space height, and a metal plate with screws is attached to fixate the adjusted adjacent levels to mediate fusion of the adjacent vertebral bone [3-9].
Interbody spacers made of iliac crest autograft bone and cadaver-derived structural allograft bone were once the gold standard for ACDF procedures, adding stability and maintaining intervertebral disc space height during fusion, while also providing osteoinductive and osteoconductive properties, respectively [8-15]. The desire to eliminate the need for a second surgical site with potential for host site morbidity motivated the shift towards allograft spacers [12,15]. While structural allograft interbody spacers are popular for 1-level fusions due to their perceived high fusion rates [8,10,15], fusion rates decrease with multi-level fusions and allograft spacers subside or collapse more than autograft spacers [12,16]. In addition, the time it takes for full fusion to occur is debatable, and allograft spacer use presents a race between resorption of the non-viable spacer and fusion across the intervertebral space [13], which can result in loss of intervertebral height either due to resorption or subsidence [12,13,16]. Additionally, in patients with comorbidities such as smoking or diabetes, non-unions are more likely to occur [17].
Synthetic interbody devices have gained popularity due to concerns of host-site morbidity with autograft spacers and donor variability with allograft spacers [12,18]. Polyetheretherketone (PEEK) is a popular polymer for spine and orthopaedics use due to its radiolucency, which allows for easy radiographic assessment of fusion and a modulus of elasticity that closely mimics that of native cortical bone [19]. While PEEK interbody spacers have gained popularity, traditional smooth PEEK devices do not typically support osseointegration and can potentially induce fibrous tissue responses leading to radiolucencies around the device or even a “PEEK-halo effect” in radiographs that indicates poor osseointegration [20]. This lack of osseointegration of the device can cause micro-motion of the device in the intervertebral space leading to delays in fusion and motion of the segment in the intervertebral space. This can then be followed by loss of segmental lordosis, non-unions, or migration of the spacer out of the intervertebral space, possibly leading to physical pressure of the spacer on sensitive nervous tissue.
In order to provide synthetic interbody devices that actually promote an osteogenic response to facilitate fusion, the use of titanium and titanium alloys, which can be readily modified to create rough or porous surfaces shown to be osteoconductive, have gained traction in the spine as well. Specifically, porosity has consistently been shown to elicit a greater osteoconductive response and stronger implant osseointegration compared to either micro- or nano roughness [21-24]. However, titanium is radioopaque on X-ray and CT imaging and has a modulus of elasticity that is 50 times greater than native cortical bone [19]. This modulus mismatch between titanium and bone can promote undesired consequences such as subsidence and implant migration that can potentially lead to loss of intervertebral height, and compression on surrounding neural tissue. Given these shortcomings of titanium, recent efforts have focused on improving the ability for PEEK to osseointegrate to bone by making it porous.
Novel PEEK interbody devices with surface porosity have been developed that present a seamless transition from solid PEEK to surface porous PEEK that mimics the structure of vertebral bone and is resistant to expulsion, compression, and shear [25-28]. Preclinical data shows that a specifically optimized porous architecture at the interbody surface promotes bone in-growth with an osseointegration strength that is stronger than rough titanium surfaces and just as strong as that achieved with porous titanium devices [25,29,30]. Clinically, these porous PEEK devices for cervical fusion have exhibited desirable outcomes in a prospective case series of 50 consecutive patients including those with debilitating comorbidities that typically inhibit fusion [31]. Despite these clinical data and extensive preclinical data showing interbody devices with rough or porous surfaces improve osseointegration no published comparative study currently shows a clear clinical improvement due to these osteoconductive surfaces. The objective of this single site retrospective study was to compare early radiographic and clinical outcomes in patients undergoing ACDF treated with a porous PEEK device to those treated with either a structural allograft spacer or a smooth PEEK spacer with no differences in anterior fixation instrumentation or graft aperture packing material.
Methods
The present study is a retrospective comparative study to evaluate the role of three different interbody fusion materials on early outcomes in patients undergoing ACDF. This study includes patients on which ACDF was performed at Baptist Health Hospital and Mercy Health-Lourdes Hospital with all pre-operative and post-operative procedures performed at The Orthopaedic Institute of Western Kentucky in Paducah, Kentucky.
ACDF was performed on 189 consecutive patients in whom a total of 380 cervical levels were operated. All patients received a standard cervical interbody fusion procedure in which the interbody spacer was packed with a cellular allograft material and accompanied by anterior fixation with standard plates and screws [5,6,8,32]. The only difference among the patients was the choice of interbody material used in the fusion procedure. The first 42 patients enrolled (93 levels) received a structural allograft interbody spacer (ALLOFUSE® Cervical Graft, AlloSource, Centennial, Colorado, USA), the next 61 patients (115 levels) received a standard smooth PEEK interbody spacer (BRECKENRIDGE™, Zimmer-Biomet, Warsaw, Indiana, USA), and the final 86 patients (172 levels) received a porous PEEK interbody fusion device (COHERE®, NuVasive, San Diego, California, USA). All patients were followed up to 12 months post-operatively. Inclusion and exclusion criteria are provided in Table 1.
| Inclusion Criteria | ≥ 18 years of age |
| Degenerative disc disease with cervical radiculopathy as diagnosed by a spine surgeon based on patient history, physical examination, and radiographic assessment | |
| Exclusion Criteria | Corpectomy partial corpectomy |
| Cervical trauma with no previously diagnosed degenerative disc disease | |
| Posterior fixation | |
| Osteoporotic | |
| Morbid obesity (BMI ≥ 45) | |
| BMI: Body Mass Index | |
Table 1: Inclusion and Exclusion Criteria.
The medical procedures performed by the study surgeons followed standard-of-care procedures for these cases and did not expose patients to more-than-minimal-risk. Institutional Review Board (IRB) approval was obtained, but due to the retrospective nature of the study and non-randomization, informed consent by the IRB was not required. Patients were not given a choice of interbody material and the interbody material used was purely based on which interbody material was preferred by the surgeon at the time of the procedure. All patient data were de-identified in compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
Demographics, comorbidities, and operative details
Patient demographics, relevant comorbidities, and operative details were analyzed in the patient data set to determine if any of these variables were differentially distributed among the three patient groups. With regards to patient demographics, age at time of surgery, body mass index (BMI), and patient sex were included. As for relevant comorbidities, smoking status and whether a patient was diabetic, hypertensive, or arthritic were analyzed among all groups. Finally, the number of operated levels among the three groups was included.
Outcome measures
At pre-op and post-op visits, patients were evaluated for neck function by the Neck Disability Index (NDI), a modification of the Oswestry Lower Back Disability Index [33], and the Visual Analog Pain Scale (VAS) for neck and left/right arm pain. Data for NDI scores and pain scores are presented as improvement at each follow-up compared to baseline pre-op scores due to patient-specific differences in baseline scores.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 8.0. Categorical data such as patient demographics, comorbidities, and operative details were examined by Fisher’s exact test. Time point specific outcome data were analyzed by one-way Browne-Forsythe and Welch analysis of variance (ANOVA) followed by the Tamhane T2 test for multiple comparisons due to unequal variances and unequal sample sizes among the groups. NDI and neck pain score improvements over time were analyzed by least-sum-of-squares non-linear regression to determine a best-fit curve function.
Results
A total of 189 total patients between January 2015 and December 2017 underwent a standard ACDF procedure. The first 42 patients (93 levels) received a structural allograft interbody spacer, the next 61 patients (115 levels) received a standard smooth PEEK interbody spacer, and the final 86 patients (172 levels) received a porous PEEK interbody fusion device. A patient enrollment chart is provided in Figure 1. From the group of patients treated with structural allograft, 1 patient was excluded because a corpectomy was required, 1 was excluded because the patient suffered cervical trauma with no previously diagnosed degenerative disc disease (DDD), and 1 patient was excluded due to a BMI greater than 45 (morbidly obese). From the group of patients treated with smooth PEEK, 6 patients were excluded due to corpectomies or partial corpectomies, 1 was excluded due to cervical trauma with no previously diagnosed DDD, 1 was excluded due to osteoporosis, and 3 were excluded due to morbid obesity. From the group of patients treated with porous PEEK, 4 were excluded due to corpectomies or partial corpectomies, 3 were excluded due to osteoporosis, and 1 was excluded due to morbid obesity.
Demographics and patient characteristics
Demographics, comorbidities, and operative details are provided in Table 2. The average age at the time of surgery (allograft, 53.8 ± 8.8; smooth PEEK, 54.8 ± 9.6; porous PEEK, 55.0 ± 8.9) was not significantly different among the three groups. The average BMI (allograft, 31.0 ± 5.1; smooth PEEK, 29.9 ± 5.4; porous PEEK, 29.9 ± 6.2) were also not statistically significantly different among the treatment groups. As for patient sex, each group had consistently more female than male patients and the distribution was not statistically significantly different among the groups (Table 2). Among comorbidities analyzed including smoking status, or whether the patients were diabetic, hypertensive, or arthritic, none were significantly altered in distribution among the three groups (Table 2).
| Patient characteristics | All patients after exclusion (167) | p-values for significant differences in demographics, comorbidities, and number of levels | Analysis performed | ||||
|---|---|---|---|---|---|---|---|
| Demographics | Allograft (39) | Smooth PEEK (50) |
Porous PEEK (78) |
Allograft vs Smooth PEEK | Allograft vs Porous PEEK | Smooth PEEK vs Porous PEEK | |
| Average Age (SD) | 53.8 (8.8) | 54.8 (9.6) | 55.0 (8.9) | n.s. | n.s. | n.s. | ANOVA |
| Average BMI (SD) | 31.0 (5.1) | 29.9 (5.4) | 29.9 (6.2) | n.s. | n.s. | n.s. | ANOVA |
| Female (%) | 22 (56.4) | 31 (62.0) | 44 (56.4) | n.s. | n.s. | n.s. | Fisher's exact test |
| Male (%) | 17 (43.6) | 19 (38.0) | 34 (43.6) | ||||
| Comorbidities | |||||||
| Smoker (%) | 8 (20.5) | 10 (20.0) | 15 (19.2) | n.s. | n.s. | n.s. | Fisher's exact test |
| Diabetic (%) | 8 (20.5) | 9 (18.0) | 9 (11.5) | n.s. | n.s. | n.s. | Fisher's exact test |
| Hypertensive (%) | 25 (64.3) | 20 (45.9) | 41 (54.7) | n.s. | n.s. | n.s. | Fisher's exact test |
| Arthritic (%) | 20 (51.3) | 19 (38.0) | 34 (43.6) | n.s. | n.s. | n.s. | Fisher's exact test |
| Operative details (Number of levels) | |||||||
| Multi-level (%) | 38 (97.4) | 33 (66.0) | 62 (79.5) | 0.0001 | 0.0105 | n.s | Fisher's exact test (1-level vs. Multi-level) |
| 1-level (%) | 1 (2.6) | 17 (34.0) | 16 (20.5) | ||||
| 2-level (%) | 29 (74.4) | 25 (50.0) | 50 (64.1) | 0.0002 | 0.0002 | n.s | Fisher's exact test (1-level vs. 2-level) |
| 3-level (%) | 9 (23.1) | 8 (16.0) | 11(14.1) | n.s. | n.s. | n.s. | Fisher's exact test (2-level vs. 3-level) |
| 4-level (%) | 0 (0) | 0 (0) | 1 (1.3) | n/a | n/a | n/a | n/a |
| Total Levels | 86 | 91 | 153 | n/a | n/a | n/a | n/a |
Table 2: Demographics, comorbidities and operative details after patient exclusion.
As for operative details (Table 2), specifically the number of levels fused per patient (from 1-level fusions to 4-level fusions), there were significantly fewer 1-level fusions in the structural allograft group (1 patient, 2.6% of allograft patients) compared to both the smooth PEEK group (17 patients, 34.0% of smooth PEEK patients) and the porous PEEK group (16 patients, 20.5% of porous PEEK patients). The majority of patients in all three groups were either 2-level or 3-level ACDFs (greater than 65% in all three groups). There was only one 4-level fusion, which was within the porous PEEK group.
Clinical outcomes
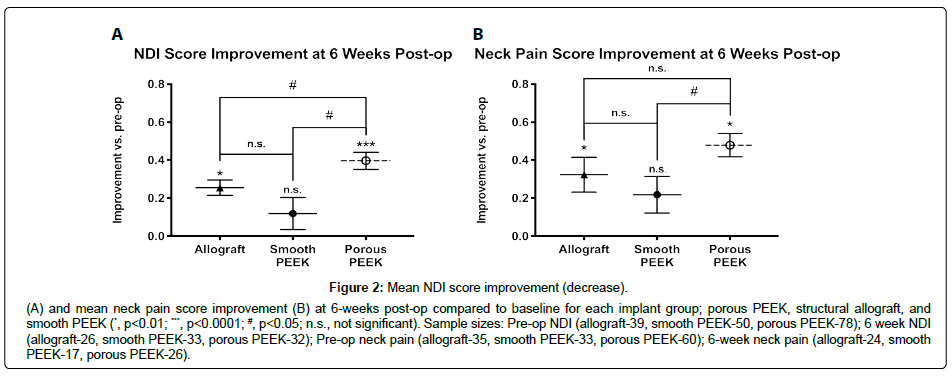
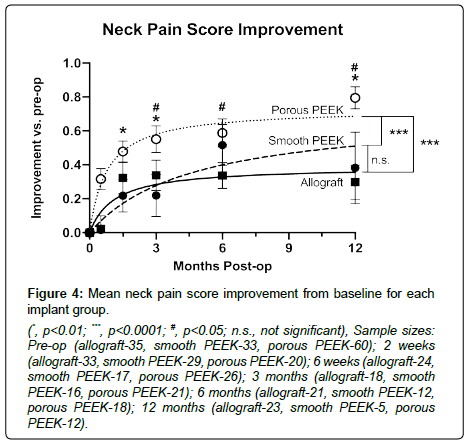
Differences in sample sizes (reported with figures) at each time point arose from incomplete data due to patient loss-to-follow-up. Porous PEEK patients achieved greater mean improvements in both NDI and neck pain scores by 6 weeks post-op over it’s pre-op baseline (Figure 2A and 2B respectively), and this was maintained to 12 months post-op when compared to both smooth PEEK and structural allograft (NDI, Figure 3; neck pain, Figure 4). Furthermore, by 6 weeks post-op, porous PEEK exhibited statistically significant improvements in NDI scores compared to both allograft and smooth PEEK patients at 6 weeks post-op (Figure 2A). Smooth PEEK did not show statistically significant improvement in NDI scores over baseline at 6 weeks post-op (Figure 2A) but began to improve at 3 months post-op and continued to improve at similar rates to allograft until 12 months post-op (Figure 3). Interestingly, porous PEEK trended towards improvement as early as 2 weeks post-op (p=0.0107 compared to porous PEEK pre-op); however, neither allograft nor smooth PEEK significantly improved over their respective pre-op scores, and porous PEEK was not significantly different than either allograft or smooth PEEK at 2 weeks post-op (data not shown). Among all patients, improvements in NDI scores (Figure 3) and neck pain (Figure 4) were observed in all groups from pre-op to 12 months post-op. In addition, as can be seen by the data for all groups, the best-fit curve for the porous PEEK group is statistically significantly different from both the smooth PEEK and allograft groups (p<0.0001) with a rates of increase over time statistically greater (p<0.0001), suggesting that porous PEEK patients improve faster at early time points and continue to improve faster than both smooth PEEK and allograft at later post-op times. These best-fit-curve models may hold at later time points, but 24 month or later follow-up data are required to determine the effect of the different interbody materials on longer term clinical outcomes. While all patients in this dataset presented with radiculopathy, left and right arm pain scores were also analyzed and exhibited similar trends (data not shown).
Because of statistically significant differences in distribution of 1-level vs. multi-level fusions between structural allograft and smooth PEEK (Table 2; p=0.0105) or porous PEEK (Table 2, p=0.0001), we also performed analysis of outcome data with all 1-level fusion data excluded to determine if inclusion of this data may have confounded results. After excluding all 1-level ACDF data, multi-level data exhibited the same trends in NDI and pain score improvements (data not shown). Improvements in NDI scores and neck pain scores were still observed at earlier times in porous PEEK patients than both smooth PEEK and structural allograft patients (data not shown).
Adverse events
No adverse events were reported during the course of this study. Estimated blood loss (EBL) was minimal for all patients (<250mL) and no post-operative infections were reported.
Discussion
While most studies place an emphasis on radiographic fusion rates, those results do not necessarily show an improvement in functional clinical outcomes in patients. Therefore, the objective of this study was to evaluate early functional clinical outcomes such as improvements in neck function and pain, . These data are the first evidence that interbody devices designed to enhance bone in-growth into a porous architecture incrementally and significantly improve clinical outcomes compared to traditional implants [25,30]. Unlike structural allograft cages that need to remodel during the fusion process [13,15,18,34] or smooth PEEK interbody spacers that do not osseointegrate [20,35,36], porous PEEK interbody fusion devices are non-resorbable and can osseointegrate with the vertebral endplates [28,30,31].
This single-center retrospective study comparing early outcomes of ACDF in 189 consecutive patients with 380 operated levels is the first to demonstrate a definitive early and sustained clinical benefit of porous PEEK compared to traditional smooth PEEK and structural allograft interbody devices in which the interbody material was the only procedural difference between cohorts. Every patient received the same standard ACDF procedure using the same instrumentation for anterior fixation and the same cellular allograft material to fill the graft aperture prior to implantation in the intervertebral space. While some patients were excluded from analysis due to potential confounding variables, the overall cohort was still represented by 167 patients with a total of 330 operated cervical levels. Patients who were treated with porous PEEK interbody fusion devices achieved clear improvements in NDI scores and pain by 6 weeks post-op, while patients treated with smooth PEEK only began to show improvements at 3 months post-op. While structural allograft patients began to improve over baseline by 6 weeks, porous PEEK patients showed statistically significant improvements over baseline by 2 weeks and significantly greater improvements than allograft and smooth PEEK patients by 6 weeks post-op. This early improvement was maintained at all time points up to 12 months post-op, demonstrating clear superior outcomes in patients treated with porous PEEK interbody fusion devices. These data along with preclinical data that demonstrate early bone in-growth into porous PEEK [25,28-30] suggest that porous PEEK promotes earlier functional fusion, possibly due to early bone in-growth minimizing micro-motion during the fusion process. This early stabilization may allow fusion to proceed across the device more easily than a smooth PEEK device that does not osseointegrate or a structural allograft device that must remodel during the fusion process. Sustained mean NDI and pain score improvement of porous PEEK patients compared to allograft and smooth PEEK patients at 12 months post-op further supports accelerated fusion and maintenance of intervertebral height previously demonstrated in a clinical case series of ACDF patients treated with porous PEEK [31].
This comparative study demonstrates that porous PEEK implants that are durable under clinical loads [37], closely mimic the modulus of elasticity of bone, and support bone in-growth, promote better early outcomes by 6 weeks post-op than traditional smooth PEEK and structural allograft implants in patients that require cervical fusion. However, it is unknown how porous PEEK may perform clinically compared to a porous architecture made from a material with a higher modulus of elasticity such as titanium or silicon nitride. A previous study suggests that bone in-growth into porous PEEK is stress-shared, whereas bone in-growth into porous titanium is stress-shielded [38]. Furthermore, clinical data have demonstrated that stiffer implants such as those made of titanium have a greater risk of subsidence and implant migration [39]. Therefore, the improved outcomes attributed to porous PEEK in this study along with the preclinical data exhibiting improved osteoconductive response not only contradicts the assumption that PEEK itself does not osseointegrate, but these data suggest that porous PEEK may be the optimal surface modification for early and sustained clinical improvement.
The data presented in this study may also be particularly important to consider for patients such as those with confounding comorbidities or multi-level fusions in which fusion is more difficult to achieve. In a prior comprehensive analysis of 50 consecutive patients among which 24% were smokers, 40% were obese, 14% were diabetic, and 26% had previously failed fusions, all achieved radiographic fusion by 6 months with porous PEEK [31]. Along with this study and its indication for use in multi-level ACDFs with allograft filler, porous PEEK presents a safer alternative to osteoinductive biologics traditionally used to promote fusion under challenging conditions such as the use of BMP-2, which is not indicated for use in the cervical spine.
Conclusion
This is the first retrospective case study directly comparing porous PEEK, smooth PEEK, and structural allograft interbody devices for ACDF, and furthermore, it is the first study to show that porous PEEK patients have greater early clinical improvements than smooth PEEK or structural allograft patients. The early comparative improvement in NDI and pain scores in patients treated with porous PEEK are consistent with preclinical results showing bone in-growth into the porous endplates [25,30] and clinical results in which early fusion (6-12 months postoperative) was achieved in a challenging patient population potentially due to mechanical interlock eliminating micro-motion [31].
Acknowledgements
The authors thank Rebekah Vinson, CMA and Benjamin Kirchner, PA for technical and clinical support. The authors would like to thank Reyhaan Chaudhri, PhD from NuVasive Inc. for his administrative, editorial, and statistical support of this manuscript. The authors received no direct compensation for their work on this manuscript. NuVasive Inc. also provided financial support for the open-access availability of this manuscript.
References
- Dillin W, Uppal GS (1992) Analysis of medications used in the treatment of cervical disk degeneration. Orthop Clin North Am 23: 421-433.
- Southwick WO, Robinson RA (1957) Surgical approaches to the vertebral bodies in the cervical and lumbar regions. J Bone Joint Surg Am 39-A: 631-644.
- Bohlman HH, Emery SE, Goodfellow DB, Jones PK (1993) Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 75: 1298-1307.
- Brodke DS, Zdeblick TA (1992) Modified Smith-Robinson procedure for anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 17(10 Suppl): S427-430.
- Wang JC, McDonough PW, Kanim LE, Endow KK, Delamarter RB (2001) Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 26: 643-646.
- Katsuura A, Hukuda S, Imanaka T, Miyamoto K, Kanemoto M (1996) Anterior cervical plate used in degenerative disease can maintain cervical lordosis. J Spinal Disord 9: 470-476.
- Gore DR, Sepic SB (1984) Anterior cervical fusion for degenerated or protruded discs. A review of one hundred forty-six patients. Spine (Phila Pa 1976) 9: 667-671.
- Miller LE, Block JE (2011) Safety and effectiveness of bone allografts in anterior cervical discectomy and fusion surgery. Spine (Phila Pa 1976) 36: 2045-2050.
- Suchomel P, Barsa P, Buchvald P, Svobodnik A, Vanickova E (2004) Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: a prospective study with respect to bone union pattern. Eur Spine J 13: 510-515.
- Shapiro S (1996) Banked fibula and the locking anterior cervical plate in anterior cervical fusions following cervical discectomy. J Neurosurg 84: 161-165.
- Epstein NE (2012) Iliac crest autograft versus alternative constructs for anterior cervical spine surgery: Pros, cons, and costs. Surg Neurol Int 3: S143-156.
- Floyd T, Ohnmeiss D (2000) A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J 9: 398-403.
- Rhee JM, Patel N, Yoon ST, Franklin B (2007) High graft resorption rates with dense cancellous allograft in anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 32: 2980-2984.
- Samartzis D, Shen FH, Goldberg EJ, An HS (2005) Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine (Phila Pa 1976) 30: 1756-1761.
- Zdeblick TA, Ducker TB (1991) The use of freeze-dried allograft bone for anterior cervical fusions. Spine (Phila Pa 1976) 16: 726-729.
- Lind BI, Zoega B, Rosen H (2007) Autograft versus interbody fusion cage without plate fixation in the cervical spine: a randomized clinical study using radiostereometry. Eur Spine J 16: 1251-1256.
- Brown CW, Orme TJ, Richardson HD (1986) The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine (Phila Pa 1976) 11: 942-943.
- Jurgensmeier D, Hart R (2010) Variability in tissue bank practices regarding donor and tissue screening of structural allograft bone. Spine (Phila Pa 1976) 35: E702-707.
- Heary RF, Parvathreddy N, Sampath S, Agarwal N (2017) Elastic modulus in the selection of interbody implants. J Spine Surg 3: 163-167.
- Phan K, Hogan JA, Assem Y, Mobbs RJ (2016) PEEK-Halo effect in interbody fusion. J Clin Neurosci 24: 138-140.
- Cheng A, Cohen DJ, Boyan BD, Schwartz Z (2016) Laser-sintered constructs with bio-inspired porosity and surface micro/nano-roughness enhance mesenchymal stem cell differentiation and matrix mineralization in vitro. Calcif Tissue Int 99: 625-637.
- Cheng A, Cohen DJ, Kahn A, Clohessy RM, Sahingur K, et al. (2017) Laser sintered porous Ti-6Al-4V implants stimulate vertical bone growth. Ann Biomed Eng 45: 2025-2035.
- Cheng A, Humayun A, Boyan BD, Schwartz Z (2016) Enhanced osteoblast response to porosity and resolution of additively manufactured Ti-6Al-4V constructs with trabeculae-inspired porosity. 3D Print Addit Manuf 3: 10-21.
- Wang X, Gittens RA, Song R, Tannenbaum R, Olivares-Navarrete R, et al. (2012) Effects of structural properties of electrospun TiO2 nanofiber meshes on their osteogenic potential. Acta Biomater 8: 878-885.
- Evans NT, Torstrick FB, Lee CS, Dupont KM, Safranski DL,et al. (2015) High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater 13: 159-167.
- Evans NT, Irvin CW, Safranski DL, Gall K (2016) Impact of surface porosity and topography on the mechanical behavior of high strength biomedical polymers. J Mech Behav Biomed Mater 59: 459-473.
- Evans NT, Torstrick FB, Safranski DL, Guldberg RE, Gall K (2016) Local deformation behavior of surface porous polyether-ether-ketone. J Mech Behav Biomed Mater 65: 522-532.
- Torstrick FB, Evans NT, Stevens HY, Gall K, Guldberg RE (2016) Do surface porosity and pore size influence mechanical properties and cellular response to PEEK? Clin Orthop Relat Res 474: 2373-2383.
- Torstrick FB, Lin ASP, Gall K, Guldberg R, editors Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating in PEEK: in vitro and in vivo analysis. Orthopaedic Research Society (ORS) Annual Meeting; 2017; San Diego, CA, USA.
- Torstrick FB, Safranski DL, Burkus JK, Chappuis JL, Lee CS, et al. (2017) Getting PEEK to stick to bone: The Development of porous PEEK for interbody fusion devices. Techniques in Orthopaedics 32: 158-166.
- Burkus JK (2018) Early outcomes of anterior cervical discectomy and fusion using a porous PEEK interbody fusion device. J Spine Neurosurg 7(2).
- Connolly PJ, Esses SI, Kostuik JP (1996) Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord 9: 202-206.
- Vernon H, Mior S (1991) The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther 14: 409-415.
- Mroz TE, Lin EL, Summit MC, Bianchi JR, Keesling JE Jr, et al. (2006) Biomechanical analysis of allograft bone treated with a novel tissue sterilization process. Spine J 6: 34-39.
- Cho DY, Liau WR, Lee WY, Liu JT, Chiu CL, et al. (2002) Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery 51: 1343-1349.
- Yson SC, Sembrano JN, Santos ER (2017) Comparison of allograft and polyetheretherketone (PEEK) cage subsidence rates in anterior cervical discectomy and fusion (ACDF). J Clin Neurosci 38: 118-121.
- Torstrick FB, Klosterhoff BS, Westerlund LE, Foley KT, Gochuico J, et al. (2018) Impaction durability of porous polyether-ether-ketone (PEEK) and titanium-coated PEEK interbody fusion devices. Spine J 18: 857-865.
- Carpenter RD, Klosterhoff BS, Torstrick FB, Foley KT, Burkus JK, et al. (2018) Effect of porous orthopaedic implant material and structure on load sharing with simulated bone ingrowth: A finite element analysis comparing titanium and PEEK. J Mech Behav Biomed Mater 80: 68-76.
- Seaman S, Kerezoudis P, Bydon M, Torner JC, Hitchon PW (2017) Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J Clin Neurosci 44: 23-29.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi