Research Article, J Pharm Drug Deliv Res Vol: 13 Issue: 1
Development and Characterization of Prednisolone Microspheres for the Treatment of Ulcerative Colitis
Mribha Manandhar1, Sajan Maharjan2* and Beny Baby3
1SR Drug Laboratories Pvt Ltd, Satungal, Kathmandu, Nepal
2Department of Pharmaceutical Sciences, CiST College, Kathmandu, Nepal
3Department of Pharmaceutics, Karnataka College of Pharmacy, Bangalore, India
*Corresponding Author: Sajan Maharjan
Department of Pharmaceutical Sciences, CiST College, Kathmandu, Nepal
E-mail: maharjansajan02@gmail.com
Received date: 24 April, 2023, Manuscript No. JPDDR-23-94873;
Editor assigned date: 27 April, 2023, PreQC No. JPDDR-23-94873 (PQ);
Reviewed date: 11 May, 2023, QC No. JPDDR-23-94873;
Revised date: 27 December, 2023, Manuscript No. JPDDR-23-94873 (R);
Published date: 03 January, 2024, DOI: 10.4172/2325-9604.1000255
Citation: Manandhar M, Maharjan S, Baby B (2024) Development and Characterization of Prednisolone Microspheres for the Treatment of Ulcerative Colitis. J Pharm Drug Deliv Res 13:1.
Abstract
The aim of this study was to formulate a microspheres of prednisolone sodium phosphate with a controlled release of the drug. The FTIR and DSC spectra revealed that there was no interaction between polymers and drug. Microspheres of prednisolone sodium phosphate were successfully prepared using chitosan and sodium tripolyphosphate polymers by ion gelation method. The percentage yield of all microspheres increased as the amount of polymer was decreased in each preparation method. The entrapment efficiency was good in all the cases. The particle size of optimized formula was 35.5 μm. SEM analysis of the optimized formula revealed that the formulation was spherical with smooth surface. In vitro release of prednisolone sodium phosphate decreased as concentration of polymer increased. Stability studies for two months revealed that the formulation was stable in 40°C ± 2°C and 75% ± 5% RH. Hence, the prepared microspheres of prednisolone sodium phosphate may prove to be potential candidate for safe and effective controlled drug delivery over an extended period of time.
Keywords: Microspheres; Prednisolone sodium; Controlled drug delivery; Ulcerative colitis
Introduction
Colon specific drug delivery system must be capable of releasing the drug into the colon i.e. drug release and absorption should not occur in the stomach as well as intestine, neither the bioactive agent should be degraded in either of the dissolution site but only released and absorbed once reaches to the colon [1].
Microspheres are one of the most useful devices to deliver materials in an effective, prolonged and safe manner. Microspheres offer a novel approach for developing sustained release drug delivery system that has potential for colonic drug delivery [2].
Prednisolone sodium phosphate is a corticosteroid drug useful for the treatment of a wide range of inflammatory and autoimmune conditions such as ulcerative colitis and Crohn's disease. It suppresses inflammation by blocking the early manifestations of inflammation, including enhanced vascular permeability, vasodilation, and infiltration by neutrophils as well as the later consequences of inflammation, including fibroblast activation, vascular proliferation, and deposition of collagen It has an intermediate duration of action allowing once daily dosing [3].
The use of microspheres in preference to single unit dosage forms for colon drug delivery showed that microspheres enabled the drug to reach the colon quickly and were retained in the ascending colon for relatively long period of time. It also reduces dosing frequency, the drug discharge in the stomach and reduces the local unwanted effects [4].
Materials and Methods
Pre-formulation studies
Solubility determination: The solubility of the selected drug was determined in distilled water, pH 7.4, pH 6.8 and 0.1 N HCl using standard method [5].
Melting point determination: Melting point of the drug was determined by taking a small amount of drug in a capillary tube closed at one end and was placed in Thiel’s melting point apparatus and the temperature at which the drug melts was noted. Average of triplicate readings was taken [6].
Drug excipient compatibility studies: The pure drug and its formulation were subjected to IR studies. In the present study, the potassium bromide disc (pellet) method was employed [7].
Preparation of microspheres: Prednisolone sodium phosphate microspheres were prepared by ion gelation method. An accurately amount of prednisolone sodium phosphate was dissolved in 5 ml of water using sonication for 5 minutes. Specified amount of chitosan were dispersed in aqueous solution of glacial acetic acid and stirred by magnetic stirr for 1 hr the aqueous solution of drug is poured slowly into the aqueous solution of glacial acetic acid containing chitosan with continuous stirring and 20 mg of sodium benzoate is dissolved in 2.5 ml of water which is used as preservative and transfer to the formulation with continuous stirring for 1 hr at 600 rpm and different concentration of TPP solution was transfer to the formulation [7]. After that it starts to form particles which are homogenized at 4000 rpm to breakdown the particles into micro form. Micro particles were dried by using freeze bed drier (lyophilizes) at 0.320 pressures at -105°C for 3 days (Table 1) [8].
| Formulation code | Drug (mg) | Chitosan (mg) | Sodium tripolyphosphate (%) | Glacial acetic acid (5% v/v) (ml) | Sodium benzoate (mg) |
|---|---|---|---|---|---|
| F1 | 500 | 500 | 0.5 | 100 | 20 |
| F2 | 500 | 1000 | 1 | 100 | 20 |
| F3 | 500 | 1500 | 1.5 | 100 | 20 |
| F4 | 500 | 2000 | 2 | 100 | 20 |
| F5 | 250 | 500 | 0.5 | 100 | 20 |
| F6 | 250 | 1000 | 1 | 100 | 20 |
| F7 | 250 | 1500 | 1.5 | 100 | 20 |
| F8 | 250 | 2000 | 2 | 100 | 20 |
Table 1: Formulation chart of prednisolone sodium phosphate microspheres.
Evaluation parameters
Scanning Electron Microscopy (SEM): Scanning electron microscopy was carried out for formulation dry microspheres were placed on an electron microscope brass stub coated with gold in an ion sputter. Then picture of microspheres were taken by random scanning of the stub [8]. The SEM analysis of the microspheres was carried out by QUNTA-200 FEI (Netherland) using analytical scanning electron microscope. The microspheres were viewed at an accelerating voltage of 20 KV [9].
Particle size analysis: Particle size were performed on a Malvern Zetasizer 3000 instrument (Malvern instrument Ltd, Malvern UK) at 25°C and at a scattering angle of 90°C to obtain the size of microspheres [10].
Percentage yield: Microspheres after drying were weighed to calculate the percentage yield of microspheres using the following formula [11].
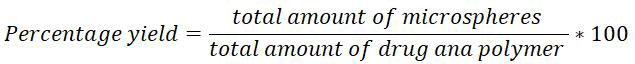
Drug content and entrapment efficiency: Accurately weighed microspheres equivalent to 100 mg of prednisolone sodium phosphate, were suspended in a 100 ml of phosphate buffer pH 7.4 and kept for 24 hrs and sonicated for 30 minutes and it was filtered and analyzed by using UV-Visible spectrophotometer after suitable dilution at 246 nm [12].
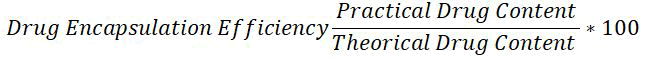
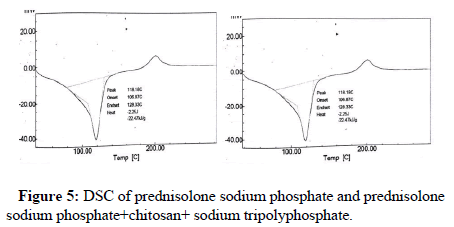
Differential Scanning Calorimetry (DSC): A differential scanning calorimetry (JADE DSC Perkin Elmer, USA) was used to study the thermal analysis of drug excipient compatibility. Firstly binary mixtures of prednisolone sodium phosphate and drug excipient mixture was scanned in the temperature range of 100°C-200°C under an atmosphere of nitrogen. The heating rate was 20°C/min and the obtained thermograms were observed for any type of interaction [13].
Degree of swelling: The swellability of microspheres in physiological media was determined by allowing the microspheres to swell in the phosphate buffer pH 7.4. 100 mg of accurately weighed microspheres were immersed in little excess of phosphate buffer pH 7.4 for 24 hrs [14]. The degree of swelling was determined by using the following formula:
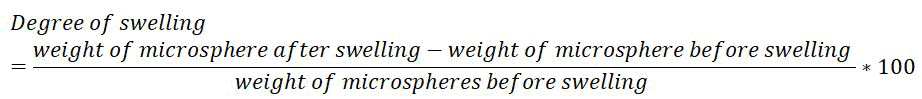
In vitro release studies: In vitro release study of microspheres was performed in pH progression medium using paddle type dissolution apparatus. First 900 ml of 0.1 N HCl, was used as dissolution media maintained at 37°C ± 0.5°C respectively, and the paddle was rotated at a constant speed of 100 rpm for 2 hrs [10]. Accurately weighed amount of microspheres equivalent to 40 mg were placed. After 2 hrs the same sample was place in 900 ml of phosphate buffer pH 7.4 and was rotated at a constant speed of 100 rpm for 10 hrs. Aliquots of sample were withdrawn at the interval of 1 hour. The samples withdrawn were filtered, diluted suitably and analyzed at 247 nm spectrophotometrically for drug release [15].
Kinetics release study: To analyze the mechanism of the drug release rate kinetics of the dosage form, the data obtained were plotted as:
• Cumulative percentage drug released vs. time (in vitro release plots).
• Cumulative percentage drug released vs. square root of time (Higuchi‘s
plot).
• Log cumulative percentage drug remaining vs. time (first order plots).
• Log percentage drug released vs. log time (Peppas plots) [16].
Validation of the model
The model was validated using ANOVA calculation, and then the estimation pure error was done. The variance of these observations pooled over all to get an estimate of pure error of variance. The F-test on regression and lack of fit was used for judging descriptive properties of a model and the significance of model terms [11].
Predictions using the selected model
Once a model was selected and validated, the brute force method was applied for the prediction of response. With the help of 3D response surface or a 2D contour diagram, the prediction was done using these graphics either by grid search or feasibility search methods.
Stability studies: The selected formulations were packed in amber colored bottles, which were tightly plugged with cotton and capped with aluminum. They were then stored at 40°C/75% RH for 30 days and evaluated for their drug content and in vitro dissolution study [17].
Results and Discussion
Solubility study
Prednisolone sodium phosphate was found to be slightly soluble in 0.1 N HCl, freely soluble in water and phosphate buffer pH 7.4 (Table 2).
| S. No. | Solutions | Solubilities |
|---|---|---|
| 1 | Water | 0.45 ± 0.12 µg/ml |
| 2 | 0.1 NHCl | 0.21 ± 0.12 µg/ml |
| 3 | pH 6.8 | 0.35 ± 0.12 µg/ml |
| 4 | pH 7.4 | 0.42 ± 0.12 µg/ml |
Table 2: Solubility studies.
Melting point
The melting point of the obtained drug was found to be 215°C which is found to be within reported range of 210°C-220°C. It complies with the standards thus indicating the purity of the drug sample [12].
Compatibility study by FTIR
FTIR spectra of pure drug, polymer and their physical mixtures (stored at 40°C ± 2°C/75% ± 5% RH for 2 months) were recorded. The drug, polymer and physical mixtures of drug and polymers were scanned for absorbance (Figure 1). The spectra obtained from the physical mixtures showed all the principal peaks at or around the requisite wave number of pure drug [13]. Thus it may be inferred that there was no interaction between drug and polymer, the purity and integrity of drug was maintained in the physical mixtures (Figure 2).
Evaluation parameters
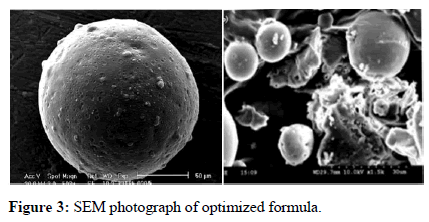
Scanning electron microscope: From the SEM photographs, it can be concluded that microspheres were spherical in shape with smooth surface (Figure 3).
Percentage yield
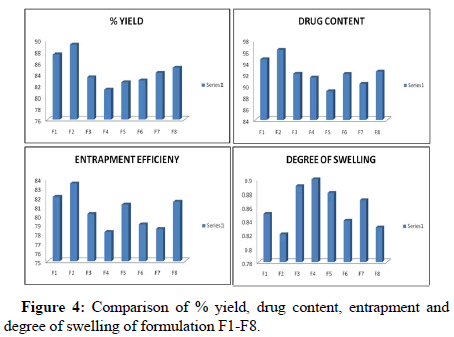
The percentage yields of microspheres prepared by ion gelation method were found to be 83.45 to 89.20. It was found that the percentage yield of microspheres prepared as F2 formulation showed the greater percentage yield than the other prepared concentrations [14].
Drug content
The drug content of microspheres prepared by ion gelation method were found to be 89.02% to 96.30%. It was found that the drug content of microspheres prepared as F2 formulation showed the greater percentage yield than the other prepared concentrations [15].
Entrapment efficiency
The entrapment efficiency were in the range 78.19% to 83.52%. It showed that entrapment efficiency decreases with increase in drug to polymer ratio.
Degree of swelling
The degree of swelling was in the range 78.19% to 83.52%. It showed that degree of swelling increases with increase in drug to polymer ratio (Figure 4).
Differential scanning calorimetry: In DSC studies melting peak appeared at 118.18°C for prednisolone sodium phosphate. There was no change in melting point of binary mixture of prednisolone sodium phosphate, chitosan and sodium tripolyphosphate which indicate that there is no interaction between drug and polymers (Figure 5).
In vitro drug release
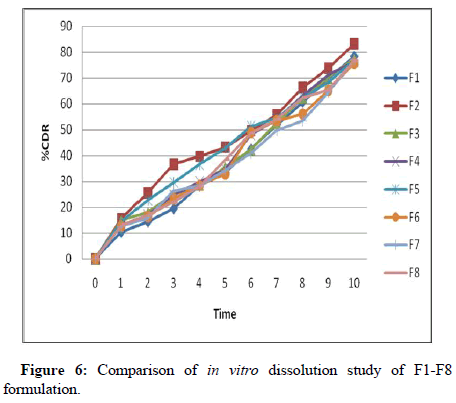
The in vitro release of prednisolone sodium phosphate from the prepared microspheres formulation was studied in phosphate buffer pH 7.4 for 10 hours. As the concentration of polymer increased, the drug release also decreased proportionally. This may be because chitosan retards release to more extent. Chitosan permits a protection for therapeutic agent from the hostile conditions of the upper gastrointestinal tract and release the entrapped agent specifically at the colon through degradation of the glycosidic linkages of chitosan by colonic microflora [16]. It forms the stable complexes with sodium tripolyphosphate. Controlled drug delivery systems follow several mechanisms and it is still ambiguous. Here it is followed either purely diffusion or erosion controlled (Figure 6).
Kinetics of drug release
The drug release data was fitted into the different models like korsmeyer peppas, zero order and Higuchi equation. It suggests that the release of drug from the formulations may follow any one of these models. The r2 values of zero order of all the formulations have shown higher value which indicates the drug release is directly proportional to time which means release of prednisolone phosphate sodium follows zero order. But n values range from 0.764 to 0.926 which indicate non-Fickian diffusion mechanism.
Optimization
In the numerical optimization techniques, the desirability approach was used to generate the optimum settings for the formulation (Table 3). For the optimized formulation the drug release at 1st hr the drug release at 5th hr and drug release at 10th were kept at minimum and % yield, entrapment efficiency were kept maximum. The composition of optimized formula for microsphere is prednisolone sodium phosphate (500 mg), chitosan (1000 mg), sodium tripolyphosphate (1%). The optimized formulation was prepared according to predicted model and evaluated for responses.
| Ingredients | Quantity |
|---|---|
| Drug (mg) | 500 |
| Chitosan (mg) | 1000 |
| Sodium tripolyphosphate (%) | 1 |
| Glacial acetic acid (ml) | 100 |
Table 3: Optimized formula (F9).
Evaluation of optimized prednisolone sodium phosphate microsphere drug content: The drug content of the formulation F9 was found to be 97.8%.
Particle size analysis: The particle size of formulation F9 was found to be 35.5 μm (Table 4).
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 0 | 16 | 26.1 | 37.6 | 40.6 | 44.9 | 51.1 | 57.8 | 67.6 | 75.2 | 84.3 |
Table 4: In vitro release studies for optimized formula.
Stability studies
These studies revealed that formulation F9 was stable in drug content; there was no change in the physical appearance of the formulation and in vitro release after storage for the two months at 40°C ± 2°C and 75% ± 5% RH (Table 5). The drug content of the formulation F9 stored at 40°C ± 2°C and 75% ± 5% RH was 94.89% and 94.32% drug release at 10th hr of the formulation F9 stored at 40°C ± 2°C and 75% ± 5% RH showed 83.67% and 82.87%.
| Time | % CDR at 1st hr | % CDR at 5th hr | % CDR at 10th hr | Drug content % | Entrapment efficiency |
|---|---|---|---|---|---|
| Initial | 15.65 | 43.23 | 84.12 | 95.32 | 82.94 |
| 1st month | 14.23 | 42.89 | 83.67 | 94.89 | 81.78 |
| 2nd month | 13.29 | 42.11 | 82.87 | 94.32 | 81.23 |
Table 5: Stability studies of formulation F9.
Conclusion
The present study has been a satisfactory attempt to formulate microspheres of prednisolone sodium phosphate with controlled release of the drug. Microspheres of prednisolone sodium phosphate were successfully prepared using chitosan and sodium tripolyphosphate polymers by ion gelation method. The particle size of optimized formula was in the range i.e. 35.5 μm. Hence, the prepared microspheres of prednisolone sodium phosphate may prove to be potential candidate for safe and effective controlled drug delivery over an extended period of time.
References
- Qureshi AM, Momin M, Rathod S, Dev A, Kute C (2013) Colon targeted drug delivery system: A review on current approaches. Indian J Pharm Biol Res 1:130-147.
- Dashora A, Jain CP (2009) Development and characterization of pectin-prednisolone microspheres for colon targeted delivery. Int J Chem Tech Res 1:751.
- Sharma H, Sharma K. Principles of Pharmacology. Paras Medical Publisher, 2007.
- Alagusundaram M, Chetty MS, Umashankari K, Badarinath AV, Lavanya C, et al. (2009) Microspheres as a novel drug delivery system: A review. Int J Chem Tech Res 1:526-534.
- Wong T, Lee H, Chan L, Heng P (2002) Drug release properties of pectinate microspheres prepared by emulsification method. Int J Pharm 2242:233-237.
[Crossref] [Google Scholar] [PubMed]
- Roy S, Panpalia SG, Nandy BC, Rai VK, Tyagi LK, et al. (2009) Effect of method of preparation on chitosan microspheres of mefenamic acid. Int J Pharm Sci Drug Res 1:36-42.
- Chandran S, Sanjay KS, Asghar LF (2009) Microspheres with pH modulated release: Design and characterization of formulation variables for colonic delivery. J Microencapsul 26:420-431.
[Crossref] [Google Scholar] [PubMed]
- Berthold A, Cremer K, Kreuter JS (1996) Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for anti-inflammatory drugs. J Control Release 39:17-25.
- Bhusnure OG, Gholve S, Manoj B, Todkar V, Giram PS (2015) Analytical method development and validation of prednisolone sodium phosphate by Qbd approach. IOSR J Pharm Biol Sci 10:64-75.
- Sheth Z MS, Singh M, Gupt M (2010) Design and development of 5-Fluorouracil loaded biodegradable microspheres. Int J Ayurveda Pharm 160-168.
- Ganesh N (2011) Comparative evaluation of lornoxicam microspheres using natural and synthetic polymers. J Pharm Res 4:3212-3215.
- Parashar V, Ahmad D, Gupta SP, Upmanyu N, Parashar N, et al. (2010) Formulation and evaluation of biodegradable microspheres of tinidazole. Int J Drug Deliv. 2:238-241.
- Paharia A, Yadav AK, Rai G, Jain SK, Pancholi SS, et al. (2007) Eudragit-coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS PharmSciTech 8:E87-E93.
- Kushwaha P, Fareed S, Nanda S, Mishra A (2011) Design and fabrication of tramadol HCL loaded multi particulate colon targeted drug delivery system. J Chem Pharm Res 3:584-595.
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan based micro and nanoparticles in drug delivery. J Control Release 100:5-28.
[Crossref] [Google Scholar] [PubMed]
- Srivastava AK, Ridhurkar DN and Wadhwa S (2005) Floating microspheres of cimetidine: Formulation, characterization and in vitro evaluation. Acta Pharm 55:277-285.
[Google Scholar] [PubMed]
- Navneet G, Akanksha G and Neetesh J (2011) Formulation design and in vitro evaluation of metformin microspheres using ionotropic gelation technique. J Pharm Res 4:2103-2106.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi