Research Article, J Nucl Ene Sci Power Generat Technol Vol: 9 Issue: 1
Determination of Levels of Radioactivity of Uranium and Radium in Environmental Samples by Liquid Scintillation Counting and Alpha Spectroscopy
AM Abdelmonem1,2*, MA Abd El-Samad3,4, HA Hanafi4,5, AMH Ibrahim4,6 and Mohamed Abbas1,7
1Department of Physics, Jouf University, Al-Jouf, KSA
2Reactor Physics Department, Laboratories for Detection of Landmines and Illicit Materials, Nuclear Research Center, Atomic Energy Authority, Cairo, Egypt
3Radioactive Environmental Pollution Department, Hot Laboratory Center, Atomic Energy Authority, Egypt
4College of science and Humanities - Alquwayiyah-Shaqra University, KSA
5Cyclotron Project, Nuclear Research Center, Atomic Energy Authority, Cairo, Egypt
6Nuclear Fuel Technology Department, Atomic Energy Authority, Cairo, Egypt
7Physics Department, Ain Shams University, Egypt
*Corresponding Author : AM Abdelmonem, Department of Physics
Faculty of Science and Arts, Jouf University, Al-Jouf, KSA
E-mail: ashrafkhater72@yahoo.como.com
Received: February 13, 2020 Accepted: February 29, 2020 Published: March 05, 2020
Citation: Abdelmonem AM, El-Samad MAA, Hanafi HA, Ibrahim AMH, Abbas M (2020) Determination of Levels of Radioactivity of Uranium and Radium in Environmental Samples by Liquid Scintillation Counting and Alpha Spectroscopy. J Nucl Ene Sci Power Generat Technol 9:1. doi: 10.4172/2325-9809.1000191
Abstract
The present study aims to use the α-spectrometry using silicon surface-barrier detector Si (Li), as a radiometric technique to estimate U-isotopes and Ra-226 that were carried out for two rock samples from Gabal Gattar, north-eastern desert area, Egypt and two references soil samples of international atomic energy agency, IAEA. A secular equilibrium between U-238 and U-234 and disequilibrium between U-238 and Ra-226 were observed. There is no significant change of radioactivity levels of U-238, U-234 and Ra-226; this means that there is secular equilibrium between U-238 and its relative daughters. Specific dose equivalent rate of sample #1 equal about three times the corresponding value of sample #2 and doses equivalent rate for both two samples are negligible after about 6 cm without shield.
Keywords: Uranium isotopes; α-spectrometry; Environmental rock sample; Liquid scintillation spectrometry
Introduction
Liquid Scintillation Spectrometry (LSS) was one of the first techniques used for the detection of ionizing radiation. This technique is based on the detection of the pulses of light that are emitted by some substances when ionizing radiation passes through them [1]. A simple method to determine the isotopic composition of uranium in a series of standard sources is by alpha spectroscopic measurements, using silicon surface-barrier detectors. Alpha spectroscopy was also employed to determine the isotopic composition of uranium, protactinium, and thorium in different matrix materials [2]. Evaluate the capability of using barium as a carrier for Ra-226 determination and estimated the amount added during the radiochemical separation from rock samples. The activity concentration of Ra-226 was estimated in rock samples using LSS using its short-lived daughters after radiochemical separates [3]. The chemical yields of different metal isotopes were determined by alpha spectroscopy and isotopic dilution, respectively. The results showed that uranium in pitchblende minerals is in equilibrium with its relative daughters [4,5].
Alpha spectrometry, finds also several applications using the determination of the specific activity of different uranium isotopes, separation chemistry of α-emitter tracer uranium-232 as a tracer, and in preparation of α-sources for nuclear measurements [6]. The determination of radon-222 is also important in radium-226 analysis and lead-210 dating since the inhalation of radon-222 is a health hazard. This was made by using spiked solutions for yield determinations and using a silicon surface-barrier detector for alpha spectrometry [5]. The different factors affecting the quantitative separation of radium-226 by electrodeposition with the alpha-particle spectrometry to determine the radioactivity levels of radium-226 separated under different conditions are studied [7].
Different geological and other environmental samples containing uranium are regularly investigated in the laboratory for environmental radioactivity of the Physikalisch-Technische-Bundesanstalt, Germany, through active participation in several international comparisons runs, related to determine different uranium isotopes in environmental samples and the framework of certified reference materials for uranium concentration and related isotopic composition [6]. A simple method for analyzing alpha spectra from natural and enriched or depleted uranium samples were developed, considering low-energy tail and branching-ratio corrections for accurate calculation of peak area corresponding to each uranium isotopes [8,9].
For the measurements of natural radioactivity of rock samples, it is of interest to have a system with high counting efficiency. Since alpha liquid scintillation spectrometry has been counting in 4 π geometry, it occupies a prestigious benefit in this field. Liquid Scintillation Counting (LSC) is the main technique for the measurement of pure beta, alpha emission and electron capture emitting radionuclides. The main advantage of this technique is that it has a very low background with high resolution and can be used for measuring very low radioactivity of alpha-emitting radionuclides. LSC has been sure practically to be a quick, multi-use, efficient and accurate instrument for radiometric investigation. Gross alpha and beta, radium and uranium isotopes activity can be estimate with easy procedures and quite sensitivity can be tailored to ensure reasonable agreement with other estimated levels. By preparing a thin source to determine the mixture of alpha-emitting nuclides, Alpha spectroscopy was used for both qualitative and quantitative measurements with a high degree of precision. It is used also to determine the radioactivity levels of uranium isotopes, uranium-234, uranium-235, and uranium-238 in environmental samples. It is a conventional radiometric technique, but still, a competitive method compared to mass spectrometry especially for short-lived radionuclides. The disadvantage of this system is that it needs very precise thin source preparation; it needs long counting periods for getting precise results with low uncertainty.
The present study aims to use the α-spectrometry, for analysis of U-isotopes and Ra-226 that were carried out for two rock samples from Gabal Gattar northeastern desert area, Egypt and two reference soil samples of IAEA. Estimation of natural terrestrial radiation is necessary from both radioactivity and environmentally points of interest and must be considered. The locally derived soils/rocks usually indicated the U-content of country rocks. The excess exposure to the ionizing radiations such as α, β, and γ-rays emitted from the radioactive material may lead to some health risks, based on the fact that the soil-plant-living organisms are recognized as one of the most impactful pathways for transfer of the radionuclides to organism's body [10].
Experimental Details
Alpha spectroscopic measuring system description, energy calibration, and efficiency are presented.
Alpha spectroscopic measuring system
Alpha spectroscopic measuring system is a technique commonly used for determining most alpha-emitting radionuclides through calibration of the measuring system to determine the counting efficiency at specific geometry. The nuclear spectroscopic setup used for recognition of different uranium isotopes and subsequent measurement of their radioactivity levels, nuclides a Si (Li) detector, model 7401 for Canberra industries; Inc. Meriden, Connecticut, USA; Situated in a vacuum chamber connected to vacuum pump model 7401, located the rear paneled the rear panel. The system includes a unified preamplifier connection terminal for different signal transmission. Figure 1 illustrates a block diagram of the whole α- spectroscopic system. The efficiency of use detector is about 25% for detector- source spacing of less than 10 mm and energy resolution ≤ 20 KeV at FWHM with the detector-source spacing equal to the detector diameter, based on the use of two sources of standard americium-241. The electronic system consists of bias supply ranging from 1-198 V, DC with positive polarity and an amplifier connected with pulse pile up register to keep the noise of the whole setup less than 5 mV.

Figure 1: Block diagram of the whole α- particle spectroscopic system.
Energy and efficiency calibration of α-spectroscopy: Energy calibration of the Si (Li) spectroscopic system was established by determining the position of alpha particle peak, selected for full energy of the largest peak height to background ratio (the region of interest, ROI). For this purpose, two certified reference sources of Am-241 and natural Uranium were measured. α-energy line of 5.5 MeV, 4.19 MeV, 4.39 MeV and 4.77 MeV were evaluated for Am-241, U-238, U-235, and U-234, respectively.
To keep constant geometry, both energy calibration standard sources and sample sources were positioned at the same distance from the detector surface the counting efficiency of the used detector and the geometrical measuring condition.
In the present work, for the determination of the α-counting efficiency of the used detector, two ultrathin certified standard sources of Am-241(t1/2=433y) with different radioactivity levels were measured at a different distance from the used detector.
Table 1 represents the radioactivity levels of the two Am-241 sources corresponding to 4151 and 3867 Bq at the production date (July 1999).
Table 1: Change in counting efficiency of used system with distance between measured source and used source detector; 4122 and 3837 Bq, respectively.
The counting efficiency of the used system (ε) depends on several factors, including those represented in equation (1).
ε=R/A* I (1)
Where R: count rate, A: The absolute disintegration rate, I: The relative intensity of the concerned energy line.
The percent, counting efficiency for specific geometrical conditions ε, can thus be calculated under each geometry in terms of the measured count rate R, corresponding calculated absolute radioactivity levels A and the relative intensity I of americium-241 α-line at 5.5 MeV (I=0.84). Counting efficiency is inversely proportional to (detector- source) separating distance.
Liquid Scintillation Counting system, LSC, is a type of nuclear spectroscopic technique used to determine the low radioactivity level or both α and β emitting radionuclides. These instruments have been classified as low-level instruments because their background has been reduced and the minimum detectable activity can be less than one Bq/L of water. Low-level LSC is now commonly used for measurements of natural series radionuclides such as U-238, Ra-226, and Th-232. The following part will illustrate the instrumental and calibration of the LSC model Tri-Carb supplied from CANBERRAPACKARD USA.
Instrumentation
Time-Resolved Liquid Scintillation Counter, TR-LSC, is a potential electronic technique used to reduce background count rates, by discrimination out the unquenchable component (originating outside the cocktail) of background from true beta events and the quenchable background based on the number of pulses which follow prompt pulse events. The theory of LSC counting is mainly depending on the interaction of radiation that comes from α and/or β emitting radionuclides with the organic cocktail (organic materials that produce scintillation or pulse after interaction). The photoelectrons from photocathodes are multiplied by using Photomultiplier Tube (PMT) and counted through digital or computer systems. The number of pulses is directly related to the number of particles that are emitted from the radionuclides.
Calibration of working system: Calibration of LSC includes counting region optimization, cocktail choice, alphabeta discrimination and counting efficiency determination.
Cocktail choice: Liquid Scintillators should be prepared with reagents that are low in natural radioactivity. Organic cocktails should have a low background and high sample loading capacity. In the present work, a universal α-β organic scintillator was used (ultima GOLD AB) for acidic samples.
Alpha-beta discrimination: To optimize alpha/beta separation performance, it is essential to determine the correct Pulse Decay Discriminator (PDD). On the Packard Tri-Carb model, with alpha/beta discrimination, the optimum setting is the setting where there is an equal and minimum spill of alpha pulses into the beta MCA and beta pulses into the alpha MCA. In the present work two standards of both Am-241, (alpha emitter, which produces alpha particles in the 5.4 to 5.5 MeV, and Cl-36 (beta emitter) which has Emax of 710 KeV placed in Packard Ultima Gold AB cocktail were measured. The instrument determines the optimum setting those results in the minimum misclassification of alpha and beta activity and will generate the presence of a misclassification plot on demand.
Counting efficiency of LSC for Ra-226 measurements: The 4π counting geometry of LSC results in approximately 100% counting efficiency for alpha-emitting radionuclides, coupled with low alpha background count rates. In the present work, three standards of Radium-226 with different radioactivity levels (1.7 Bq/ml) prepared in 0.1 HNO3, and 2 ml of each were transferred to suitable vials and 15 ml of organic cocktail (α-β insta-Gel) was added to each one. With slight mixing, the three vials containing standards and cocktails were measured against quenched blank using low-level counting mode. The counting time was optimized to be 20 min/cycle (10 cycles were performed).
Sample preparation: Rock samples preparation were carried out by different sequential step including leaching, spiking, and acid digestion. Several steps of sample preparation, radiochemical separation, and source preparation were performed before analysis. 2 mL of Ra-226 standard (1.7 Bq/mL) prepared in 0.1 HNO3 was transferred to a glass vial and then 15 mL of organic cocktail (Ultima GOLD AB) was added with gentle mixing until a homogeneous clear solution appeared. The vial containing both Ra-226 standard and the organic cocktail was introduced to the counting room of Tri-carb. LSC and measured against blank (2 ml water in 0.1 HNO3+15 ml organic cocktail) using low-level counting mode, and counting time 20 min/ cycle (10 cycles is used to get a minimum counting error 2.0%). The counting efficiency of the detector was determined based on the net count rate of U-232 radiotracer at energy 5.34 MeV and its activity. The separation process of uranium was performed using UTEVA column conditioned by 0.1 M of ammonium oxalate or deionized water. Two rock samples from Gabal Gattar were chosen because they are characteristics of their high activity concentration. Rock samples preparation was carried out by different sequential steps including aching, spiking and acid digestion. Figure 2 illustrates a geological map of Gabal Gattar area, Northeastern Desert, Egypt.

Figure 2: Geological map of Gabal Gattar area, north eastern desert, Egypt.
Leaching and spiking: Leaching of samples is very important in dealing with radioisotopes. The leaching step is essential to destroy the organic material and to keep the radioisotopes that have relatively low evaporation temperature. Tables 2 and 3 illustrate the specific data of both analyzed samples and radiotracers, respectively, concerning half lifetime, main energy in KeV, major intensity and mode of decay. Complete leaching of each sample was recorded after getting a constant weight reading. Each sample was spiked with 100 μL of Barium-133radiotracer (410 Bq/ml), 20 mg of barium carrier, 200 μL of uranium-232 (5.561 dpm/300 μL). Table 2 main nuclear characteristics of some radionuclides of interest [11].
Table 2: Main nuclear characteristics of some radionuclides of interest.
Table 3: Specific data of radiotracers for analysis.
Sample digestion: A representative weight of each sample was heated with 12.5 ml of 40% HF in Teflon beaker until dryness. Another 6 ml of 40% HF and 12 ml of 70% HNO3 were added and evaporated to dryness. 10 mg of Ba carrier as Ba nitrate followed by 20 ml of 70% HNO3 twice were added to the mixture and evaporation to dryness. 0.5 g of boric acid and 15 ml of HCl were added to remove the excess of HF and dissolve CaF2 precipitate that might be formed during the digestion process. The sample solution was evaporated to reduce its volume to 3 mL-5 mL. The sample mixture was washed with 30 mL of 2M HCl then transferred to a centrifugal tube. Mostly clear the solution of each sample using fast filter paper. The two samples were prepared in 50 ml 3M HNO3.
Results and Discussion
Chemical recovery of Radium-226 for rock Sample #1 and #2 from Gabal Gattar area
The chemical recovery of Ra-226 in the real sample is based mainly on the net count rate of Ba-133 standard solution, and the net count rate of Ba-133 radiotracer that spiked to the analyzed samples that were measured the same geometrical condition by γ-spectroscopy. This can be illustrated according to equation (2). The data presented in Table 4 illustrate the chemical recovery of Ra-226 using non-isotopic.
Table 4: Determining the chemical recovery of Ra-226 using Non-isotopic.

Where;
C1: The net count rate of radiotracer Ba-133 at Eγ=81.5 and 356 KeV
C2: The net count rate of Ba-133 standard at Eγ=81.5 and 356 KeV
A1: The radioactivity level of radiotracer Ba-133
A2: The radioactivity level of Ba-133 standard
Determination of radioactivity level of Ra-226, two rock samples from Gabal Gattar area using LSC: The specific radioactivity level (Bq.Kg-1) of Ra-226 in the different analyzed samples is mainly depending on the chemical recovery of Ba-133 for each sample, the net count rate of Ra-226 at energy line 186 KeV with relative intensity 5% and the sample weight in g. Table 5 illustrates the specific radioactivity level of Ra-226.
Table 5: Radioactivity levels of Ra-226.
Uranium analysis
Radiochemical separation purification and quantitative determination of different uranium isotopes in rock samples #1 and #2 were estimated. The calibration efficiency of an α-spectroscopic measuring system for uranium determination was carried out using γ uranium source containing known radioactivity level of U-232 radiotracer (5.56 dpm/300 μL). The measured U-232 sources were prepared by the micro co-precipitation process using the prepared source, the counting efficiency of the detector was determined based on the net count rate of U-232 radiotracer at the energy line 5.34 MeV and the radioactivity level of U-232. The separation process of uranium was performed using UTEVA column conditioned by 0.1 M of ammonium oxalate or de-ionized water.
Uranium measurement for two rock samples from Gabal Gattar area: Determine the specific the separation and measurement procedure of Uranium isotopes in the two investigated sample was carried out by monitoring the chemical recoveries of both the spiked sample with uranium radiotracer (U-232) and the certified reference material containing known amount of the radionuclide needed to be analyzed two IAEA sample number. IAEA-313 and IAEA-314 were included and analyzed together with two environmental samples under investigation. In all cases, these samples were spiked with U-232 radiotracer (5.56 dpm/300 μL).
Uranium measurement for rock samples from Gabal Gattar area: After being prepared by electro-deposition or by micro coprecipitation were counted by α-spectroscopic measuring system. Then analyzed measurement procedure of uranium isotopes in the two investigated samples was carried out by monitoring the chemical recoveries of bath the spiked sample with uranium radiotracer (U- 232) and the certified reference material containing a known amount of the radionuclide needed to be analyzed.
In all cases, these samples were spiked with U-232 radiotracer (5.56 dpm/300 μL). The specific radioactivity levels for each uranium isotope in each sample is mainly depending on the gross area of U-232 radiotracer at energy line 5.39 MeV, its radioactivity levels ( 5.56 dpm/300μL ) and the dry weight of the sample in grams.
A comparative study was performed between the radioactivity levels of U and its daughters. The radioactivity levels of U-238 and its relative γ-emitting radionuclide daughters, U-234, and Ra-226 were presented in Table 6. For two rock samples from Gabal Gattar, the specific radioactivity levels of U-238 are in secular equilibrium with their relative daughters has been illustrated in Table 6.
Table 6: Radioactivity levels of U-238 and its α-emitting relative daughters.
It is clear to conclude that there is no significant change of radioactivity levels of U-238, U-234 and Ra-226. This means that there is a clear secular equilibrium between U-238 and its relative daughters. The data show there is a secular equilibrium between U-238 and U-234 only and Ra-226 this may be attributed to leaching of some uranium radioactivity levels of its relative daughter Ra-226 more than uranium.
Determination of isotopic composition of Uranium: Mathematical calculation were carried out to determine the isotopic composition natural uranium in two rock samples from Gabal Gattar, these calculation are principally based on the total radioactivity levels for each isotope in the respective mixture. When dealing with two radionuclides (i and j) of the same element such as uranium in the presents work the relation between their isotopic compositions in the respective element is a function of their radioactivity levels and the half-a lifetime of each radionuclide this relationship is represented according to the equation (3);

Where; I: is the isotopic composition, T: the half live and A: the radioactivity levels of respective radionuclide.
For (n) nuclides of the concerned element, their percent isotopic composition was shown by the equation (4)

Table 7 illustrates the composition of uranium isotopic in rock using by α- spectroscopy. In the case of uranium with natural isotopic composition, the radioactivity level of U-238 is almost equal to U-234 as illustrated in Tables 6 and 8. In the case of sample #1 prepared by micro co-precipitation the isotopic composition of U-238, U-235 and U-234 were found to be 99.394%, 0.60% and 0.006% with relative uncertainty 2.44%, 3.0% and 2.41%.
Table 7: Determination of the isotopic composition of uranium in rock using by α-spectroscopy.
In the case of sample #2 by micro co-precipitation the isotopic composition of U-238, U-235 and U-234 were found to be 99.36, 0.634 and 0.006 with relative uncertainty 7.68%, 6.77% and 7.25%.
The obtained isotopic composition values represent a good agreement of both source preparation methods with the reference values of Uranium. The source prepared by micro co-precipitation is more sensitive and accurate for the resolution of the U-238, U-235 and U-234 peak. Therefore, the micro co-precipitation method is applicable for calculation of the isotopic composition, especially when dealing with samples containing a high amount of Uranium.
Table 8 shows the adjustment factor for radioactivity measurements in Uranium analysis using α-spectroscopy. Table 9 shows the dependence of the specific dose equivalent rate of U-238 in rock samples on the distance. From results obtained from the alpha spectrometer for two rock samples, there is a secular equilibrium between U-238 and its relative daughters. There is a secular equilibrium between U-238 and U-234 only and there is disequilibrium between U-238 and Ra-226. This may be attributed to the leaching of some uranium contents in the soil matrix leading to higher radioactivity levels of its relative daughter Ra-226 more than uranium (Figure 3).
Table 8: Adjustment factor for radioactivity measurements in Uranium analysis by α-spectroscopy.
Table 9: The dependence of specific dose equivalent rate (μrem.hr-1.Kg-1) of U-238 in rock samples on the distance without shield.
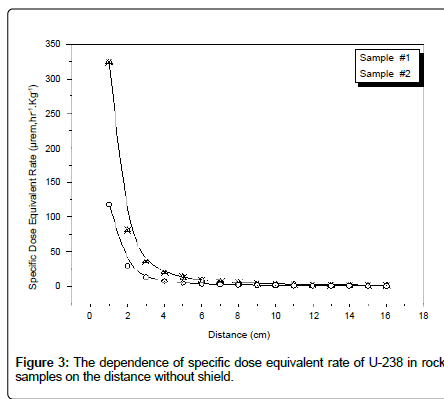
Figure 3: The dependence of specific dose equivalent rate of U-238 in rock samples on the distance without shield.
Online Rad Pro Calculator software was used to convert from specific activity (Bq.Kg-1) to a specific dose equivalent rate (μrem.hr-1.Kg-1) [12,13]. Based on γ spectrometer measurement, it is clear that value of the dose equivalent rate is negligible after about 6 cm without shield and the specific dose equivalent rate from sample #1 is about three times of sample #2, because two rock samples are taken from two different locations in Gabal Gattar area. Table 10 illustrates a comparison between α and γ spectrometer for the specific activity of U-238 in two rock samples, factor (α/γ) higher than one; this means the efficiency of α-spectrometry higher than the efficiency of γ-spectrometry. Table 11 shows that the specific radioactivity levels of U-238 for two samples were found to be in secular equilibrium with U-234.
Table 10: Comparison between α and γ spectrometer for specific activity of U-238 in two rock samples.
Table 11: Determination the radioactivity levels of Uranium isotopes in two rock samples using U-232 radiotracer by a-spectroscopy.
Conclusion
From the previous results, the following points are deduced:
1. The isotopic composition of U-238 in sample #1 and sample #2 are 99.394% ± 2.44% and 99.360% ± 7.68% and these values are matched with the reference value 99.275%
2. The isotopic composition of U-235 in sample #1 and sample #2 are 0.60% ± 3.0% and 0.634% ± 6.77% and these values are very close to the reference value 0.72%
3. The isotopic composition of U-234 in sample 1 and sample 2 are 0.006% ± 2.41% and 0.006% ± 7.25% and these values are matched with the reference value 0.005%
4. Radioactive secular equilibrium for U-238 and its relative daughters U-234 and Ra-226 this ratio were proved 1.02 and 1.074 for sample #1, and the ratio 1.09 and 0.845 for sample #2
5. Factors ratio of (α/γ) spectrometry for measuring the specific radioactivity level (Bq.Kg-1) of U-238 higher than one, this means the efficiency of α-spectrometry is high comparing with γ-spectrometry
6. The total specific activity of Uranium isotopes which is measured by α-spectroscopy for samples #1 and #2 is 82.98 nCi.Kg-1 and 28.98 nCi.Kg-1 respectively means the specific activity of sample #1 equally about three times the corresponding value for sample #2
There is a noticeable difference between the specific activities of the U-238 series in the two investigated samples; therefore, carefully surveying this area from different depths as a suggestion for future works.
Acknowledgment
The authors would like to express their thanks to prof. Yasser Lasheen for great help for measurements, discussions, and cooperation.
References
- L’Annunziata MF, Kessler MJ (2012) Liquid scintillation analysis: Principles and practice. In: L’Anninziata, M.F. (ed.) Handbook of radioactivity analysis. San Diego. Academic Press.
- ALY HF, EL-Dessouky MM (1985) Isotopic composition of Uranium, Thorium, Protactinium, and applications on dating Uranium glass, Cairo-Egypt, paper No: 74,357.
- Abdellah, WM (2019) Determination of Radium-226 in rock samples by liquid scintillation counter. Open Journal of Applied Sciences. 9: 270-284.
- Scott RD, Mackenzie AB (1984) Detection of radon-222 by use of silicon surface-barrier detectors. Int J Appl Radiat Isot 35: 301-309.
- Roman D (1984) Electrodeposition of radium on stainless steel from aqueous solutions. Int J Appl Radiat Isot. Great Britain 35: 990-999.
- Kromphorm G (1996) Determination of the activity of Uranium isotopes Uranium-234, Uranium-235 and Uranium-238 in environmental samples alpha-spectrometry. Chemistry Materials and Earth Sciences. Germany, p: 27.
- Umemto S (1967) Determination of the activity of Uranium-234/Uranium-238 in natural materials. Japan, p: 107.
- Sanchez AM, Tome FV, Bejarano JD, Vargas MJA (1992) Rapid method for determination of the isotopic composition of Uranium Samples by alpha spectroscopy. Nuc Inst Meth phy Res 219.
- Zantuti F, Al-Medehem B, Silin VL, Perwtrukhin VF (1991) Electrodeposition of actinide traces from aqueous alkaline solutions and tributyl phosphate. J Radioanal Nucl Chem Articles 147: 51-60.
- IAEA, International Atomic Energy Agency (1982) Generic models and parameters for assessing the environmental transfer of radionuclides from routine releases. Safety Series, No. 57: 96-97.
- Rytz A (1991) Recommended energy and intensity values of alpha particles from radioactive decay. At Data Nucl Data Tables 47: 205.
- Basic equations for decay, half-life, specific activity, shielding, conversions, etc. From: Gollnick, Daniel A (2006) "Basic Radiation Protection Technology, 5th Edition", Pacific Radiation Corporation.
- Gamma exposure-rate equations From: Chabot, George, CHP, PHD, “Relationship Between Radionuclide Gamma Emission and Exposure Rate”, The Health Physics Society, “Ask the Experts”.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 

