Research Article, J Regen Med Vol: 8 Issue: 2
CD133 Clinical Trials: Safety and Efficacy
Ullah M, Pocard M and Mirshahi M*
CAP-Paris Tech., INSERM U1275, Hôpital Lariboisière, Paris, France
*Corresponding Author: Massoud Mirshahi CAP-Paris Tech., INSERM U1275, Hôpital Lariboisière, Paris, France, Tel: 33664373220; E-mail: massoud.mirshahi@inserm.fr
Received: April 16, 2019 Accepted: May 16, 2019 Published: June 04, 2019
Citation: Ullah M, Pocard M , Mirshahi M (2019) CD133 Clinical Trials: Safety and Efficacy. J Regen Med 8:2.
Abstract
In human body there are cells that can proliferate and differentiate into various kinds of adult cells known as “Stem cells”. These cells express a membranous protein known as “Prominin-1” or “CD133”. CD133 exhibits 7 isoforms, distributed in different tissues of the body. The isolation and understanding of these cells helped develop an innovative and new kind of therapeutic approach called “Stem Cell Therapy”. Mutations in Prominin-1 gene are known to cause genetic disorders like Stargardt disease and retinal macular dystrophy. On the other hand, in clinical trials, CD-133+ cells are being used to treat certain diseases. These clinical trials are targeted for variety of diseases such as Duchenne muscle dystrophy, severe combined immunodeficiency syndrome, degenerative diseases like Asherman’s syndrome, cerebral palsy, liver cirrhosis and cardiovascular diseases like ischemia and myocardial infarction. CD133+ cells, owing to their stem cell properties, not only help in regenerating the damaged tissues, but also enhance the healing process via decreased inflammatory reactions and slowing down of apoptotic processes. CD133+ has a significant role in cancer studies. In cancer cells, a subpopulation that express CD133 is termed as cancer stem cells (CSCs). These CSCs are rare, proliferative and resistant to chemotherapy and can survive drug treatments, resulting often in relapse of the disease. Many studies have reported the presence of CD133+ cells with the decreased survival rate in cancer patients. Hence, CD133+ cells play an important role in prognosis and outcome of cancer treatment.
Keywords: CD133; Prominin-1; Stem cell therapy; Disease targeting; Regenerative medicine
Keywords
CD133; Prominin-1; Stem cell therapy; Disease targeting; Regenerative medicine
Introduction to Stem Cells
A single human body contains trillions of cells consisting of multiple types based on physiology and function. There are types of cells that are related to achieving specific physiological roles for example neurons are signal sending cells, bone cells are specialized in maintaining integrity of the skeletal structure, while muscle cells are specialized in coordinating body movements. In addition to these specialized cells there exist other types of cells that apparently do not exhibit any specific function. These cells can be induced to acquire specific functions through differentiation. Such cells are known as Stem cells, and can be defined as cells that have the capability of selfdivision as well as differentiation into specialized cells.
The term “stem cell” finds its first mention in scientific-literature by a German biologist Ernst Haeckel. He used the term ‘‘Stammzelle’’ to describe the unicellular organism presumed to be the ancestor of the multicellular organism. Haeckel also used the same word to describe fertilized egg that gives rise to a more complex multicellular organism [1]. Later, in the late 19th century Weismann proposed a theory of continuity of the germ-plasm. The theory suggests a separate kind of cell that remains segregated in embryonic development known as germ cells. Boveri, in 1892, proposed that the germ cells are the ones which can lead to primordial germ cells and from which other primordial somatic cells originate, giving a definition to the stem cells which is quite near to their modern definition [2].
The modern concepts in stem cells come from the advancement of research in the hematopoietic system. Specially, the boost to the stem cell research was provided by the development of staining for various kinds of blood cells by Ehlrich. Although, Peppenheim in 1896, suggested the common precursor of red blood cells and white blood cells, yet Maximow is credited for coining the term of stem cells in 1909 [3,4]. The definitive evidence was finally provided by Till and McCullough in 1963 when they published about the cells in hematopoietic tissue giving rise to colonies that contain cells of different lineages i.e. erythrocytes, granulocytes and megakaryocytes [2,5-7].
Stem cell types and characteristics
Stem cells can be divided either on the basis of origin or on their characteristic ability to produce other type of cells. On the basis of origin, Stem cells are divided into two types: embryonic stem cells, isolated from embryo, and adult stem cells obtained from the adult tissues [8].
On the basis of ability to generate different lineages of cells, stem cells can be: Totipotent, producing every type of cells including germ cells, Pluripotent: that can produce all type of cells except embryonic germ cells, Multipotent: the stem cells that have the ability to differentiate into different types of mature cells, Self-regenerating stem cells: that can divide and produce large quantity of stem-cells and Plastic stem cells, that can differentiate into type other than they were originally isolated from [7,9,10].
Stem cell therapy
As indicated by the name, Stem cell therapy is the usage of stem cells in order to treat diseases. Using the stem cells, because of their ability to regenerate and differentiate, in order to treat physiological disorders seemingly appears to have promising perspectives [11]. Stem cell therapy is applied in a number of disorders. Various clinical trials are being carried out to establish the safety and efficacy of stem cell therapy as presented in Table 1.
Stem cell therapy is of great interest because of its applications in tissue engineering, regenerative medicine and gene therapy because of their therapeutic potential. The main objectives in the field of stem cell research for the coming years are to identify the therapeutic targets, cell differentiation and physiological mechanisms, safety and efficacy for use of stem cells as therapy [12,13].
The most important practical application is found in the treatment of patients with leukemia or lymphoma using bone marrow derived stem cells [14]. Chemotherapy can kill rapidly-dividing cells without discriminating neoplastic cells from the healthy ones. This is a major problem inherent in the use of chemotherapy. The side effect of conventional chemotherapy can be reversed through stem-cell transplant by administering healthy bone marrow functional stem cells to replace the damaged stem cells in the host body [15]. One of the major sideeffect associated with such transplant is that these stem cells (especially if cells are of heterogeneous origin) have the ability to provoke immune response that can result in graft-vs-host disease [16].
A total of eight hematopoietic stem-cell products derived from umbilical cord blood has been already approved by FDA for blood and immunological disease treatment [13,17,18]. The European Medicines Agency in 2014, approved the use of limbal stem cell for people suffering from severe limbal stem cell deficiency vital for epithelial regulation in cornea [19,20].
CD133 or Prominin-1
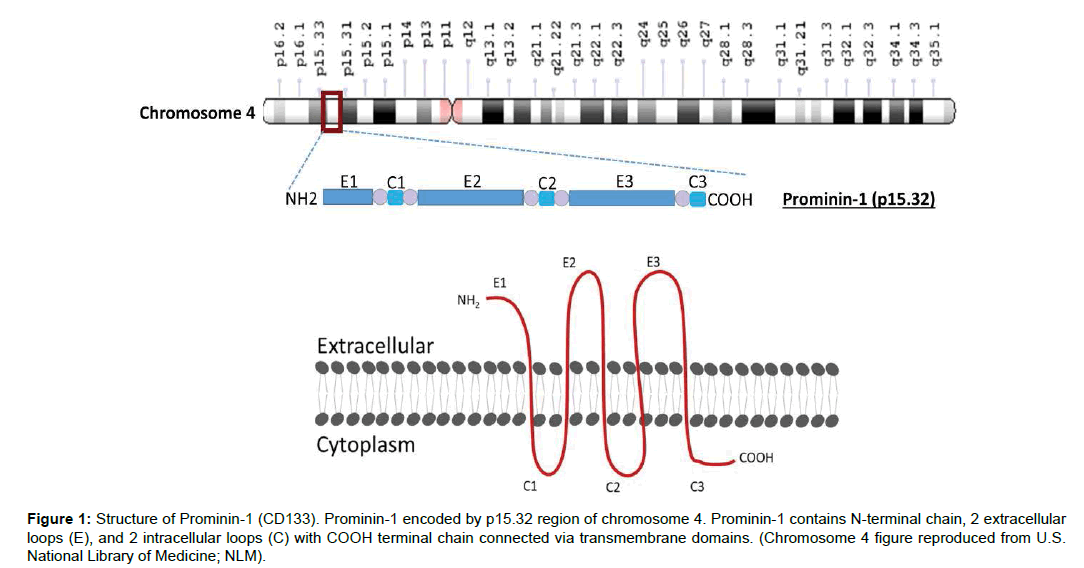
Hematopoietic stem cells express a pentaspan glycoprotein present on membrane known as CD133 or Prominin-1. The role of Prominin-1 is well established in organizing membrane structural integrity [21]. In human, a gene on chromosome 4 encodes Prominin-1. CD133 is a transmembrane glycoprotein. It has five transmembrane domains, two extracellular loops and two cytoplasmic loops an N-terminal chain which is extracellular and a C-terminal cytoplasmic tail, comprising total of 865 amino acids and have a molecular weight of 120 kDa. The N-terminal chain consists of 105 amino acids, two extracellular loops (one larger than other) contain around 258 and 279 amino acids, while two intracellular domains that are smaller in size compared to extracellular loops, have only 29 and 21 amino acids and a cytoplasmic tail is of 59 amino acids. These loops are connected by transmembrane domains containing 23 amino acids each (Figure 1). There are seven different isoforms of CD133 has been reported that are distributed in different parts of the body [22-24].
Figure 1: Structure of Prominin-1 (CD133). Prominin-1 encoded by p15.32 region of chromosome 4. Prominin-1 contains N-terminal chain, 2 extracellular loops (E), and 2 intracellular loops (C) with COOH terminal chain connected via transmembrane domains. (Chromosome 4 figure reproduced from U.S. National Library of Medicine; NLM).
CD133 distribution in human body
CD133 was initially identified in 1997 by two independent studies while examining murine neuroepithelial (NE) cells and human hematopoietic stem cells. Weigmann showed that prominin-1 is localized in microvilli on various epithelial cells, like brain ependymal layer and the brush border membrane of kidney tubules in the adult mouse [25-27]. In 2000, it was presented that prominin-1 is located in the cholesterol-based lipid micro domains present in apical plasma membrane.
Prominin-1 is expressed in epithelial cells in numerous tissues including the mammary gland, testis, digestive tract, trachea and placenta [28]. Although identified in epithelial cells, Prominin-1 is not limited to neuroepithelial progenitor cells but can also be found in non-epithelial cells [29], for example, rod photoreceptor cells, as well as in many types of cancers including gastric, breast, melanoma, lung ovarian, pancreatic, colon, prostate, glioma and hepatocellular cancers [27].
Various roles have been proposed for prominin-1 that includes stem cell and cancer stem cell biomarker, plasma-membrane organization, maintenance of epithelial cells, biogenesis of the photoreceptive disc and mechanism of multi-drug resistance, and the capacity for self-renewal and tumor formation [23,30].
The presence of prominin-1 also varies during the growth stages. The protein is expressed in different parts of the body starting from the embryonic stage to maturity. For example, prominin-1 is expressed in trophoblasts and in epithelia of all three germinal layers at the embryonic stage. However, in adults prominin-1 is expressed in the kidneys, the epididymis, the ductus deferens, the seminal vesicle and the prostate [31].
Although CD133 is employed as cell surface marker for cancer stem cells, the expression of prominin-1 is not limited to cancer stem cells, only. For example CD133 is also expressed in hematopoietic stem cells [32], endothelial progenitor cells [33], glioblastoma, neuronal and glial stem cells [34]. Prominin-1 has been identified in various pediatric brain tumors [35], adult kidney, mammary glands, trachea, salivary glands, uterus, placenta, digestive tract, testes, and other cell types [27,36].
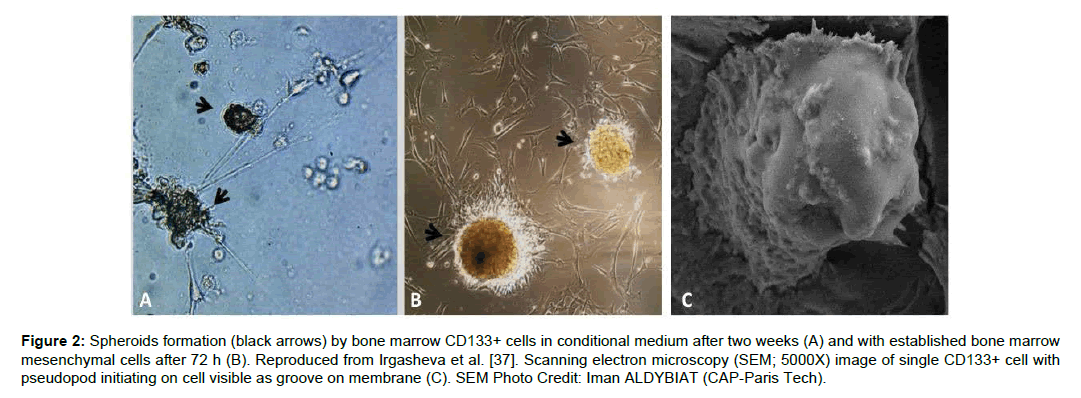
One of the most common sources to isolate CD133+ cells is the bone marrow mononuclear cells. CD133+ cells can be isolated using magnetic beads with purification levels up to 87+.6% [37]. These cells when cultured in-vitro forms a sphere as shown in Figure 2.
Figure 2: Spheroids formation (black arrows) by bone marrow CD133+ cells in conditional medium after two weeks (A) and with established bone marrow mesenchymal cells after 72 h (B). Reproduced from Irgasheva et al. [37]. Scanning electron microscopy (SEM; 5000X) image of single CD133+ cell with pseudopod initiating on cell visible as groove on membrane (C). SEM Photo Credit: Iman ALDYBIAT (CAP-Paris Tech).
Functions of CD133
Prominin-1 plays an important role in cell differentiation, proliferation and apoptosis [38]. It binds to cholesterol in cell membrane and serves a role in the organization of the epithelial cells in apical plasma membrane. It acts as an important regulator in disk morphogenesis through early retinal development at embryonic stage [27].
Prominin-1 has been shown to play role in epididymal stereocilia, hence role in biogenesis of spermatozoa, in a study by Fargeaset et al. who also confirmed the presence of Prominin-1 in testis [39].
Another important function of Prominin-1 is its involvement in regulation of signaling pathways like MAPK and Akt [40]. CD133 also plays an important role in angiogenesis and neovascularization by regulating the expression of vascular endothelial growth factor (VEGF) through WNT signaling pathway [27,41]. This increased angiogenesis can help in the faster wound healing.
Genetic diseases caused by mutations in Prom-1 gene
Stargardt (STGD) disease: As mentioned earlier, CD133 is also found in non-epithelial cells, such as rod photoreceptor cells and bone marrow cells. This shows that CD133 plays an important role in the formation of photoreceptor discs. Photoreceptors are the cells present in retina and are involved in vision. Although, the exact mechanism of CD133 in photoreceptor disc morphogenesis is not known, but it seems to play role owing to its high lipid binding particularly cholesterol [42]. One of the most common cause of blindness is the dystrophy of these photoreceptor cells known as Stargardt disease (STGD ; macular degeneration), which is caused by the mutation in CD133 gene [43]. In STGD, mutation in Prominin-1 blocks its migration in photoreceptor cells from myoid region to outer segment. The absence of Prominin-1 results in dystrophy of photoreceptor cells [44], hence causes STGD.
Retinal macular dystrophy 2 (MCDR2): Retinal macular dystrophy (MCDR2) is a genetic disorder which is characterized by slow progressive Bull’s eye maculopathy (BEM) that results in declined vision [43, 44]. Michaelides et al. screened the patients from different origin for the presence of PROM1 mutation. It was found that R373C mutation was present heterozygously in all the affected patients. PROM1 gene mutation has also been shown to cause a severe form of autosomal recessive retinitis pigmentosa (RP) in two families of Indian and Pakistan decent [44].
Clinical application of CD133+ cells
Stem cell therapy, with its discovery brought the similar changes in the medical field as the internet has brought to human society. Stem cells have the potential to revolutionize the field of regenerative medicine. However, due to certain regulatory issues and hurdles the development remains slow. Most of the stem cell therapies are currently in trial phases. As, stem cells once administered become a part of the body, hence, extensive safety measures have to be undertaken prior to practical implementation in clinical settings. CD133+ cells have been extensively used to treat a number of diseases; from genetic disorders to heart diseases and cancer. Owing to their angiogenic properties, CD133+ cells have been applied for muscle injuries in clinical trials [45]. There have been several other clinical trials that administered bone marrow derived CD133+ stem cell therapy in patients for liver regeneration and to repair the tissues following hepatic fibrosis, myocardial infarction, chronic occlusion and ischemia (Table 1). CD133+ has also been shown to serve as an independent prognostic marker in several types of cancers. Table 1 shows 30 clinical trials that have been completed or are being carried out in order to evaluate the safety or efficacy of CD133+ stem cell therapy.
| Condition | Product used | Purpose | Stage of Clinical Trial | Phase | NCT ID | |
|---|---|---|---|---|---|---|
| Cancer | Acute Myeloid Leukemia | CD133 antigen CAR-T cells | Treatment | Recruiting | Phase 1 | NCT03473457 |
| Colorectal Cancer | (Exploratory studies) | Prognosis | Recruiting | N/A | NCT03002727 | |
| CD133+ infusion | Treatment | Recruiting | Phase 2 | NCT03803241 | ||
| Malignant Glioma | CD133 antigen CAR-T cells | Treatment | Recruiting | Phase 1 | NCT03423992 | |
| Neuroblastoma | CD133+ cells | Treatment | Completed | Phase 1 | NCT02130869 | |
| CD133+ hematopoietic stem cell | Treatment | Completed | N/A | NCT00152126 | ||
| CNS | Cerebral Palsy | CD133+ cells | Treatment | Completed | Phase 1 | NCT01404663 |
| CD133+ cells (intramyocardial injection) | Treatment | Completed | Phase 2 | NCT01763255 | ||
| CVD | Anemia | CordIn(TM) | Treatment | Recruiting | Phase 2 | NCT03173937 |
| Chronic Ischemia | ABM CD133+ cells | Treatment | Recruiting | Phase 1 | NCT01120925 | |
| Chronic Myocardial Ischemia | ABM CD133+ cells | Safety and Preliminary efficacy | Recruiting | Phase 1 | NCT02059681 | |
| Coronary Artery Bypass Surgery | ABM CD133+ cells | Safety and efficacy | Completed | Phase 2 | NCT01467232 | |
| ABM CD133+ cells | Safety and efficacy | Completed | Phase 2 | NCT01033617 | ||
| CD133+ cells | Treatment | Completed | Phase 3 | NCT00462774 | ||
| CD 133+ cells Implantation (Transepicardial with Transseptal ) | Treatment | Completed | Phase 4 | NCT02870933 | ||
| CD133+ cells | Treatment | Completed | Phase 2 | NCT00694642 | ||
| Lower Extremity Ischmeia | CD133+ cells | Treatment | Completed | Phase 2 | NCT00753025 | |
| Myocardial Infarction | CD133+ cells | Treatment | Completed | Phase 2 | NCT00400959 | |
| Stable Angina | CD133+ cells | Treatment | Completed | Phase 2 | NCT01660581 | |
| Miscellaneous | Liver Cirrhosis | CD133+ cells | Treatment | Completed | Phase 2 | NCT00713934 |
| CD133+ cells | Treatment | Recruiting | Phase 3 | NCT03109236 | ||
| CD133+ hematopoietic stem cell | Treatment | Completed | Phase 2 | NCT01120925 | ||
| Asherman Syndrome | Bone Marrow CD133+ cells | Treatment | Completed | Phase 4 | NCT02144987 | |
| CD133+ cells isolation | Safety and Tolerance | Active, Not recruiting | N/A | NCT03665649 | ||
| Azoospermia | ABM CD133+ cells | Treatment | Recruiting | Phase 2 | NCT02641769 | |
| Osteonecrosis | CD133+ cells | Treatment | Completed | Phase 1 | NCT01198080 | |
| Retinitis Pigmentosa | ABM CD133+ cells | Treatment | Active, Not recruiting | Phase 2 | NCT02709876 | |
| Sepsis | (Exploratory studies) | Primary study | Recruiting | N/A | NCT02589535 | |
| Severe Combined Immunodeficiency | CD133+ hematopoietic stem cell | Treatment | Completed | Phase 1 | NCT00152100 | |
| Spinal Cord Injury | CD133+ cells | Treatment | Recruiting | Phase 2 | NCT02687672 | |
| ABM CD133+ cells = Autologous Bone Marrow CD133+ cells | ||||||
Table 1: Clinical trials administering CD133+ cells.
Genetic disorders
Stem-cells, owing to their ability to differentiate into various types of cells especially hematopoietic cells, find extensive application in immune deficiency disorders. Stem cell therapy helps repair immune system and overcome immune deficiencies. One of the examples is Severe Combined Immunodeficiency syndrome (SCID). It is a rare yet fatal condition characterized by the absence of T-cells and B-cells. Children born with SCID have very low immunity against disease and possess life threatening situations. At least, 13 different defects in genes are reported to cause SCID. Stem-cell therapy has provided promising results in children suffering from SCID. Laurie et al. reported the normalized T-cell function following stem cell therapy at neonatal stage in a study that involved 21 infants out of which 20 survived [46].
Recently, a phase-I clinical trial regarding the safety and efficacy of CD-133 in SCID children has been completed at St. Jude’s Children’s Research Hospital (NCTID: NCT00152100; the results have not been published yet). CD133 stem cells are available as cryopreserved stem cell-based product known as CordIn™. CordIn™ is being implied in a clinical trial to treat patients with hemoglobinopathies like Sickle cell disease and thalassemia. The trial is open and is in Phase-II study period. This is a multicenter clinical trial with studies conducted upon patients in USA and France (NCTID: NCT02504619).
Duchenne muscular dystrophy
Duchenne muscular dystrophy (DMD) is a disastrous muscle disorder linked to X gene caused by a defect in the gene that codes dystrophin. The absence of functional dystrophin in the muscles results in fragility of the muscle fiber membrane, as well as progressive muscle weakness resulting in premature death. There is no cure for DMD and current treatment options focus primarily on respiratory assistance, comfort care, and delaying the loss of ambulation. As it involves the degeneration of muscles, using the stem cells for muscle repair can bring great benefits. Torente et al. administered CD133+ cells derived from muscle, to check for safety and efficacy of stem cell therapy in DMD. The trials were conducted as double blind in eight children (mean age of 126.75+21.28 months) over a seven-month period time. Patients presented increased capillary ratio per muscle fibers (that may help in muscular regeneration) with no adverse effect either locally or systemically [47]. These promising results are indeed encouraging in the area of cell therapy for Duchenne muscle dystrophy.
CD133 and cerebral palsy
Cerebral Palsy (CP) is a disorder characterized in children as an impairment in cognitive function such as movement, hearing, seeing, learning and thinking. It involves damage to several types of brain cells, hence making pharmacological treatment difficult. Currently, the treatment of the disorder is limited to supportive and management strategies. However, there is a long time suggestion for using stem cell therapy in order to improve the cognitive function. A phase I clinical trial (NCTID: NCT01763255) has been completed in children with cerebral palsy. The children were administered with intrathecal CD133 enriched bone marrow stem cells. A total of 12 Children aged 4 to 12 years, were injected with stem cells, intrathecally. The patient neurological scores were observed. The neurological measures were taken as baseline and 6 months after the injection. Zali et al. reports possible short term improvement in neurological function (NCTID: NCT01404663). Further, there was no adverse event reported except for seizure in 1 of the children, hence, the therapy was termed completely safe with a potential of increased neural health [48].
CD133 and Asherman’s syndrome
Asherman’s syndrome is characterized by endometrial regeneration. It results in the formation of scar tissue in uterine cavity, most often developed following uterine surgery. The presence of scar tissue may cause amenorrhea, miscarriages and can lead to infertility. This is generally treated by surgery which involves the removal of scar tissue. However, in 2016, Santamaria et al. reported the use of stem cell therapy in 11 patients (NCTID: NCT02144987), using CD133+ bone marrow derived cells. Increased thickness and angiogenesis of endometrium with increased volume and duration of menses was reported following CD133+ cells in conjunction with hormonal replacement, during first three months of treatment [49]. CD133 and liver cirrhosis Liver cirrhosis occurs at an end stage following chronic liver injuries. It may lead to severe hepatic dysfunction resulting in life-threatening condition. Currently, the only suggested treatment is the liver transplant, which is associated with many problems. One of the major issue is availability of the matching donor and risk of graft rejection. Recently, Stem-cell therapy has shown promising result in repairing the damage to liver tissue. Several studies have implied bone marrow stem cells as well as hepatic stem cells for the repair of cirrhosis. Stem cells not only help in repair of the damaged tissue but also help in suppressing inflammatory response, reduced apoptosis and helps in increasing hepatocyte regeneration. These complex mechanisms helps in reducing the liver fibrosis and help improve restore hepatic function [50].
In 2015, Pietro et al., in Italy reported the safety of CD133+ bone marrow stem cells reinfusion in phase-1 clinical trial (NCTID: NCT01025622). 16 patients were enrolled with Model for End-stage Liver Disease (MELD) score between 17 and 25. An initial worsen score was observed for the patients, following mobilization of stem cell (a process that involves the movement of stem cell from bone marrow into blood). However, temporary improvement was found after reinfusion of stem cells in the patients. No serious adverse event was reported following the procedure. The investigators suggest that stem cell therapy can act as a “bridge to transplant” and can help the period required to find matching donors [51].
The phase-I clinical trial held in 2012 (NCTID: NCT01729221) in Egyptian patients (n=90) with HCV-associated liver cirrhosis suggests significant improvement in liver functions (during 12 month follow up period), when patients were infused with CD133+CD34+ purified stem cells. This improvement was further increased in patients who were given two sessions of stem cells infusions (second infusion administered after 4-months). The maximum improvement in model of end-stage liver disease (MELD) score and INR (international normalized ratio) was seen after 3-months following the stem cell infusion [52].
Another Phase-I study evaluated the efficacy and safety of transplanting autologous CD133+ bone marrow stem cells in patients presenting with decompensated cirrhosis. The transplantation of stem cells was found safe with no adverse event reported in six patients [53]. The investigators from Singapore hospital has started phase-3 clinical trial in order to determine the potential of CD133+ cells to reveres fibrosis and improve clinical outcome for patients with end stage cirrhosis. The clinical trial is currently at stage of recruiting patients, and is supposed to be completed in 2021 involving 23-33 patients with end stage cirrhosis (NCTID: NCT03109236).
CD133 in hypercholesterolemia
Familial hypercholesterolemia (FH) is a genetic disorder in which patients have increased levels of low density lipoprotein cholesterol (LDL-C) in blood circulation that leads to increased rate of atherosclerosis. Lipoprotein apheresis (removal) has proven to be an effective treatment for FH patients and has shown reduced cardiovascular morbidity and mortality. FH patients has been found to have higher baseline circulating levels of CD34+ /CD133+ and CD34+/CD133+/CXCR4+ cells compared to hyperlipidemic patients (HLP) and healthy subjects. This suggests the activation of reparative procedure in FH patients. There was no significant change in circulating progenitor cells (CPCs) following apheresis in FH patients. It is hypothesized that in addition to a reduction in atherogenic lipids, the cardiovascular benefits, from lipoprotein apheresis therapy is mediated by enhanced vascular reparative capacity through mobilization of CPCs [54].
Statins (HMG-CoA reductase inhibitors) are the choice medications for treatment of FH. It is reported that therapy with statins was associated with high baseline levels of CD133+ cells [55] and early endothelial progenitor cells (CD133+VEGFR2+) [56]. This suggests that the treatment with statins also helps in activating reparative process in the patient’s body.
Cardiovascular diseases
Circulating progenitor cells (CPCs) are markers of overall vascular health. The diminished levels have been associated with decreased reparative potential and poor outcomes. These cells express both CD34 as well as CD133 markers. An increased level of CD133 in circulation has been found to be significantly correlated with reduced hospitalization event as well as first major cardiovascular event [55]. Several studies have reported increased amount of circulating CD133+ cells in response to ischemic area formed following myocardial infarction [55,57-59]. CD133+ cells have strong angiogenic capacity and via WNT pathway activation or direct interaction with VEGF potentiate its effect and helps in revascularization of the injured area. These factors could be involved in angiogenesis/ revascularization after cell therapy [37,60].
Ischemia
Ischemia is a condition presenting decreased blood supply to tissues, resulting in oxygen deficiency, hence disturbing cellular mechanism. Stem cells exhibit certain cytokines like angiopoietin, VEGF that can help repair the vasculature and can improve the blood supply to the ischemic zone, hence have a great potential to help in recovery of the tissues injured following blood or oxygen loss. The phase-III clinical trials are underway in order to establish the CD133+ stem cells efficacy in Ischemia. Stamm et al., reported the safety and efficacy of CD133+ cells in chronic ischemic heart disease following coronary artery bypass (2007, NCTID: NCT00462774). No cell related complication was reported in a 3-years follow-up. The patient group that received CD133+ cells showed significant improved Left ventricular ejection fraction (LVEF) in a 6-months follow-up [61]. Moreover, Improvement of cardiac function has been seen in patients with end-stage chronic ischemic cardiomyopathy when stem cell therapy was given without coronary artery bypass graft (CABG). Stem cell therapy with CD133+ purified cells has shown an increased ventricular ejection fraction up to 24.8 ± 5% from 15.8 ± 5%. This shows that stem cell therapy alone has a potential to treat ischemia and improve cardiac function [62].
Myocardial infarction
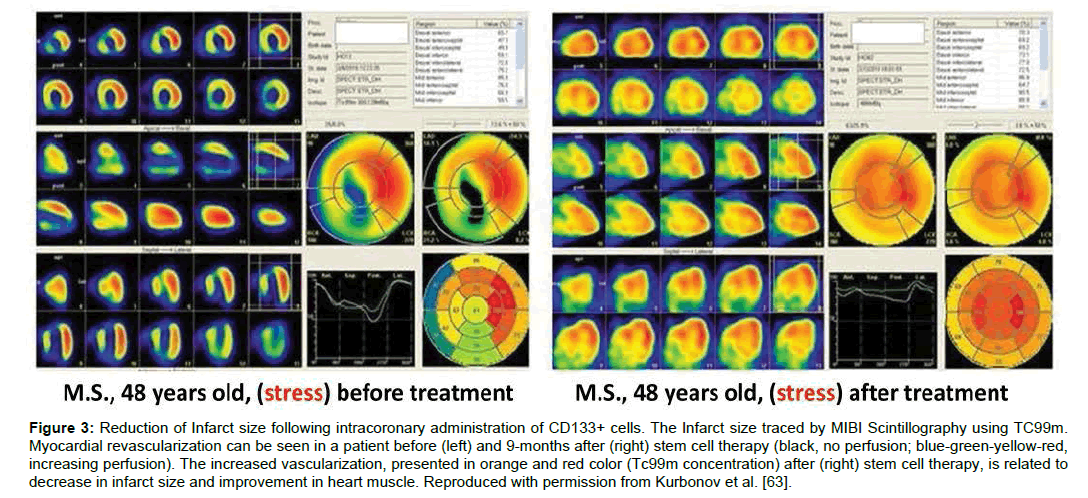
Myocardial infarction (MI), commonly known as heart attack results in decreased blood flow or complete blockage of blood to the heart tissue, causing damage to the heart muscles. Stem cell therapy, having a potential to regenerate the tissue, can help in heart repair, hence decrease the risk of MI in future. The studies show that several factors need to be considered that can influence the usefulness of stem cells in myocardial infarction. These factors involve the route of administration, optimal cell type, safe concentration and stem cells ability of homing to the damaged region. CD133+ stem cells have been safely used in several studies hence, safety of CD133+ cells administration in MI patients has been well established [63- 67]. Administration of CD133+ cells through remodeling of the infarcted area, helps repair the heart muscles [64]. Intracoronary administration of CD133+ enriched mesenchymal stem cells by Kurbonov et al. has been shown to decrease infarct size in 11 out of 15 patients [63] (Figure 3). Although, intracoronary administration of CD133+ has produced promising results, however it is associated with the increase in coronary events like ventricular tachycardia [65,66].
Figure 3: Reduction of Infarct size following intracoronary administration of CD133+ cells. The Infarct size traced by MIBI Scintillography using TC99m. Myocardial revascularization can be seen in a patient before (left) and 9-months after (right) stem cell therapy (black, no perfusion; blue-green-yellow-red, increasing perfusion). The increased vascularization, presented in orange and red color (Tc99m concentration) after (right) stem cell therapy, is related to decrease in infarct size and improvement in heart muscle. Reproduced with permission from Kurbonov et al. [63].
CD133 and CABG (Coronary Artery Bypass Grafting)
Coronary artery bypass is a surgical procedure carried out to improve blood flow to heart muscle by diverting blood around narrowed or clogged parts of the major arteries. For CABG, preferred route of CD133+ stem cell administration is through intramyocardial injection. It has been proved a safe method with no indication of any adverse effect appearing during follow-up [61,68,69]. The studies until now has been focused on establishing the safety of CD-133 transplantation and not much data is available proving the efficacy of CD-133+ stem cell therapy. However, in safety studies, Systolic wall thickening [68-70] and increase in left ventricular ejection fraction [61] has been observed.
CD133 in cancer
Cancer is one of the major causes of deaths in modern world. It is caused by the abnormal growth and uncontrolled proliferation of cells. Cancer cells have the ability to proliferate and give rise to newer population of cancer cells. Cancer cells share these properties in common with stem cells. However, not every cancer cell can be considered stem cell or is a stem cell. Cancer cells contain heterogeneous sub group of cells that are known as cancer stem cells (CSCs). These CSCs display the properties similar to normal stem cells that include self-renewal, proliferation and ability to differentiate to other cell types. CSCs are responsible for the initiation of tumor formation, metastasis and display increased resistance to chemotherapy [71,72]. An important point to note here is that the term “Cancer Stem Cell” is not implied to state that cancer originates from these stem cells. However, some cells of a tumor may undergo genetic or epigenetic modifications in the signaling pathway that lead to a phenotype similar to stem cells [73-75].
There are several markers that can be used to identify CSCs. CD133 is the most commonly used marker to isolate CSCs. Studies have shown that CD133+ (positive) tumor cells have better ability to initiate tumor in immune-compromised mice compared to CD133- (negative) tumor cells [76]. Hence, the role of CD133+ is inevitable in tumor initiation. The more recent focus is to target the CD133+ cells in order to destroy the tumor propagation capability.
Many of the human cancer cell lines of different origin have been evaluated by researchers to express CD-133+ cells (Table 2). However, it can be noted that the expression is not confined to specific cancer type. Within a given cell type some cell lines express CD133 while others do not. Moreover, CD-133 expression has also been reported in tissues from several types of tumors like intrahepatic cholangiocarcinoma [77], hepatocellular carcinoma [77], colorectal carcinoma [78], rectal cancer, endometrial cancer [79] and in other types of cancers [80-83].
| Cancer origin | Cell line name | % age of CD-133 expression | Reference |
|---|---|---|---|
| Cervical carcinoma | Hela | 0.8 | [96] |
| Colon Cancer | Colo205 | 6.9 | [97] |
| HCT116 | 69.62 | [97] | |
| HT-29 | 74 | [98] | |
| SW480 | 0.23 | [97] | |
| Colon Cancer (Meta static) | SW-620 | 30 | [99] |
| Colorectal adenocarcinoma | Caco-2 | 79 | [100] |
| Colorectal cancer | DLD-1 | 20 | [101] |
| Gastric Cancer | KATO-III | 80.2 | [102] |
| MKN45 | 0.2 | [102] | |
| MKN74 | 0.6 | [102] | |
| NCI-N87 | 0.1 | [102] | |
| SNU-1 | 0.7 | [102] | |
| SNU-216 | 37.7 | [102] | |
| SNU-601 | 32.2 | [102] | |
| SNU-638 | 0.4 | [102] | |
| SNU-688 | 0.1 | [102] | |
| Glioblastoma | T98G | 0.3 | [96] |
| U87 | 1.9 | [103] | |
| U87MG | 0.2 | [96] | |
| Head and Neck Squamous carcinoma cell line | HN-12 | 0.4 | [96] |
| HN-30 | 0.1 | [96] | |
| NA-SCC | 5.9 | [100] | |
| UMSCC-1B | 4 | [100] | |
| Hepatocarinoma | Hep3b | 96.8 | [96] |
| Lung Cancer | EKVX | 0.39 | [104] |
| H1299 | 95 | [81] | |
| HTB-182 | 1.07 | [104] | |
| LC-42 | 56.89 | [104] | |
| SELS | 0.43 | [104] | |
| Lung adenocarcinoma | H23 | 5.36 | [96] |
| Lung fibroblast | WI38 | 2.4 | [96] |
| Melanoma | FEMX-I | 100 | [103] |
| HO-1 | 1.4 | [96] | |
| Mewo | 0.5 | [96] | |
| Osteosarcoma | HOS | 37.7 | [96] |
| SAOS-2 | 10.86 | [96] | |
| U2OS | 1.06 | [96] | |
| Ovarian Cancer | Ovcar-3 | 65.2 | [105] |
| Ovcar-4 | 59.64 | [106] | |
| Ovcar-5 | 26.35 | [106] | |
| Pancreatic adenocarcinoma | Mia-PaCa-2 | 0.08 | [103] |
| Prostate adenocarcinoma | DU145 | 0.6 | [96] |
| LNCap | 1 | [96] | |
| PC3 | 0.229 | [103] |
Table 2: CD133 expression in different cell lines.
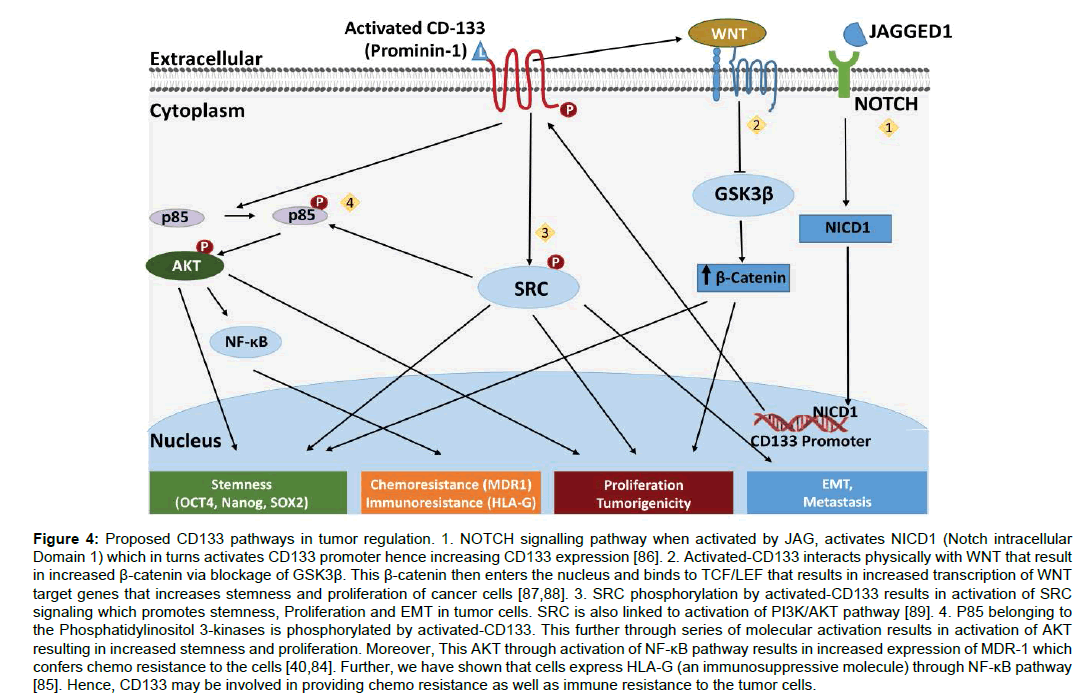
Several mechanisms have been proposed for CD133 role in cancer proliferation (Figure 4). It activates PI3k/AKT pathway and SRC signaling that helps in tumorigenesis, tumor stemness and via epithelial-mesenchymal transition helps in tumor metastasis. Further, through NF-κB pathway activation it helps cancer to exhibit chemo resistance via increased expression of MDR1 [84] and immune resistance through expression of HLA-G via IL-1β [85] (Figure 4).
Figure 4:Proposed CD133 pathways in tumor regulation. 1. NOTCH signalling pathway when activated by JAG, activates NICD1 (Notch intracellular Domain 1) which in turns activates CD133 promoter hence increasing CD133 expression [86]. 2. Activated-CD133 interacts physically with WNT that result in increased �-catenin via blockage of GSK3�. This �-catenin then enters the nucleus and binds to TCF/LEF that results in increased transcription of WNT target genes that increases stemness and proliferation of cancer cells [87,88]. 3. SRC phosphorylation by activated-CD133 results in activation of SRC signaling which promotes stemness, Proliferation and EMT in tumor cells. SRC is also linked to activation of PI3K/AKT pathway [89]. 4. P85 belonging to the Phosphatidylinositol 3-kinases is phosphorylated by activated-CD133. This further through series of molecular activation results in activation of AKT resulting in increased stemness and proliferation. Moreover, This AKT through activation of NF-?B pathway results in increased expression of MDR-1 which confers chemo resistance to the cells [40,84]. Further, we have shown that cells express HLA-G (an immunosuppressive molecule) through NF-?B pathway [85]. Hence, CD133 may be involved in providing chemo resistance as well as immune resistance to the tumor cells.
CD133+ cells immunogenicity
The mice In-vivo studies have shown that isolated CD133+ cells can provoke immune response. It has been reported that CD133+ melanoma cells express RNA helicase DDX3x, which is an immunogenic protein, hence can be used to produce vaccines. T cells isolated from mice injected with irradiated CD133<sup>+</sup>cells from melanoma cells have resulted in significant decrease in parental melanoma cells. Moreover, it was identified that CD4<sup>+</sup> cells rather than CD8+ cells isolated from lymph node, helped in eradicating cancer cells [90]. Currently, two phase I clinical trials are in recruiting stage to evaluate the efficacy of CAR-T cells (NCTID: NCT03473457) in Acute myeloid leukemia (AML) and recurrent malignant gliomas with expression of CD133 antigen (NCTID: NCT03423992).
CD133 and chemo resistance
Cancer stem cells (CSCs) like adult stem cells have the ability to protect themselves against genetic modifications or damage by chemicals. The most commonly used treatment against cancer are radiotherapy and chemotherapy. Cancer stem cell membrane has specialized proteins that prevent the drug molecules from entering the cell, hence protecting them against chemotherapy. CSCs have the specialized enzyme to protect against radiation-induced reactive oxygen species, hence blocking the effect of radiotherapy [91]. In addition, CSCs also have enhanced repair activity of DNA which results in reduced apoptotic events. Human glioma cells have found to express increased CD133 expression following radiation therapy [92]. This increased CD133 expression has been found to be linked with decreased sensitivity to radiotherapy and decreased rate of apoptosis [93,94].
Chemo resistant CD133 cells are also known to have high levels of ATP-binding cassette (ABC) transporter proteins [95]. These are the proteins responsible for nullifying the effect of chemotherapy by transporting chemotherapeutic agents out of the cell. Moreover, CD133 cells upon radiation show higher levels of phosphorylated- Akt, especially in glioma stem cells. This high Activated AKT increases resistance to 5-FU, Adriamycin, mitomycin C, cis-platinum and paclitaxel [96-109] (Table 2).
CD133+ bone marrow cells helps in cancer metastasis
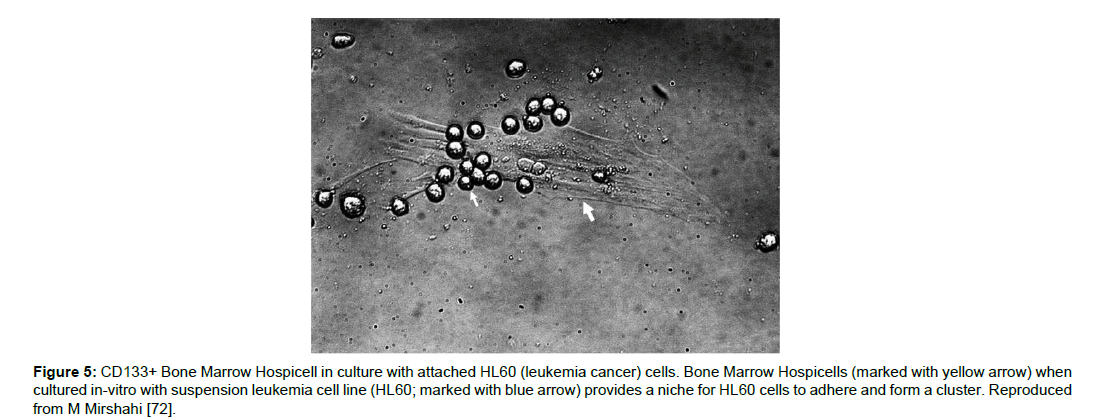
Recently, specialized types of bone marrow mononuclear cells have been identified [110]. These cells possess pods that have high beta-actin concentration, and these pods serve as cell attractant. These cells are named “Hospicells” for their ability to adhere other cells. An increased concentration (about 3-folds) of these hospicells has been found in patients with Acute Myeloid Leukemia. This increased presence of hospicells was also associated with decreased survival rate. In-vitro studies have shown the adherence of leukemic cells (HL60) and form a cluster being attached to CD133+ BMH (Bone marrow hospicells) [111]. Further, these hospicells not only showed increased resistance towards Aracytine(AraC) or Daunorubicin (DnR) themselves, but also protected the leukemic cancer cells (HL60) against the chemotherapy [72]. Targeting of BMH using anti-CD94mAB and anti-CD11a mAB, in-vitro significantly decreased HL60 adhesion on BMH. This suggests that not only the Cancer CD133+ cells but also the BMH can help tumors resist against chemotherapy. BMH can be a novel target to eradicate cancer Figure 5.
Figure 5:CD133+ Bone Marrow Hospicell in culture with attached HL60 (leukemia cancer) cells. Bone Marrow Hospicells (marked with yellow arrow) when cultured in-vitro with suspension leukemia cell line (HL60; marked with blue arrow) provides a niche for HL60 cells to adhere and form a cluster. Reproduced from M Mirshahi [72].
CD133 and prognosis in cancer
As already mentioned, presence of CD133+ cells result in increased resistance against anti-cancer therapy. This increased resistance is linked to a decreased treatment response leading to shorter survival rate and poor prognosis in cancer patients. Further, CD133+ cells are also linked to increased proliferative capacity [112] and metastatic activity of tumor [113], hence, presence of more CD133+ cells results in tumors with high proliferative index and increased metastasis. CD133+ has in general a high prognostic impact in cancer. Presence of CD133+ cells has been linked to decreased overall survival rate. This has been reported in many of the Cancer types e.g. colon cancer [114], gastric carcinoma [115], hepatocellular carcinoma (also a prognostic factor for liver transplantation [116]), lung cancer [117], Breast cancer [118,119], Ovarian Cancer [120,121], non-mucin producing intrahepatic cholangiocarcinoma [122], Brain Cancer [123], Kidney, and cutaneous squamous cell carcinoma [124].
Targeting CD133 for cancer treatment
Increased expression of CD133 linked to shortened survival enourages targeting CD133 as a therapeutic approach against cancer. It may help reduce cancer progression and improve the efficiency of chemotherapy. In-vivo ovarian cancer model using pseudomonas exotoxin fused to anti-CD133 (for CD133 targeting) has shown the decreased ovarian cancer progression in mice [125]. Similarly, this exotoxin [126] has been reported to mediate apoptosis in myeloid leukemia cells. CD-133 targeted delivery of potent cytotoxic drug monomethyl auristatin F (MMAF) has been shown in-vitro to induce apoptosis in hepatocellular carcinoma and gastric carcinoma cell lines (Hep3B and Kato-III). In mice model, the treatment showed significant delay in Hep3B tumor growth [127].
CD133 cells can be targeted through differentiation in order to reduce their stem cell properties. Several compounds like DIF (differentiation-inducing factors) [128] and arsenic trioxide [129] can be used to decrease CD133 expression. In hepatocellular carcinoma (mice model), reduced expression of CD133 was found, following differentiation using arsenic trioxide. Further, decreased recurrence rates and prolonged survival was observed in a mouse model. Other targeted therapies involve elimination of CD133+ tumor cells, using oncolytic viruses [130], inhibiting PPARγ agonists to inhibit the growth and proliferation of CD133+ stem cells [131] and blocking the NOTCH [132] pathway specially for brain tumors, where glioblastoma cells has increased NOTCH activity in CD133 positive tumor cells.
Wang et al. reported the use of CD-133 targeted CAR-T cells in Phase-I trial, completed in 23 patients with advanced malignancies. CAR-T cells (following multiple cell infusion cycles from 2 to 4) showed effective activity in destroying CD-133+ cells (confirmed through biopsies). No de novo lesion was detected in 21 out of 23 patients following administration of CAR-T cells. 3 of the patients showed partial remission, 14 patients presented with stable disease for 9 weeks to 15.7 months while 3 patients showed response till last follow-up in early 2018. In conclusion, CD-133+ targeted CAR-T cells are potential candidate for effective and safe therapy [133].
Conclusion
CD133 or Prominin-1 is an important stem cell marker. CD133+ stem cells, hence, play an important role in stem cell therapy. While alteration in CD133 gene is known to cause genetic defects, CD133+ purified stem cells can be used in regenerative medicines. Majority of the clinical trials currently completed or in process are focused on safety studies of CD133+ stem cell therapy, however, the ones which are completed, has reported the efficacy of CD133+ stem cells especially in heart and liver disease. Further, in cancer, CD133+ stem cells have a great potential to be used as a prognostic marker as well as a target for tumor eradication.
Acknowledgement
We would like to thank Professor Amu Therwath for his valuable time.
References
- Maehle AH (2011) Ambiguous cells: the emergence of the stem cell concept in the nineteenth and twentieth centuries. Notes Rec R Soc 65: 359-378.
- Ramalho-Santos M, Willenbring H (2007) On the origin of the term “stem cell”. Cell stem cell 1: 35-38.
- Ho AD, Wagner W, Mahlknecht U (2005) Stem cells and ageing: The potential of stem cells to overcome age-related deteriorations of the body in regenerative medicine. EMBO reports 6: S35-S38.
- Zahorec P, Koller J, Danisovic L, Bohac M (2015) Mesenchymal stem cells for chronic wounds therapy. Cell and tissue banking 16: 19-26.
- Wu J, Greely HT, Jaenisch R, Nakauchi H, Rossant J, et al. (2016) Stem cells and interspecies chimaeras. Nature 540: 51-59.
- Irgasheva J, aK Baratov M (2018) Human stem cells and cardiac revitalization (New experience In Tajikistan). Vestnik Avitsenny [Avicenna Bulletin] 20: 176-180.
- Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell stem cell 2: 313-319.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147.
- Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, et al. (2015) Establishing criteria for human mesenchymal stem cell potency. Stem Cells 33: 1878-1891.
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, et al. (2010) Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci 107: 4335-4340.
- Brunt KR, Weisel RD, Li RK (2012) Stem cells and regenerative medicine-future perspectives. Can J Physiol Pharmacol 90: 327-335.
- Fadini GP, Agostini C, Avogaro A (2010) Autologous stem cell therapy for peripheral arterial disease: meta-analysis and systematic review of the literature. Atherosclerosis 209: 10-17.
- Rosemann A (2014) Why regenerative stem cell medicine progresses slower than expected. J cell bio 115: 2073-2076.
- Lane SW, Scadden DT, Gilliland DG (2009) The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood 114: 1150-1157.
- Maze R, Carney JP, Kelley MR, Glassner BJ, Williams DA, et al. (1996) Increasing DNA repair methyltransferase levels via bone marrow stem cell transduction rescues mice from the toxic effects of 1, 3-bis (2-chloroethyl)-1-nitrosourea, a chemotherapeutic alkylating agent. Proc Natl Acad Sci U S A 93: 206-210.
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, et al. (2006) Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108: 2114-2120.
- Ballen KK, Gluckman E, Broxmeyer HE (2013) Umbilical cord blood transplantation: the first 25 years and beyond. Blood 122: 491-498.
- Halme DG, Kessler DA (2006) FDA Regulation of Stem-Cell-Based Therapies. New England Journal of Medicine 355: 1730-1735.
- Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, et al. (2016) Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology 62: 216-225.
- Shapiro SA, Smith CG, Arthurs JR, Master Z (2019) Preparing regenerative therapies for clinical application: proposals for responsible translation. Regen Medi 14: 2
- Gurudev N, Florek M, Corbeil D, Knust E (2013) Prominent role of prominin in the retina Prominin-1 (CD133): New Insights on Stem & Cancer Stem Cell Biology. Springer 55-71.
- Jászai J, Fargeas CA, Florek M, Huttner WB, Corbeil D (2007) Focus on molecules: prominin-1 (CD133). Exp Eye Res 85: 585-586.
- Fargeas C (2006) Prominin–1 (CD133): from progenitor cells to human diseases. Future Lipidology 1: 213-225.
- Yu X, Lin Y, Yan X, Tian Q, Li L, et al. (2011) CD133, stem cells, and cancer stem cells: myth or reality? Curr Colorectal Cancer Rep 7: 253.
- Maw MA, Corbeil D, Koch J, Hellwig A, Wilson-Wheeler JC, et al. (2000) A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet 9: 27-34.
- Corti S, Nizzardo M, Nardini M, Donadoni C, Locatelli F, et al. (2007) Isolation and characterization of murine neural stem/progenitor cells based on Prominin-1 expression. Exp Neurol 205: 547-562.
- Shmelkov SV, Clair RS, Lyden D, Rafii S (2005) AC133/CD133/Prominin-1. Int J Biochem Cell Biol 37: 715-719.
- Karbanová J, Missol-Kolka E, Fonseca AV, Lorra C, Janich P, et al. (2008) The stem cell marker CD133 (Prominin-1) is expressed in various human glandular epithelia. J Histochem Cytochem 56: 977-993.
- Marzesco AM, Janich P, Wilsch-Bräuninger M, Dubreuil V, Langenfeld K, et al. (2005) Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 118: 2849-2858.
- Corbeil D, Röper K, Fargeas CA, Joester A, Huttner WB (2001) Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic 2: 82-91.
- Kania G, Corbeil D, Fuchs J, Tarasov KV, Blyszczuk P, et al. (2005) Somatic stem cell marker prominin‐1/CD133 is expressed in embryonic stem cell–derived progenitors. Stem Cells 23: 791-804.
- Irollo E, Pirozzi G (2013) CD133: to be or not to be, is this the real question? Am J Transl Res 5: 563.
- Horn PA, Tesch H, Staib P, Kube D, Diehl V, et al. (1999) Expression of AC133, a novel hematopoietic precursor antigen, on acute myeloid leukemia cells. Blood 93: 1435-1437.
- Sanai N, Alvarez-Buylla A, Berger MS (2005) Neural stem cells and the origin of gliomas. N Engl J Med 353: 811-822.
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, et al. (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63: 5821-5828.
- Mizrak D, Brittan M, Alison M (2008) CD133: molecule of the moment. J Pathol 214: 3-9.
- Irgasheva J, Aldybiat I, Shukurov FA, Mirshahi M (2017) Physiological role of bone marrow adult stem cell CD133+. Вестник Авиценны 19: 177-182.
- Mak AB, Pehar M, Nixon AM, Williams RA, Uetrecht AC, et al. (2014) Post-translational regulation of CD133 by ATase1/ATase2-mediated lysine acetylation. J Mol Biol 426: 2175-2182.
- Fargeas CA, Joester A, Missol-Kolka E, Hellwig A, Huttner WB, et al. (2004) Identification of novel Prominin-1/CD133 splice variants with alternative C-termini and their expression in epididymis and testis. J Cell Sci 117: 4301-4311.
- Wei Y, Jiang Y, Zou F, Liu Y, Wang S, et al. (2013) Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc Natl Acad Sci USA 110: 6829-6834.
- Bogaerts E, Heindryckx F, Vandewynckel YP, Van Grunsven LA, Van Vlierberghe H (2014) The roles of transforming growth factor-β, Wnt, Notch and hypoxia on liver progenitor cells in primary liver tumours. Int J Oncol 44: 1015-1022.
- Zacchigna S, Oh H, Wilsch-Bräuninger M, Missol-Kolka E, Jászai J, et al. (2009) Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci 29: 2297-2308.
- Yang Z, Chen Y, Lillo C, Chien J, Yu Z, et al. (2008) Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest 118: 2908-2916.
- Michaelides M, Gaillard MC, Escher P, Tiab L, Bedell M, et al. (2010) The PROM1 Mutation p. R373C Causes an Autosomal Dominant Bull's Eye Maculopathy Associated with Rod, Rod-Cone, and Macular Dystrophy. Invest Ophthalmol Vis Sci 51: 4771-4780.
- Paprocka M, Krawczenko A, Dus D, Kantor A, Carreau A, et al. (2011) CD133 positive progenitor endothelial cell lines from human cord blood. Cytometry A 79: 594-602.
- Myers LA, Patel DD, Puck JM, Buckley RH (2002) Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood 99: 872-878.
- Torrente Y, Belicchi M, Marchesi C, D'antona G, Cogiamanian F, et al. (2007) Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell transplantation 16: 563-577.
- Zali A, Arab L, Ashrafi F, Mardpour S, Niknejhadi M, et al. (2015) Intrathecal injection of CD133-positive enriched bone marrow progenitor cells in children with cerebral palsy: feasibility and safety. Cytotherapy 17: 232-241.
- Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, et al. (2016) Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod 31: 1087-1096.
- Schuppan D, Afdhal NH (2008) Liver cirrhosis. The lancet 371: 838-851.
- Andreone P, Catani L, Margini C, Brodosi L, Lorenzini S, et al. (2015) Reinfusion of highly purified CD133+ bone marrow-derived stem/progenitor cells in patients with end-stage liver disease: a phase I clinical trial. Dig Liver Dis 47: 1059-1066.
- Zekri A-RN, Salama H, Medhat E, Musa S, Abdel-Haleem H, et al. (2015) The impact of repeated autologous infusion of haematopoietic stem cells in patients with liver insufficiency. Stem Cell Res Ther 6: 118.
- Nikeghbalian S, Pournasr B, Aghdami N, Rasekhi A, Geramizadeh B, et al. (2011) Autologous transplantation of bone marrow-derived mononuclear and CD133+ cells in patients with decompensated cirrhosis. Arch Iran Med 14: 12-17.
- Sandesara P, Ramjee V, Ghasemzadeh N, Guo Y, Bhatia N, et al. (2018) Circulating progenitor cells in patients with familial hypercholesterolemia. J Clin Apher 33: 404-408.
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, et al. (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999-1007.
- Golab-Janowska M, Paczkowska E, Machalinski B, Meller A, Kotlega D, et al. (2018) Statins Therapy is Associated with Increased Populations of Early Endothelial Progenitor (CD133+/VEGFR2+) and Endothelial (CD34-/CD133-/VEGFR2+) Cells in Patients with Acute Ischemic Stroke. Curr Neurovasc Res 15: 120-128.
- Schömig K, Busch G, Steppich B, Sepp D, Kaufmann J, et al. (2006) Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J 27: 1032-1037.
- Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Ribó M, et al. (2010) Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res 80: 317-323.
- Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, et al. (2005) Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 105: 199-206.
- Lohela M, Bry M, Tammela T, Alitalo K (2009) VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 21: 154-165.
- Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, et al. (2007) Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg 133: 717-725.
- Klein H, Ghodsizad A, Ruhparwar A, Marktanner R, Poll L, et al. (2007) Intramyocardial implantation of CD133+ stem cells improved cardiac function without bypass surgery. Heart Surg Forum 10: 28.
- Kurbonov U, Dustov A, Barotov A, Khidirov M, Mirojov G, et al. (2013) Intracoronary Infusion of Autologous CD133. Stem Cells Int 2013: 582527
- Manginas A, Goussetis E, Koutelou M, Karatasakis G, Peristeri I, et al. (2007) Pilot study to evaluate the safety and feasibility of intracoronary CD133+ and CD133-CD34+ cell therapy in patients with nonviable anterior myocardial infarction. Catheter Cardiovasc Interv 69: 773-781.
- Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, et al. (2007) Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol 50: 1761-1767.
- Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, et al. (2005) Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: Feasibility and safety. Circulation 112: 178-183.
- Goussetis E, Manginas A, Koutelou M, Peristeri I, Theodosaki M, et al. (2006) Intracoronary infusion of CD133+ and CD133− CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells 24: 2279-2283.
- Forcillo J, Stevens L-M, Mansour S, Prieto I, Salem R, et al. (2013) Implantation of CD133+ stem cells in patients undergoing coronary bypass surgery: IMPACT-CABG pilot trial. Can J Cardiol 29: 441-447.
- Mocini D, Staibano M, Mele L, Giannantoni P, Menichella G, et al. (2006) Autologous bone marrow mononuclear cell transplantation in patients undergoing coronary artery bypass grafting. Am Heart J 151: 192-197.
- Noiseux N, Mansour S, Weisel R, Stevens LM, Der Sarkissian S, et al. (2016) The IMPACT-CABG trial: a multicenter, randomized clinical trial of CD133+ stem cell therapy during coronary artery bypass grafting for ischemic cardiomyopathy. J Thorac Cardiovasc Surg 152: 1582-1588.
- Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, et al. (2010) CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non‐small cell lung cancer patients. Int J Cancer 126: 950-958.
- Mirshahi M (2019) Identification of an adherent cell that interact with leukemic cells and induced chemo resistance. Proceedings of The European Congress on Leukemias - Between Cutting Edge and Grey Zone.
- Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. New Eng J Medi 355: 1253-1261.
- Kim Y, Kaidina A, Chiang JH, Yarygin K, Lupatov AY (2017) Cancer stem cell molecular markers verified in vivo. Biochemistry (Moscow), Supplement Series B: Biomedical Chemistry 11: 43-54.
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, et al. (2004) Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64: 7011-7021.
- Lai IC, Shih P-H, Yao CJ, Yeh CT, Wang-Peng J, et al. (2015) Elimination of cancer stem-like cells and potentiation of temozolomide sensitivity by Honokiol in glioblastoma multiforme cells. PLoS One 10: 0114830.
- Chen YL, Lin PY, Ming YZ, Huang WC, Chen RF, et al. (2017) The effects of the location of cancer stem cell marker CD133 on the prognosis of hepatocellular carcinoma patients. BMC Cancer 17: 474.
- Kazama S, Kishikawa J, Kiyomatsu T, Kawai K, Nozawa H, et al. (2018) Expression of the stem cell marker CD133 is related to tumor development in colorectal carcinogenesis. Asian J Surg 41: 274-278.
- Ding DC, Liu HW, Chang YH, Chu TY (2017) Expression of CD133 in endometrial cancer cells and its implications. J Cancer 8: 2142.
- Zhang KJ, Parikh SR, Sigelmann-Danieli N, Hafron JM, Liu JL, et al. (2016) Diagnostic Values of a Progenitor Cell Marker CD133 Expression in Various Types of Adenocarcinoma. J Mol Biomark Diagn 7: 299.
- Le H, Zeng F, Xu L, Liu X, Huang Y (2013) The role of CD133 expression in the carcinogenesis and prognosis of patients with lung cancer. Mol Med Rep 8: 1511-1518.
- Xie Y, Huang J, Wu M, Zhou Y (2018) Expression of CD133 protein in osteosarcoma and its relationship with the clinicopathological features and prognosis. J Cancer Res Ther 14: 892.
- Huang M, Zhu H, Feng J, Ni S, Huang J (2015) High CD133 expression in the nucleus and cytoplasm predicts poor prognosis in non-small cell lung cancer. Dis Markers 2015: 8.
- Xi G, Hayes E, Lewis R, Ichi S, Mania-Farnell B, et al. (2016) CD133 and DNA-PK regulate MDR1 via the PI3K-or Akt-NF-κB pathway in multidrug-resistant glioblastoma cells in vitro. Oncogene 35: 241.
- Ullah M, Azazzen D, Kaci R, Benabbou N, Lauraine EP, et al. (2019) High Expression of HLA-G in Ovarian Carcinomatosis: The Role of Interleukin-1β. Neoplasia 21: 331-342.
- Kumar D, Kumar S, Gorain M, Tomar D, Patil HS, et al. (2016) Notch1-MAPK signaling axis regulates CD133+ cancer stem cell-mediated melanoma growth and angiogenesis. J Invest Dermatol 136: 2462-2474.
- Wang R, Sun Q, Wang P, Liu M, Xiong S, et al. (2016) Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget 7: 5754.
- Zhou L, Xu M, Yang Y, Yang K, Wickett RR, et al. (2016) Activation of β-catenin signaling in CD133-positive dermal papilla cells drives postnatal hair growth. PLoS One 11: e0160425.
- Chen Y-S, Wu M-J, Huang C-Y, Lin S-C, Chuang T-H, et al. (2011) CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS One.6: e28053.
- Miyabayashi T, Kagamu H, Koshio J, Ichikawa K, Baba J, et al. (2011) Vaccination with CD133+ melanoma induces specific Th17 and Th1 cell–mediated antitumor reactivity against parental tumor. Cancer Immunol Immunother 60: 1597-1608.
- Song Y, Jang J, Shin TH, Bae SM, Kim JS, et al. (2017) Sulfasalazine attenuates evading anticancer response of CD133-positive hepatocellular carcinoma cells. J Exp Clin Cancer Res 36: 38.
- Tamura K, Aoyagi M, Ando N, Ogishima T, Wakimoto H, et al. (2013) Expansion of CD133-positive glioma cells in recurrent de novo glioblastomas after radiotherapy and chemotherapy. J Neurosurg 119: 1145-1155.
- Rycaj K, Tang DG (2014) Cancer stem cells and radioresistance. Int J Radiat Biol 90: 615-621.
- Angelastro JM, Lamé MW (2010) Overexpression of CD133 promotes drug resistance in C6 glioma cells. Mol Cancer Res 8: 1541-7786.
- Li Z (2013) CD133: A stem cell biomarker and beyond. Exp Hematol Oncol 2: 17.
- Kelly SE, Di Benedetto A, Greco A, Howard CM, Sollars VE, et al. (2010) Rapid selection and proliferation of CD133 (+) cells from cancer cell lines: Chemotherapeutic implications. PLoS One 5: e10035.
- Wang C, Xie J, Guo J, Manning HC, Gore JC, et al. (2012) Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer. Oncol Rep 28: 1301-1308.
- Sahlberg SH, Spiegelberg D, Glimelius B, Stenerlöw B, Nestor M (2014) Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PLoS One 9: e94621.
- Yang Z, Wang Z, Fan Y, Zheng Q (2012) Expression of CD133 in SW620 colorectal cancer cells is modulated by the microenvironment. Oncol Lett 4: 75-79.
- Waldron NN, Kaufman DS, Oh S, Inde Z, Hexum MK, et al. (2011) Targeting tumor-initiating cancer cells with dCD133KDEL shows impressive tumor reductions in a xenotransplant model of human head and neck cancer. Mol Cancer Ther 10: 1829-1838.
- Ferrand A, Sandrin MS, Shulkes A, Baldwin GS (2009) Expression of gastrin precursors by CD133-positive colorectal cancer cells is crucial for tumour growth. Biochim Biophys Acta (BBA)-Molecular Cell Research 1793: 477-488.
- Lee HJ, Choi YS, Kim SJ, Moon HJ (2010) CD44 and CD133 as cancer stem cell markers for gastric cancer. Int J Mol Cell Med 10: 99-105.
- Steponkiene S, Kavaliauskiene S, Purviniene R, Rotomskis R, Juzenas P (2011) Quantum dots affect expression of CD133 surface antigen in melanoma cells. Int J Nanomedicine 6: 2437.
- Wang P, Suo Z, Wang M, Høifødt HK, Fodstad Ø, et al. (2013) In vitro and in vivo properties of CD133 expressing cells from human lung cancer cell lines. Exp Hemat Oncology 2: 16.
- Ducros E, Mirshahi S, Azzazene D, Camilleri-Broët S, Mery E, et al. (2012) Endothelial protein C receptor expressed by ovarian cancer cells as a possible biomarker of cancer onset. Int J Oncol 41: 433-440.
- Cioffi M, D’Alterio C, Camerlingo R, Tirino V, Consales C, et al. (2015) Identification of a distinct population of CD133+ CXCR4+ cancer stem cells in ovarian cancer. Sci Rep 5: 10357.
- Oki E, Baba H, Tokunaga E, Nakamura T, Ueda N, et al. (2005) Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer 117: 376-380.
- Murakami D, Tsujitani S, Osaki T, Saito H, Katano K, et al. (2007) Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer 10: 45-51.
- Kim SH, Juhnn YS, Song YS (2007) Akt involvement in paclitaxel chemoresistance of human ovarian cancer cells. Ann NY Acad Sci 1095: 82-89.
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, et al. (2009) Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457: 603-607.
- Varin R, Garderet L, Benabbou N, Marie JP, Pocard M, et al. (2019) Isolation of cells from adherent bone marrow mononuclear cells that favored malignant hematopoietic and solid tumor cell adhesion in vitro. Proceedings of The European Congress on Leukemias - Between Cutting Edge and Grey Zone.
- Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, et al. (2008) Expression of CD133‐1 and CD133‐2 in ovarian cancer. Int J Gynecol Cancer 18: 506-514.
- Nomura A, Banerjee S, Chugh R, Dudeja V, Yamamoto M, et al. (2015) CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget.6: 8313-8322.
- Horst D, Scheel SK, Liebmann S, Neumann J, Maatz S, et al. (2009) The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol 219: 427-434.
- Ishigami S, Ueno S, Arigami T, Uchikado Y, Setoyama T, et al. (2010) Prognostic impact of CD133 expression in gastric carcinoma. Anticancer Res 30: 2453-2457.
- Li Y, Jiang N, Ruan DY (2015) Stem cell surface markers CD133 expression in hepatocellular carcinoma and as single prognostic factor for liver transplantation. Gastrointestinal e15166.
- Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, et al. (2009) Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A 106: 16281-16286.
- Joseph C, Arshad M, Kurozomi S, Althobiti M, Miligy IM, et al. (2018) Overexpression of the cancer stem cell marker CD133 confers a poor prognosis in invasive breast cancer. Breast Cancer Res Treat 174: 387-399.
- Han Z, Chen Z, Zheng R, Cheng Z, Gong X, et al. (2015) Clinicopathological significance of CD133 and CD44 expression in infiltrating ductal carcinoma and their relationship to angiogenesis. World J Surg Oncol 13: 56.
- Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, et al. (2012) CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol 25: 456.
- Štemberger-Papić S, Vrdoljak-Mozetič D, Verša Ostojić D, Rubeša-Mihaljević R, Krištofić I, et al. (2015) Expression of CD133 and CD117 in 64 serous ovarian cancer cases. Coll Antropol 39: 745-753.
- Cai X, Li J, Yuan X, Xiao J, Dooley S, et al. (2018) CD133 expression in cancer cells predicts poor prognosis of non-mucin producing intrahepatic cholangiocarcinoma. J trans medi 16: 50.
- Li B, McCrudden CM, Yuen HF, Xi X, Lyu P, et al. (2017) CD133 in brain tumor: The prognostic factor. Oncotarget 8: 11144.
- Xu R, Cai MY, Luo RZ, Tian X, Chen MK (2016) The expression status and prognostic value of cancer stem cell biomarker CD133 in cutaneous squamous cell carcinoma. JAMA Dermatol 152: 305-311.
- Skubitz AP, Taras EP, Boylan KL, Waldron NN, Oh S, et al. (2013) Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol Oncol 130: 579-587.
- Schwemmlein M, Peipp M, Barbin K, Saul D, Stockmeyer B, et al. (2006) A CD33‐specific single‐chain immunotoxin mediates potent apoptosis of cultured human myeloid leukaemia cells. Br J Haematol 133: 141-151.
- Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, et al. (2008) CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer 99: 100.
- Kubohara Y (1999) Effects of differentiation-inducing factors of Dictyostelium discoideum on human leukemia K562 cells: DIF-3 is the most potent anti-leukemic agent. Eur J Pharmacol 381: 57-62.
- Zhang K-Z, Zhang Q-B, Zhang Q-B, Sun H-C, Ao J-Y, et al. (2014) Arsenic trioxide induces differentiation of CD133+ hepatocellular carcinoma cells and prolongs posthepatectomy survival by targeting GLI1 expression in a mouse model. J Hematol Oncol 7: 28.
- Bach P, Abel T, Hoffmann C, Gal Z, Braun G, et al. (2013) Specific elimination of CD133+ tumor cells with targeted oncolytic measles virus. Cancer Res 73: 865-874.
- Chearwae W, Bright J (2008) PPARγ agonists inhibit growth and expansion of CD133+ brain tumour stem cells. Br J Cancer 99: 2044.
- Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, et al. (2010) NOTCH pathway blockade depletes CD133‐positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28: 5-16.
- Wang Y, Chen M, Wu Z, Tong C, Dai H, et al. (2018) CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 7: e1440169.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi