Review Article, Jdtba Vol: 13 Issue: 1
Breast Cancer Diagnostics: Molecular Approaches
Corbin Whitsell, Parthi Bhika, Kaitlyn Bambino, Mara Carter, James J. Aboagye, Sujan Thapa, Girdhari Rijal*
1Department of Medical Laboratory Sciences, Public Health & Nutrition Science, College of Health Sciences, Tarleton State University, Texas A & M University System, Fort Worth, Texas 76036, United States of America
*Corresponding Author: Girdhari Rijal,
Girdhari Rijal, Department of Medical Laboratory Sciences, Public
Health & Nutrition Science, College of Health Sciences, Tarleton State University, A Member
of Texas A & M University System, Fort Worth, Texas 76036, United States of America
Tel: +16827037125
E-mail: rijal@tarleton.edu
Received date: 08 Jul, 2024, Manuscript No. JDTBA-24-141234;
Editor assigned date: 10 Jul, 2024, PreQC No. JDTBA-24-141234 (PQ);
Reviewed date: 26 Jul, 2024, QC No. JDTBA-24-141234;
Revised date: 02 Aug, 2024, Manuscript No JDTBA-24-141234 (R);
Published date: 09 Aug, 2024, DOI: 10.4172/2469-5653.1000303
Citation: Whitsell C, Bambino K, Bhika P, Carter M, Aboagye JJ, et al. (2024) Breast Cancer Diagnostics: Molecular Approaches. J Diagn Tech Biomed Anal 13:1.
Abstract
Breast cancer remains a significant health concern worldwide, demanding continuous advancements in diagnostic methodologies to enhance early detection and treatment outcomes. This paper delves into the realm of molecular approaches for breast cancer diagnosis, beginning with an introduction to the pressing need for improved diagnostic techniques. It explores the spectrum of molecular techniques employed in cancer diagnosis, highlighting their pivotal role in unraveling the intricate molecular signatures of breast cancer subtypes. The discussion progresses to elucidate the diverse array of breast cancer biomarkers and their applications in molecular diagnosis, underscoring their utility in personalized treatment strategies and prognostic assessment.
Furthermore, this paper evaluates the advantages and disadvantages inherent in current breast cancer diagnostic methods, shedding light on the complexities and limitations that accompany molecular diagnostic techniques. Through a comprehensive analysis, it delineates the strengths and weaknesses of these methodologies, emphasizing the imperative of refining existing approaches to mitigate diagnostic challenges and enhance clinical outcomes.
Keywords: Breast cancer; Molecular diagnostics; Biomarkers;
Molecular methods; Immunohistochemistry
Abbreviations
IDC: Invasive Ductal Carcinoma; LCIS: Lobular Carcinoma In Situ; DCIS: Ductal Carcinoma In Situ; CAPs: College Of Pathologists; FNAC: Fine Needle Aspiration Cytology; CGB: Core Group Banking; PBMC: Peripheral Blood Mononuclear Cell; QA: Quality Assurance; ILC: Invasive Lobular Carcinoma; AI: Artificial Intelligence; IHC: Immunohistochemistry; Circulating Tumor Cells (CTCs); LC-MS: Liquid Chromatography-Mass Spectrometry; ER: Estrogen Receptor; PR: Progesterone Receptor; ELISA: Enzyme-Linked Immunosorbent Assay; WES: Whole Exome Sequencing; WGS: Whole Genome Sequencing; NGS: Next-Generation Sequencing; aCGH: Array Comparative Genomic Hybridization; PBS: Phosphate Buffered Saline; NBF: Neutral Buffered Formalin; IHC: Immunohistochemistry
Introduction
Breast tissue, a complex mammary gland, is an anatomical collection of ducts and lobules, fat and connective tissues supported by the muscles with profuse infiltration and a comprehensive network of blood and lymphatic vessels (Figure 1) [1,2]. Breast cancer is characterized by the unbounded development and division of abnormal cells within breast tissue, affecting millions of women worldwide and is a leading cause of cancer-related deaths among women in the United States [3]. It progresses by time to a complex tumor mass with diverse molecular and genetic alterations. Breast cancer can be categorized into two major groups; non-invasive and invasive. Non-invasive types do not metastasize outside the breast tissue, for example, Ductal Carcinoma In Situ (DCIS) and Lobular Carcinoma In Situ (LCIS). When the cancer remains in the ducts or in the lobules of the breast tissue, they are usually considered as a stage “0” carcinoma. Most DCIS is highly progressive towards the invasive stage, known as Invasive Ductal Carcinoma (IDC).
Figure 1: Demonstration of lactiferous ducts and lobules in both normal and cancerous forms. Note: Normal lactiferous duct has a clear passage-way for the flow of the fluid, whereas, there will be abnormal (cancerous) cells in Ductal Carcinoma In Situ (DCIS) that progresses towards the Invasive Ductal Carcinoma (IDC), blocking the flow of the fluid (A). Lobule Carcinoma In Situ (LCIS) is mostly localized, however, it progresses to Invasive Lobule Carcinoma (ILC) (B) with the demonstration of the breast tissue (C).
About 70%-80% of women suffer from IDC annually [4]. The LCIS also progresses towards a certain invasiveness compared to DCIS, known as Invasive Lobular Carcinoma (ILC) that comprises 10%-15% of all breast cancer cases. Invasive breast cancer is highly metastasized. Four main subtypes of invasive breast cancer are Luminal A, Luminal B, and HER2 enriched and Tipple-negative/ Basal-like Breast Cancers [5]. In diagnostic aspects, invasive breast cancer is highly challenging for the detection compared to noninvasive in terms of both clinical symptoms and diagnostics [6]. Failure in physical and screening tests of early state breast cancer, may be because of its tiny-size especially in dense breast tissues without clinical symptoms, supports its progression from non-invasive to the invasive cancer [7]. However, improved cancer screening methods available help reduce the mortality rate, even from the population with the history of breast cancer [8]. Precise and accurate molecular diagnostic methods are essential for the confirmation of breast cancer to minimize the challenges or errors that are encountered from conventional methods [9].
Cancer screening
Many processing/screening methods are used in the early diagnosis of breast cancer. A doctor's ability to review images and spot the emergence of breast cancer at an early stage using Artificial Intelligence (AI) and Image Processing (IP) dramatically increase the screening sensitivity of cancer. Further, optimization in X-ray diagnostic systems along with the application of innovative technologies has facilitated the early identification of disease, facilitating early initiation of treatment [8]. The binarization system creates essential criteria for the study of mammographic pictures through the development of digital image processing techniques [10]. Additionally, it increases the precision in the detection of cancers, anatomical alterations, and structural defects, providing a clear diagnostic for choosing a patient's course of prevention or treatment. Various instruments, including thermography, ultrasound, and mammography, have used to screen the breast cancer. In this regard computers make use of AI and IP to assist radiologists in more accurately identifying chest anomalies [11]. These technologies enable medical professionals to examine images and detect the disease's emergence at an early stage which fortunately enhances the likelihood of a successful outcome [12].
Clinical Specimen Sources, Collection, Storage and Processing Methods
Clinical specimen sources
Proposals to use clinical specimens, such as tissue microsection, must be approved by laboratory directors as per the guidelines of College of Pathologists (CAPs) or other accredited agencies. Considering clinical samples very important and rare, their handling and use must be carefully performed [13]. A diagnostic committee should be established using predetermined policies or the guidelines provided by CAPs or related agencies that help review the protocols from the sample handing and the acceptance or the rejection of samples to the result interpretation. Breast tissue biopsy, blood, surgical samples, Fine Needle Aspiration Cytology (FNAC) and any other possible cancer related specimen from different parts of the body could be collected.
Collection
In case of blood sample, Core Group Banking (CGB) can significantly speed up and standardize the collection of high-quality samples across sites, reducing process variation, facilitating on-site processing (e.g. such as splitting) and improve required collection compliance by sending pre-assembled kits to sites (Figure 2) [14]. Group banks should use pre-assembled collection kits whenever possible.
Figure 2: Outline of the blood collection procedure, DNA isolation, sequencing and data analysis. Note: Generally peripheral blood is collected for the DNA analysis and collected in the tube with anticoagulant, commonly EDTA. After DNA extraction and purification, DNA is sequenced using DNA sequencer and data obtained will be analyses using software.
When getting high-quality blood, plasma, or serum, the most common question is whether the requesting facility can process the sample quickly on site or whether the blood components can remain stable during the transport to a laboratory or not [15]. Both positive and negative outcomes can be found in the performance-based problems that could significantly affect the final results. Sampling processes and final products become more diverse if samples are processed by multiple organizations without standardized protocols [16]. For instance, changes in centrifugation speed, extraction time, aliquot method, and frozen temperature can alter the final results.
The fresh or frozen tissue has a finite window for the acceptance after collection. However, the general principle of quick collection, transport, freezing, and seamless integration with conventional pathologic assessment are applied [14]. Standardized and calibrated methods are applied to establish the Quality Assurance (QA), and they should show feasibility in a variety of diagnostic settings. And it is made accessible to ensure quality check on every sample collection sites to the sample processing labs, applying the pre and post-analytical quality control protocols for sample collection and processing [14]. A fresh breast cancer sample, for example, a triple-negative tumor core biopsy, is collected and processed through the approved method and it is suitable for single-cell transcriptome analysis to identify the cellular diversity in the tumor immune milieu, including a T cell subpopulation that is prognostically meaningful. Freshly collected biopsy sample may support for a high-yield system to produce a rich tissue repository for a wide range of cross-disciplinary study [15]. Having the patient undergo a refraction x-ray is another method of collecting breast cancer specimens. The x-ray used assists medical professionals and researchers in deciding how to collect samples for additional tests to find a cancer cure.
Specimen storage
The varied differences in the facilities, tools, and staffing levels of the institutions submitting specimens make it very difficult to follow standard procedures for collecting clinical samples. Variability in specimen processing at submitting institutions may lead to significant heterogeneity in specimen quality and likeness. According to biospecimen policy review committees, the clinical trial specimen bank inventory has been found to benefit greatly from efforts to harmonize specimen processing, at the institutional level. For specimens that will be placed into frozen storage, the storage aspect should be able to withstand -80ºC [17]. It is important to note that specimens should either be stored in a biohazard container or bag inside refrigerator for a short-term or inside deep fridge for a longterm that help preserve the cancer specimen till processing.
Processing methods
It is important to identify and differentiate various samples that need special techniques [18]. Trainings for sample handling, transport, storage and processing are required to meet the standard guidelines. For instance, isolation of Peripheral Blood Mononuclear Cell (PBMC) requires very different techniques and much more expertise than preparing buffer-coated WBC [8]. Most community hospitals cannot perform PBMC in isolation, even though academic institutions may be set up to do so. More demanding specifications for PBMC sample submission will restrict collection unless these samples can be sent to a central processing location [8]. Therefore, processing methods should follow the standard guidelines provided in the lab.
Discussions
Molecular techniques applied in cancer diagnosis
There are many molecular methods used for the detection and diagnosis of cancer. Each method distinguishes the genetic mutations, alterations, and biomarkers unique to cancer cells. These procedures have become a staple in diagnosis and research, enabling to acquire key insights into the genetic connections, heterogeneity and progression of cancer. These molecular techniques have enabled doctors for diagnosis of cancer and personalized therapies, leading to the improvement of overall patient care.
Immunohistochemistry (IHC)
IHC, the most recognized class of immunostaining, is a molecular technique that uses antibodies to detect specific antigens in a tissue sample. It serves as a vital tool in the effort to understand and combat cancer [19]. It provides spatial information about protein expression within tissue samples and is widely available in clinical laboratories. This method is routinely used for tumor characterization and diagnosis. The binding of specific antibodies to the protein of interest, for example, a cancer biomarker, leads to the development of a visible compound under microscopy through the fluorescent or chromogenic dye that is tagged in specific antibodies, which bind and localize specific biomarkers within tissue samples. It is highly specific and can help easily distinguish cancer cells from healthy cells by using specific markers, for example, HER2, ER and PR, thereby identifying and characterizing the proteins of interest of the cancer. Having the ability to distinguish the origin of these proteins, it allows us to target the area of interest, determine the prognosis and create a treatment plan for the patient [20,21].
These markers also show the precise location of where the cancer is in the tissue sample. This is especially important when investigating the characteristics of tumor cells [22]. This technique can also provide insight into the prognosis. Specific markers are used to predict disease outcomes, allowing for doctors to make informed decisions on treatment options based on the expression of these markers within the sample.
Disadvantage of using IHC is its limited array of antibodies that can target certain proteins and use in cancer diagnosis. Another drawback of IHC is its difficulty in the result decision, since data can be visualized and subjective, meaning people can interpret the results differently [23]. Sterile technique is key when performing IHC to avoid false-positives or false-negatives that might be caused by different factors, such as how the tissue was fixed, processed, where the antibodies are derived from and if the staining worked correctly [24].
IHC is mostly used with formalin fixed paraffin embedded tissue [21]. Paraffin-embedded tissue has the benefit of being easily stored. The consecutive step includes the retrieval of antigens [21]. This includes tissue being pre-treated to retrieve antigens masked by fixation and makes them more attainable to the binding of antibodies. Other methods of antigen retrieval exist and depend on the particular target antigen and antibody. Antigen retrieval mostly includes protein cross links being broken, which are caused by fixation by physical or chemical methods.
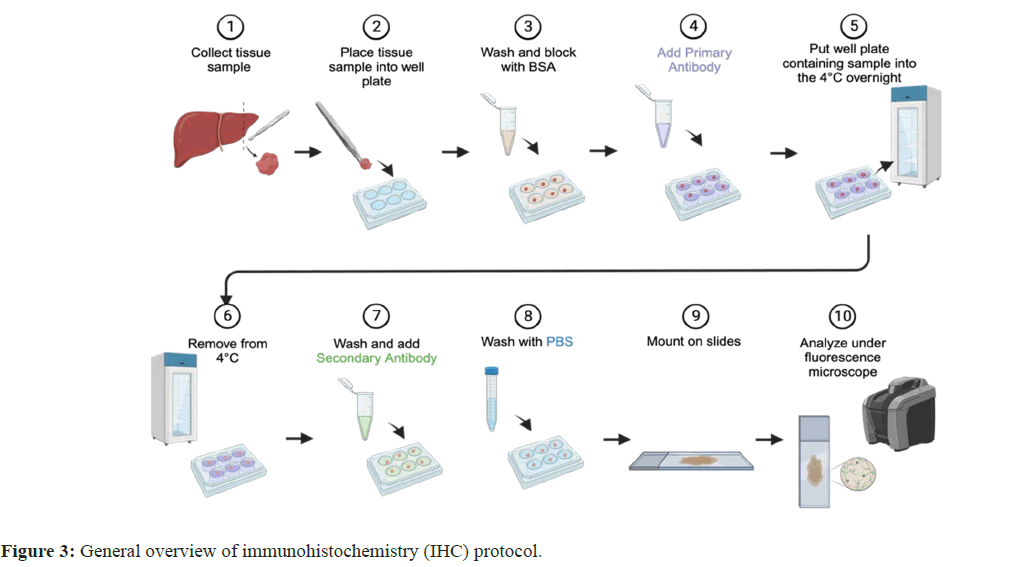
Ultrasound-mediated physical treatment or enzymatic-treated chemical methods with or without heating process are common in practice. As of now, the most widely used method is heat induced antigen retrieval by the use of microwaves, water baths, autoclaves and pressure cookers. Antigen binds with specific primary antibody that may be tagged with fluorescent or chemiluminescent dye (direct test) or secondary antibody specific to primary antibody tagged with fluorescent or chemiluminescent dye (indirect test). After subsequent follow of the approved protocols, result interpretation will be performed based on the fluorescent signal or dye retained in the tissue sections after microscopy (Figure 3) [21].
General overview of immunohistochemistry (IHC) protocol. 1) IHC begins by collecting a tissue sample from a patient. 2) Once the sample is collected it is placed into a well-plate. 3) Once in the wellplate, the sample is washed and blocked with Bovine Serum Albumin (BSA). This helps prevent non-specific binding of antibodies to the tissue sample. 4) The primary antibody is added to the well containing the sample. The primary antibody binds to the amino-acid sequence of interest. 5) The well-plate containing the tissue sample covered in the primary antibody is then placed into the 4o for 24 hours (overnight). 6) The well-plate containing the sample is removed from the 4o after 24 hours. 7) The sample is washed, and the secondary antibody is added. The 2o antibody containing a fluorophore binds to the locations where the primary antibody is also bound to, allowing for the visualization of these target areas via immunofluorescence. 8) The sample is washed with Phosphate Buffered Saline (PBS). 9) The sample is then mounted to the slides. 10) The slide containing the sample is then placed on or in a fluorescence microscope, where it can be viewed and analyzed.
Polymerase Chain Reaction (PCR)
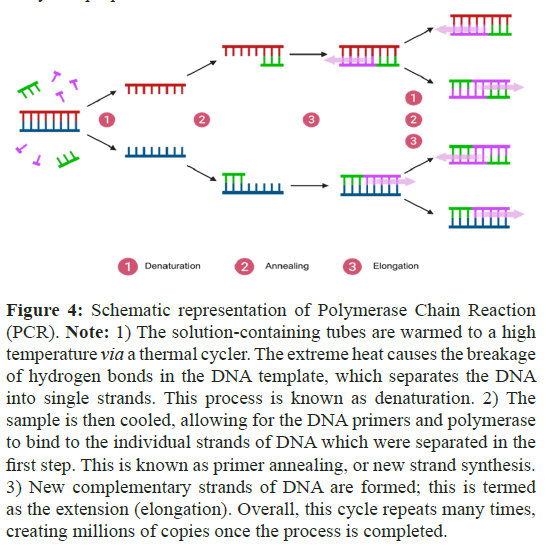
PCR is a reliable and sensitive method used in the diagnosis of cancer. It extends the capability of antibody-based studies to recognize tumor-associated markers with higher sensitivity and specificity, enabling precise tumor detection (Figure 4) [25]. In addition, it also offers a versatile and accessible method for identifying cancerous cells with genetic alterations, enabling the detection of individual cancer cell in a background of up to 100 million normal cells. PCR can be adapted for various applications, including mutation analysis, gene expression profiling, and epigenetic modifications [25]. The challenge of using this method is that PCR primers need to be highly specific to avoid amplification of non-target DNA, and designing specific primers can be challenging, especially of the highly repeated sequences or high CG contents on a target gene. It is therefore limited to the availability of primers designed for known sequences, compromising its use for detecting novel genetic alterations in diagnosis. The disadvantages of PCR include that this technique can yield false positives. RNA and DNA are both very sensitive, thus if samples are contaminated, this can lead to incorrect results. There are different variations in PCR; Reverse Transcription PCR (RTPCR), Quantitative Real-Time PCR (qPCR), Methylation-Specific PCR (MSP) and Digital PCR (dPCR) that are commonly used in the diagnostic field. Each of these methods is tailored towards different analytical purposes.
Figure 4: Schematic representation of Polymerase Chain Reaction (PCR). Note: 1) The solution-containing tubes are warmed to a high temperature via a thermal cycler. The extreme heat causes the breakage of hydrogen bonds in the DNA template, which separates the DNA into single strands. This process is known as denaturation. 2) The sample is then cooled, allowing for the DNA primers and polymerase to bind to the individual strands of DNA which were separated in the first step. This is known as primer annealing, or new strand synthesis. 3) New complementary strands of DNA are formed; this is termed as the extension (elongation). Overall, this cycle repeats many times, creating millions of copies once the process is completed.
RT-PCR is used for quantification and identification of RNA, including mRNA and the transcriptional dynamics of cancer-related genes and their expression patterns within biological samples. Realtime qPCR provides quantitative data, aiding in the assessment of gene expression levels and genomic alterations during the run time or after through the melt-curve analysis. The use of qPCR is to amplify and quantify DNA or RNA. Its data is shown through amplification curves that help determine, analyze, compare and quantify expression levels of cancer associated genes or transcripts. This is useful when monitoring disease progression and investigating treatment effectiveness.
The MSP is a unique PCR that helps examine the DNA methylation patterns on specific genes, even in CpG islands, in identifying atypical epigenetic changes or mutations that play a key role in cancer progression and development [26,27]. It is highly sensitive and specific in detecting methylated DNA sequences. But designing primers specific to methylated and unmethylated DNA can be complex, and optimization is required for accurate results. The cost and time involved in MSP can be higher compared to standard PCR techniques.
The use of dPCR is to detect and quantify specific nucleic acids. It relies on random distribution of genes in microwells, where each well acts as a micro reactor for PCR amplification [28]. It allows precise quantification of target sequences and has applications in detecting genetic variations in breast cancer. It offers high sensitivity, enabling the detection of low-abundance mutations or variants. However, dPCR can be more expensive and technically challenging compared to conventional PCR methods.
Similar to known antibody required to IHC, targets should be known in PCR to specifically design the primers to amplify them in cancer diagnosis. A mistake in primer design or experimental setup can cause inaccurate results. Unlike IHC, PCR is not able so far to amplify the target gene till this date within the cell of the tissue and therefore, it is unable to determine the exact arrangement of these cancerous cells within a tissue sample [24,29].
Fluorescence In Situ Hybridization (FISH)
FISH is a widely used method used to discern and determine a precise location of a specific DNA sequence in the cell's nucleus. Because of the use of fluorescent specific probes, it has a high level of specificity and sensitivity, which can detect genetic abnormalities, for example, chromosomal translocations, inversion, insertions, deletions, and duplications associated with cancer and its subtypes even in a very tiny clinical sample [30].
FISH allows for quantitative analysis of target sequences in the cell and helps identify disease severity. Specific probe design, fluorescent tagging, hybridization protocol and result analysis are some of the important considerations for the success of the FISH technique. FISH protocol usually involves analyzing formalin fixed paraffin-embedded tumor tissue sections using an epifluorescence microscope after subsequent deparaffinization, rehydration and hybridization of the target with fluorescent tagged specific probes (Figure 5) [31]. Compared to IHC, it provides limited information about the cancer biology, for example, protein expression and tissue morphology [32].
Figure 5: Overview of Fluorescence In Situ Hybridization (FISH). Note: 1) A tissue sample is collected from a patient. 2) Sample preparation. The cells are fixed with Neutral Buffered Formalin (NBF). Once fixed, the cells are washed with Phosphate Buffered Saline (PBS) and dehydrated (permeabilized) with ethanol. Denaturation then occurs via the incubation of these fixed cells at a high temperature and low pH. 3) The sample is then hybridized, washed with buffer and stained with DAPI. 4) The mounted sample is then examined and analyzed by imaging.
Comparative Genomic Hybridization (CGH)
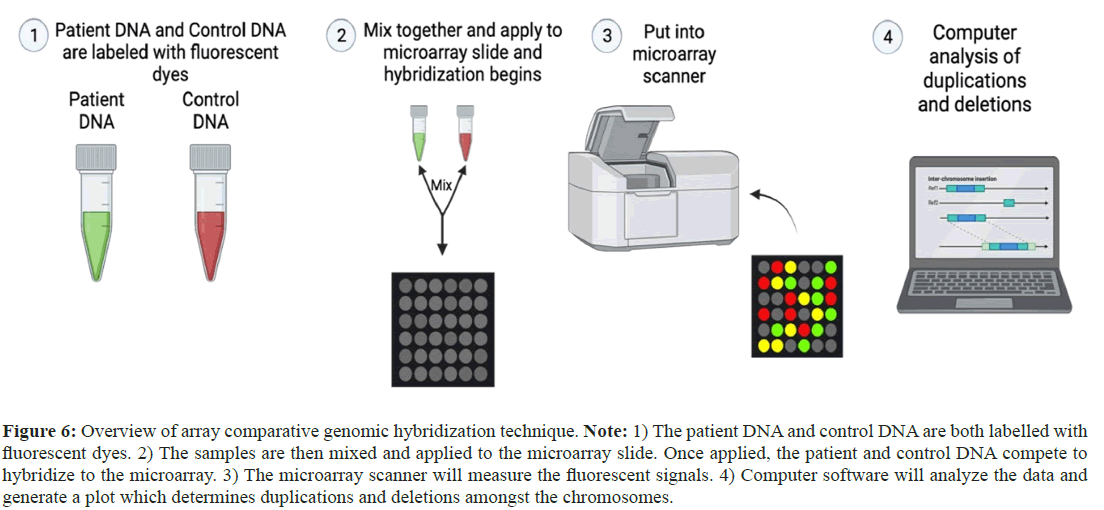
CGH is a molecular technique that is used to compare DNA copy number variants between tumor DNA and its healthy counterpart. It allows for comprehensive and genome-wide assessment of chromosomal alterations in delineating segments of DNA characterized by either amplification or deletion within cancer genomes. With extremely high-resolution mapping system, it has the ability to locate and precisely characterize genetic alterations, offering critical insights into the genomic difficult to interpret results without appropriate software and skilled staff, it has to be used along with other molecular techniques (e.g. FISH) in order to accomplish comprehensive genomic analyses. Comparatively, it is costly to run the test (Figure 6) [33].
Figure 6: Overview of array comparative genomic hybridization technique. Note: 1) The patient DNA and control DNA are both labelled with fluorescent dyes. 2) The samples are then mixed and applied to the microarray slide. Once applied, the patient and control DNA compete to hybridize to the microarray. 3) The microarray scanner will measure the fluorescent signals. 4) Computer software will analyze the data and generate a plot which determines duplications and deletions amongst the chromosomes.
Array Comparative Genomic Hybridization (aCGH), which has a high-resolution Microarray (MA) system, is used to identify Copy Number Variations (CNV’s) in a genome, permitting the concurrent in-depth analysis of many regions of an entire genome or in a target area of interest for the assessment of tumor heterogeneity in a single assay. Because of its higher sensitivity and rapid analysis system, there is a chance of false positives in a control sample, requiring confirmation via other molecular techniques. Like other techniques, they are limited to specific probes for the known targets that limit the determination of the altered targets. Sample quality without degradation or altered target is necessary to get accurate and precise results [33].
Next-Generation Sequencing (NGS)
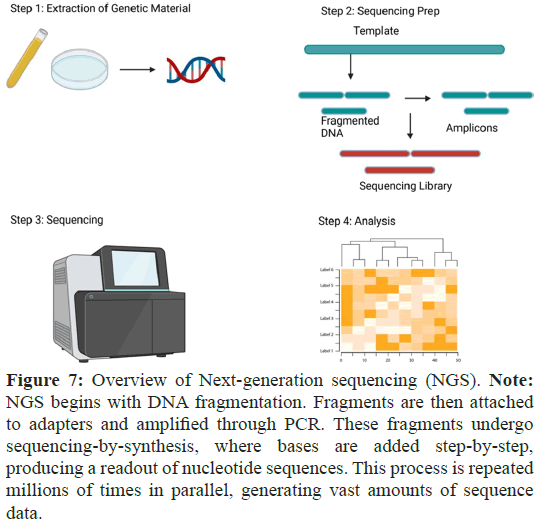
NGS enables to perform a thorough examination of a person’s DNA or RNA, providing detailed insight into their genetic makeup. NGS has proven to be an instrumental technique that is able to provide a molecular portrait of diseases states, including the most common cancer in women, breast cancer. It is a high-throughput DNA sequencing technology that allows for the rapid and costeffective analysis of DNA, RNA and other biomolecules. NGS processes millions of DNA fragments in parallel, producing a highly efficient and accurate vast amount of data. NGS has many applications including Whole Genome Sequencing (WGS), Whole Exon Sequencing (WES), targeted sequencing, metagenomics and transcriptome analysis. The technology has lowered the cost of DNA sequencing, which in turn, has broadened the range of scientific investigations and clinical applications. The technology has helped to identify disease-related genetic variants, studying the microbiome, and understanding gene expression patterns. NGS has allowed medicine to become more personalized by enabling the identification of individual genetic variations and facilitating the development of tailored treatments. With the benefit of accuracy, speed and costeffectiveness, this technology has made its mark on genetic testing (Figure 7) [34]. NGS allows for comprehensive and high-throughput analysis for an entire genome, exome, transcriptome, or targeted genomic regions, providing a detailed view of genetic alterations involved in cancer. The detection of genetic alterations, including point mutations, insertions, deletions, copy number variations, and structural rearrangements, is made easy by NGS, thus facilitating the identification of cancer mutations. It can also assess the heterogeneity inside of a tumor.
Figure 7: Overview of Next-generation sequencing (NGS). Note: NGS begins with DNA fragmentation. Fragments are then attached to adapters and amplified through PCR. These fragments undergo sequencing-by-synthesis, where bases are added step-by-step, producing a readout of nucleotide sequences. This process is repeated millions of times in parallel, generating vast amounts of sequence data.
Samples for NGS can be peripheral blood, frozen tissue, and degraded tissue samples found in formal fixed embedded samples of breast cancer biopsies. NGS general sample preparation procedures include fragmentation and end repair, addition of adaptors, PCR amplification and data analysis. Protocols vary and choices are based on the desired platform to be used. NGS is currently the preferred method in oncology research and much of the preference is due to three characteristic hallmarks: 1) Mass throughout, 2) High speed, and 3) Low cost. RNA sequencing (RNA-Seq) is a reliable method used to analyze gene expression patterns and identify fusion transcripts.
WES is confined to the analysis of protein coding regions of which comprise 1% of the genome, but interestingly exomes contain 85% of disease-causing variants. Current studies have harnessed the power of WES in identifying somatic mutations and potential passenger and driver gene contributors in breast cancer. In contrast to WES, WGS offers coverage of an entire genome, including coding and noncoding regions. This approach can capture and characterize large, small, and novel variants of insertions, deletions, copy number variants, structural variations, and repetitive DNA elements. Data derived from WGS approaches are used to detect problems in genetic repair pathways, homologous recombination repair and mismatch repair systems implicated in the pathology of breast cancer.
NGS requires special bioinformatic tools, connecting to a powerful technology that determines the nucleic acid sequences and recognizes variants in a sample, to manage massive data and for the analysis and data interpretation [35].
It also provides insights into the breast cancer genome, identifying alterations, deletions and rearrangements, offering a comprehensive view of genetic changes associated with breast cancer [36]. Taking comparatively more time for the sample processing and data analysis, it might not be suitable when rapid test is demanded [37].
It provides a technology that is fast, high, and cost-effective, similar to traditional Sanger sequencing (discussed below), for accurately identifying mutations in genes and providing clinically useful information.
Sanger sequencing
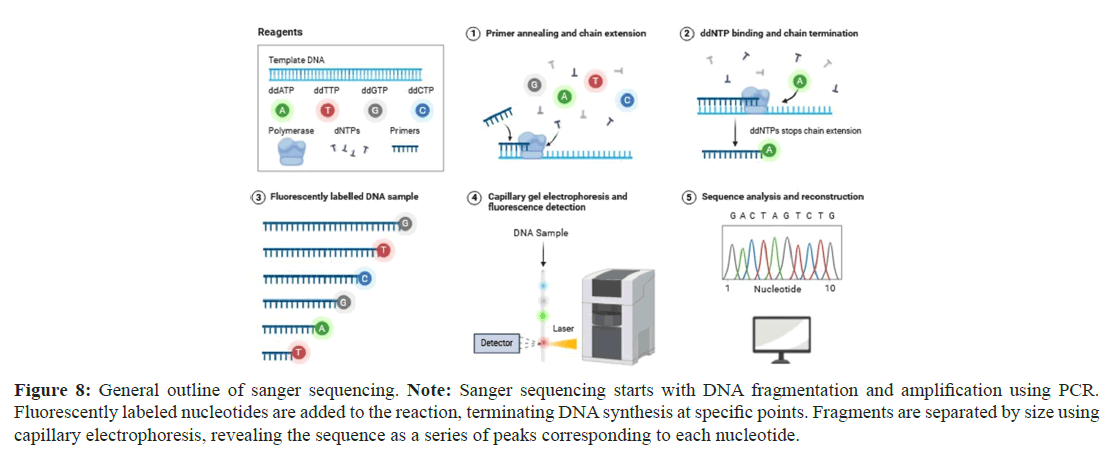
Sanger sequencing, developed in 1977, is a DNA sequencing method that is based on the chain termination principle [38]. It helps to detect mutations in genes like BRCA1 and BRCA2, associated with breast and ovarian tumors [39]. In the Sanger sequencing method, amplified DNA/complementary DNA is annealed to a primer and then is extended by the DNA polymerase enzyme [40]. This polymerase enzyme contains a mixture of 4 deoxynucleotide triphosphates or chain-terminating dideoxynucleoside triphosphates (Figure 8) [40].
Figure 8: General outline of sanger sequencing. Note: Sanger sequencing starts with DNA fragmentation and amplification using PCR. Fluorescently labeled nucleotides are added to the reaction, terminating DNA synthesis at specific points. Fragments are separated by size using capillary electrophoresis, revealing the sequence as a series of peaks corresponding to each nucleotide.
Microarray Analysis (MA)
MA is a high-throughput technique that aids in analyzing multiple samples and identifying marker frequencies in tumors. This technology enables a comprehensive study of genetic variations in breast cancer. Complementary sequences bind to each other and the DNA molecules that are unknown are cut into fragments by restriction endonucleases [41]. Then, fluorescent markers are linked to these DNA fragments. The target DNA fragments and the complementary sequences then join to the DNA probes. Emission of the fluorescence is recorded for the identification of DNA through analytic software with the detail illustration in Figure 9 [41].
Figure 9: An overview of Microarray Analysis (MA). MA involves the hybridization of DNA or RNA samples to a microarray chip containing thousands of probes. Fluorescently labeled samples bind to complementary probes on the chip, generating signals proportional to the abundance of specific nucleic acid sequences. The resulting data is analyzed to identify gene expression patterns, genetic variations, or other molecular interactions.
Liquid biopsies
A liquid biopsy is a non-invasive simple method, which can detect cancer cells in the blood samples, allowing the identification and tracking of circulating tumor DNA (ctDNA), Circulating Tumor Cells (CTCs), and microRNAs for the cancer detection and for the planning of appropriate treatment [6]. It can help to detect an early-stage tiny cancer, which is unnoticed through imaging and clinical symptoms, and to monitor the disease progression. The possibilities of false positive and negative results along with the available of less identified targets in bloodstream limit its use in the clinical diagnosis [42,43]. Circulating Tumor DNA analysis is a crucial method for identifying mutations in breast cancer genes, such as ESR1 and PIK3CA [44]. It holds the potential for tailoring therapies, detecting resistant mutations, guiding therapies and monitoring treatment response [44]. This method is based on a simple two-step procedure [45]. The first step is the sample centrifugation to remove plasma components while circulating tumor cells are captured with the anti-epithelial cell adhesion moleculeconjugated magnetic ferrofluids. The second step involves presumed circulating tumor cells which are stained and recognized using anticytokeratin antibodies, while white blood cells are identified by CD 45 staining [45].
Mass spectrometry
MS measures the mass-to-charge ratio in molecules in a sample. A subtype of MS known as Liquid Chromatography-Mass Spectrometry (LC-MS) separates, identifies and quantifies unknown and known compounds to decipher the structure and chemical properties of molecules. This technique is utilized when characterizing the levels of protein expression in both tumor tissue and healthy tissue (or blood). It is a highly sensitive technique that can detect even the smallest concentrations of biomolecules involved in cancer formation (Figure 10). It can also analyze multiple analytes in a single experimental run, allowing for the detection of many biomarkers or profiling of complex biological processes that are relevant to cancer. This technique is also used in biomarker discovery and validation, essential for developing new diagnostic tests and specialized treatments. On the other hand, MS is technically complex and requires special instruments and expertise in sample preparation, data collection, and analysis. Sample preparation is extensive and time-consuming for purification and extraction steps. MS lack specificity for the certain biomolecules due to similar mass-to-charge ratios and confirmation of those biomolecules should be performed using other molecular techniques [46].
Figure 10: Mass Spectrometry (MS) overview from sample to reading. Note: Mass spectrometry (MS) analyzes molecules based on their mass-to-charge ratio. In proteomics, for instance, proteins are fragmented into peptides and ionized. These ions are then separated based on their mass-to-charge ratio and detected, producing a spectrum. By comparing these spectra to databases, proteins can be identified and quantified, providing insights into biological processes and diseases.
Breast cancer and molecular diagnosis
Biomarkers are the groundwork upon which modern medicinal research is built on, providing scientists and healthcare workers with a quest to understand the mysteries of human health and disease. One of the most intriguing parts of biomarkers is their diversity. Genetic biomarkers, which include mutations and different variations in DNA, offer an unaverred insight into a patient’s susceptibility to a disease [47]. For reference, certain genetic markers can show an increased risk of harmful conditions, like breast cancer or Alzheimer’s disease. The knowledge biomarkers provide can help make informed decisions about a patient’s health and the ability to seek preventative measures [47].
Metabolic biomarkers, often found in urine or blood, highlight the inner workings of metabolism [48]. Raised levels of specific metabolites can show underlying metabolic disorders like diabetes or metabolic syndrome. Through the targeted analysis of these biomarkers, providers can make specific interventions to manage and mitigate the conditions effectively [48].
Imaging biomarkers, like those derived by medicinal imaging techniques like PET scans and MRI, provides visual evidence of changes physiologically in the body [48]. In neurodegenerative diseases like Alzheimer, amyloid pet scans show the accumulation of amyloid plaques in the brain, which is a hallmark of the disease [47]. These imaging biomarkers often facilitate diagnosis early on and provide disease monitoring and enabling timely interventions.
As medical research advances, biomarkers continue to stretch the boundaries of healthcare. In the world of neurodegenerative disorders, they are becoming crucial tools. The identification of biomarkers associated with conditions such as Alzheimer's or Parkinson’s diseases is critical to decipher the signs of the disease. Biomarkers provide valuable insight into the mechanisms of disease. They open doors to new and innovative therapeutic strategies and early interventions that can slow the progression of the disease.
Breast cancer biomarkers
Breast cancer biomarkers have served critical in understanding its nuances and creating treatments [47]. Estrogen Receptor (ER), Progesterone Receptor (PR), and Human Epidermal Growth Factor Receptor 2 (HER2) are the foundation of breast cancer biomarkers. ER-positive tumors are shown by cancer cells having estrogen receptors. The status of these receptors guides patient treatment decisions. These can include hormonal therapies like tamoxifen or aromatase inhibitors, which are often effective in ER-positive breast cancer [47]. Progesterone receptor affects treatment choices and can provide insights into prognosis. HER2, which is a protein that aids in cellular growth, is amplified or overexpressed in HER2-positive breast cancers. Trastuzumab and Epratuzumab are targeted therapies that have revolutionized treatment to this subtype of aggressive cancer [47].
Ki-67, which is a cellular proliferation biomarker, holds a high place in breast cancer research. High levels of Ki-67 suggest that a tumor is more aggressive with a faster growth rate. Clinicians often use this information to create treatment strategies, determining the need for more aggressive therapies in the cases of high Ki-67 [47].
Biomarkers like BRCA1 and BRCA2 mutations often highlight the interplay between breast cancer risk and genetics. These mutations are not measured within the tumor tissues, but if found, they serve as predictors of breast cancer hereditary risk. This calls for women with BRCA mutations to have more frequent screening and consider riskreduction surgeries [47].
Breast cancer biomarkers strand as critical tools for fighting one of the most prevalent and life-threatening diseases that affect men and women worldwide. Molecular indicators, like estrogen receptor, progesterone receptor and HER2 provide invaluable insights into the biology of breast cancer that guides treatment decisions and improves the patients' outcomes that are affected. This insight is provided by the ever-evolving technologies for biomarker detection [47-54].
Biomarker detection methods
The world of biomarker detection technologies is almost as diverse as the biomarkers themselves. The field has witnessed advancements at the intersection of biology, chemistry and engineering. The fields have expedited the ability from sensitive detection of proteins to the sequencing of genetic biomarkers. The landscape of biomarker detection has a diverse array of tools and technologies that empower researchers and clinicians.
Enzyme-Linked Immunosorbent Assay (ELISA) is the stalwart for biomarker detection, capitalizing on the specificity of antibodies. The technique excels in quantifying protein biomarkers that have a high sensitivity, making it a staple for diagnostics and research. The multiple different types of ELISA, including competitive, sandwich, indirect, and direct, offer a wide range of immunologic reaction detection. However, it is a slow process, it is usable for detection of multiple biomarkers at the same run [54]. Its capability is crucial in personalized medicine and comprehensive disease profiling [49].
PCR is a molecular biology technique that has changed the way scientists work with nucleic acids. It enables the precise amplification and detection of DNA and RNA sequences, and aids in the precision of genetic testing, cancer research, and infectious disease diagnostics. The greatest strength of PCR lies in its ability to amplify specific nucleic acid sequences within a complex mixture in a selective manner. In genetic testing, the role it plays in identifying genetic variations responsible for hereditary diseases was not seen before. PCR has provided researchers with the ability to target and amplify genes, which makes it possible to detect mutations that are associated with various conditions, thereby enhancing early diagnosis and personalized treatment [50].
MS is a technique that allows for the precise determination of the mass-to-charge ratio of charged particles. It offers insights into the composition and structure of molecules. In the field of molecular diagnostics, the technique is crucial for identifying biomarkers, like proteins, peptides and metabolites associated with specific diseases. The technology offers researchers information about the presence or absence of these biomarkers in samples, allowing early disease detection, monitoring and personalized treatment strategies. Additionally, MS is utilized in genomic research to analyze nucleic acids and study DNA and RNA sequences. In genetic testing, the instrument is capable of identification of genetic mutations associated with heredity diseases and various disorders [51].
LC-MS and Gas Chromatography-Mass Spectrometry (GC-MS) are analytical methods that augment MS by incorporating a separation dimension. This additional dimension greatly improves the specificity and sensitivity of biomarker detection. These two techniques excel in deciphering metabolic pathways and identifying metabolic dysregulation in diseases like cancer and diabetes. Being able to separate complex mixtures LC-MS and GC-MS enables researchers to pinpoint and quantify specific molecules, providing insights into disease mechanisms and potential diagnostic biomarkers. Despite the revolutionary capabilities the adoption of these technologies is still very limited due to the high cost of the equipment and the analytical complexities associated with the techniques [52].
Nuclear Magnetic Resonance Spectroscopy (NMR) is nondestructive and non-invasive analytical technique used for detection of biomarkers. It works by decoding the nuclear properties of molecules. This shows the structure and concentration of biomarkers. It also offers insights into protein interactions, providing information on the composition, dynamics of metabolites, and conformation of proteins and allowing scientists to further understand biochemical processes and interactions. A limitation of this technique is its relatively lower sensitivity compared to MS. This implies that NMR will require a higher concentration of biomarkers for effective detection. This sensitivity constraint can limit applications and scenarios when biomarker concentrations are low [53].
MAs have enabled the simultaneous analysis of thousands of proteins, genes, or other biomolecules within a single experiment. Their applications extend to a multitude of domains, showing to be a pivotal tool in unraveling the genetic underlying of various diseases. It works by detecting and quantifying gene expression biomarkers. MAs have significantly advanced genomic research by allowing researchers to process large datasets rapidly. They have also been a crucial technology in the development of personalized medicine by enabling tailored treatments based on an individual’s genetic profile [54]. They have become popular in glucose monitoring for diabetes management and detection of infectious diseases. They also play a significant role in the detection of cancer biomarkers. They detect specific molecules associated with cancer, such as early detection of proteins or genetic markers that aid in timely intervention. The portability and rapid results offered make them very useful for pointof- care cancer diagnostics and enhancing accessibility to critical healthcare services [55].
These technologies are the current toolkit for modern biomarker research and diagnostics, each providing its own strengths and limitations. The choice of technology depends on factors like type of biomarker, sensitivity and specificity required, sample complexity and the intended application. As technology advances, the field of biomarker detection is continuing to grow, the future techniques off a transformative potential of the molecular signposts.
New technologies like Multi-Omics Integration (MOI) use data from multiple osmic layers to uncover intricate biomarker networks. Liquid biopsies, which analyze biomarkers in the blood or other bodily fluids, is revolutionizing cancer diagnosis and monitoring cancer prognosis. The non-invasive tests give a real-time a look at tumor dynamics, empowering physicians to make time effective treatment decisions and track treatment responses. AI further helps drive algorithms for biomarker discovery and interpretation. The algorithms sift through vast datasets to identify novel biomarker candidates and predict the disease outcomes (Tables 1 and 2) [47].
| Biomarkers | Description | Function | Therapies | Gene location | References |
|---|---|---|---|---|---|
| ER | Protein in the body acts as a regulation in various physiological processes. | Mediates effects of the hormone estrogen. | Targeted therapies, Hormone therapy, Surgery | Long arm of chromosome 6 (6q25.1) | [56] |
| PR | Protein that responds to hormone progesterone. | Regulates various reproductive processes in female reproductive system | Targeted therapies | Short arm of chromosome 11 (11p15.1) | [56] |
| Hormone Replacement Therapy | |||||
| Genetic Counseling | |||||
| HER2 | Protein found on surface of same breast cancer cells. | Regulates cell growth and division. Also helps with cell growth and repair. | Specific Drugs | long arm of chromosome 17 (17q12) | [57] |
| Hormone Therapy | |||||
| Immunotherapy | |||||
| Ki-67 | Protein marker in cancer pathology for cell proliferation and growth. | In the cell cycle associated with growth and division | Used in treatment to identify aggressive faster growing cancers | NA | [58] |
| BRCA1 | Human gene associated with increased risk of breast and ovarian cancer | Tumor suppressor gene that maintains genomic stability and DNA repair | Surveillance/screening | Long arm of chromosome 17 (17q21.31) | [59] |
| Reducing Surgery Chemoprevention | |||||
| Genetic Counseling | |||||
| BRCA2 | Human gene with increased risk of breast and ovarian cancer. Plays crucial role in DNA repair | Assists in repairing double-strand DNA breaks, crucial for preventing cancerous mutation | Surveillance/ screening | Long arm of chromosome 13 (13q13.1) | [60] |
| Reducing Surgery | |||||
| Chemoprevention | |||||
| Genetic Counseling |
Table 1: Summarizing some important biomarkers used in molecular diagnostics.
| Methods | Purpose | Advantages | Others | References |
|---|---|---|---|---|
| ELISA | Used to detect and quantify protein, antibodies, antigens and hormones. | Relatively easy to perform compared to other techniques. Protocol and reagents can vary | Generally, a low cost compared to other similar techniques | [49] |
| PCR | Used to amplify DNA for other tests. Fundamental for gene cloning, genotyping and gene expression analysis | Considered easy to perform. | Cost effective technique used for routine application compared to more complex and expensive methods. | [50] |
| Contamination risk is high. | ||||
| MS-LMS | Employed for identifying and quantifying molecule based on mass and charge | More technically challenging and requires specialized experts. Sample preparation and instrument are complex | More expensive than other common techniques. Requires more powerful analytical capabilities | [51] |
| Liquid Chromat ography | Utilized for separating, identifying and quantifying compounds in a mixture. Applications in pharmaceutical, environmental and food testing. | Moderately complex. | Cost can vary depending on the system and detectors used. Varies due to the versatility | [52] |
| Specialized knowledge is required for chromatography. | ||||
| NMR | Used to study molecular structure, dynamics and interactions of compounds. | NMR is a sophisticated technique that can be technically challenging and requires specialized knowledge and experience. | The machines and maintenance are expensive. However, running them is relatively cheap. | [53] |
| IHC | Used to detect and visualize specific proteins or antigens in tissue sections. Used in histopathology and cancer diagnosis | It can be technically challenging and may require expertise in histology and immunology. | Cost can vary depending on reagents, antibodies and equipment but generally more affordable than some other advanced techniques. | [19] |
| Microarrays | This technique measures the expression levels of thousands of genes or proteins. | Experiments are technically complex and require expertise in statistical analysis and genomics. | Varies depending on the platform and number of samples. High-throughput capabilities make it suitable for large-scale | [54] |
| NGS | Specializes in rapid DNA sequencing, transcriptomic and epigenome data | NGS is highly complex technology involving library preparation, sequencing, and bioinformatics analysis | Cost is expensive due to equipment, consumables and data analysis | [34] |
| Electrochemical Biosensors | Employed for rapid and sensitive detection of specific biological molecules like proteins and DNA. | These are user- friendly and offer rapid results compared to other analytical techniques. | Varying depending on the complexity of the device and the specific application | [61] |
Table 2: Summarizing some important techniques used in molecular diagnostics.
Advantages and disadvantages of the breast cancer diagnostic methods
Molecular diagnostic methods play a critical role in understanding the molecular and genetic alterations associated with breast cancer and this aids early detection, prognosis, treatment decisions, and the monitoring of breast cancer. Each molecular diagnostic method has its own set of benefits and drawbacks.
One of the most significant benefits of molecular testing for breast cancer is the possibility of providing personalized therapy options. This is achieved by analyzing indicators such as hormone receptor status. The identification of ER and PR in tumor tissue enables targeted treatment with hormone therapy, which successfully prevents tumor cells from obtaining the hormones they require for growth. Since it tackles the individual characteristics of the tumor, this personalized approach raises the likelihood of good treatment outcomes.
Similarly, FISH can precisely identify genetic deviations and specific abnormalities in tumor cells, such as translocations, insertions, deletions, and amplifications [31]. It provides information at the cellular level, allowing for the assessment of abnormalities in individual cells. Using fluorescent probes enables visualization of genetic alterations under a microscope. However, FISH requires skilled technicians and significant labor, making it a time-consuming and expensive technique. Interpretation of FISH results can be subjective and dependent on the experience of the observer. It is also limited to the specific markers targeted by the probes used, potentially missing other important genetic alterations.
NGS is another type of diagnostic method used to diagnose breast cancer. NGS allows for a comprehensive analysis of the entire genome, providing insights into mutations, rearrangements, and variations [62]. It can process many samples simultaneously, improving efficiency and cost-effectiveness. Managing and analyzing the vast amount of sequencing data generated by NGS can be computationally intensive and requires specialized expertise. Also, the initial set-up and running costs of NGS can be high, although costs have been decreasing over time.
Sanger Sequencing is considered as gold standard for validation. Sanger sequencing is often used as a validation tool due to its accuracy and reliability in detecting specific mutations [63]. Sanger sequencing is less suitable for high-throughput applications because of its limitations in processing many samples efficiently.
MA technique aids in analyzing samples. It offers high throughput. MAs can analyze thousands of genes simultaneously, providing a broad view of gene expression patterns and genomic alterations. They aid in identifying potential biomarkers for breast cancer diagnosis, prognosis, and treatment [47]. One of the drawbacks of using hybridization-based techniques is these techniques can be sensitive to variations in hybridization conditions, affecting the accuracy of results. Circulating Tumor DNA (ctDNA) Analysis is a non-invasive method that utilizes blood samples, offering a less invasive approach to monitoring disease progression and treatment response. Sensitivity and specificity can be challenging, particularly for detecting low levels of ctDNA in early-better diagnosis, prognosis, and targeted treatment strategies. The tests and results must be carefully chosen to optimize treatment decisions for each patient and ensure the best possible outcomes in the fight against breast cancer.
Conclusion
Molecular diagnostic methods play an important role in breast cancer detection and diagnosis. Techniques like FISH, PCR, MSP, dPCR, NGS, Sanger sequencing, IHC, MA, and circulating tumor analysis. DNA analysis offer valuable insights into genetic alterations, aiding in precise tumor detection, characterization, and treatment planning.
In conclusion, this paper underscores the pivotal role of molecular approaches in revolutionizing breast cancer diagnosis, emphasizing the transformative potential of personalized medicine in improving patient care and prognosis. By elucidating the intricacies of molecular diagnostics and delineating avenues for future research, it contributes to the ongoing discourse aimed at advancing diagnostic strategies and ultimately combating breast cancer more effectively.
Acknowledgements
The authors thank the colleagues and students of the Medical Laboratory Sciences, Public Health and Nutrition Science Department, Tarleton State University, for their critical comments and suggestions.
Fundings
This review project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Lawrence RA (2022) Anatomy of the breast. Breastfeeding 38-57.
- Gupta M, Goyal N (2022) Applied anatomy of breast cancer. InBreast Cancer: Comprehensive Management 23-35.
- Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA et al.(2022) Breast cancer statistics, 2022. CA Cancer J Clin 72(6):524-541.
[Crossref] [Google scholar] [Pubmed]
- Mannu GS, Wang Z, Broggio J, Charman J, Cheung S, et al.(2020) Invasive breast cancer and breast cancer mortality after ductal carcinoma In Situ in women attending for breast screening in England, 1988-2014: Population based observational cohort study. BMJ. 27;369.
- Yin L, Duan JJ, Bian XW, Yu SC (2020) Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res 22:1-3.
[Crossref][Google scholar] [Pubmed]
- Rasool A, Bunterngchit C, Tiejian L, Islam MR, Qu Q et al.(2020) Improved machine learning-based predictive models for breast cancer diagnosis. Int J Res Public Health 19(6):3211.
[Crossref] [Google scholar] [Pubmed]
- Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R et al. (2021) Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancer 13(17):4287.
[Crossref] [Google scholar] [Pubmed]
- Younis YS, Ali AH, Alhafidhb OK, Yahia WB, Alazzam MB et al. (2022) Early diagnosis of breast cancer using image processing techniques. J Nanomater 2022(1):2641239.
- Mohiyuddin A, Basharat A, Ghani U, Peter V, Abbas S, et al. (2022) [Retracted] Breast Tumor Detection and Classification in Mammogram Images Using Modified YOLOv5 Network Comput Math Methods Med 2022(1):1359019.
- Bhushan A, Gonsalves A, Menon JU (2021) Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics 13(5):723.
- Leyland-Jones BR, Ambrosone CB, Bartlett J, Ellis MJ, Enos RA, et al. (2008) Breast international group; cooperative groups of the breast cancer intergroup of north america (tbci); american college of surgeons oncology group; cancer and leukemia group b; eastern cooperative oncology group; north central cancer treatment group; national cancer institute of canada clinical trials group; southwest oncology group; national surgical adjuvant breast and bowel project. Radiation Oncology Group 5638-44.
- Koh SB, Dontchos BN, Bossuyt V, Edmonds C, Cristea S, et al (2021) Systematic tissue collection during clinical breast biopsy is feasible, safe and enables high-content translational analyses. NPJ Precision Oncology.5(1):85.
- McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM (2016) Clinical diagnosis and management of breast cancer. J Nucl Med. 57:9S-16S.
- Omilian AR, Zirpoli GR, Cheng TY, Yao S, Stein L, et al. (2020). Storage conditions and immunoreactivity of breast cancer subtyping markers in tissue microarray sections. AMIM 28(4):267-273.
- Gerami R, Joni SS, Akhondi N, Etemadi A, Fosouli M, et al. (2022) A literature review on the imaging methods for breast cancer. Int J of Physiol Pharmacol 14(3):171.
[Google scholar] [Pubmed]
- Sadoughi F, Kazemy Z, Hamedan F, Owji L, Rahmanikatigari M, et al. (2018) Artificial intelligence methods for the diagnosis of breast cancer by image processing: a review. Breast Cancer 30:219-30.
- Guzman-Cabrera R, Guzmán-Sepúlveda JR, Torres-Cisneros M, May-Arrioja DA, Ruiz-Pinales J, et al. (2013) Digital image processing technique for breast cancer detection. Int J Thermophysics 34:1519-1531.
- Gupta S, Sinha N, Sudha R, Babu C (2019) Breast Cancer Detection Using Image Processing Techniques. IEEE 1:1-6.
- Cimino-Mathews A (2021) Novel uses of immunohistochemistry in breast pathology: interpretation and pitfalls. Mod Pathol 34:62-77. Crossref]
[Pubmed] [Google scholar]
- Hawes D, SHI SR, Dabbs DJ, Taylor CR, Cote RJ (2019) Immunohistochemistry. Modern surgical pathology 48.
[Google scholar] [Pubmed] [Crossref]
- Magaki S, Hojat SA, Wei B, So A, Yong WH (2019) An introduction to the performance of immunohistochemistry. Methods Mol Biol 1897 289-298.
[Crossref] [Google Scholar] [Pubmed]
- Kim SW, Roh J, Park CS (2016) Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med 50(6):411-418.
[Crossref] [Google Scholar] [Pubmed]
- Zaha DC (2014) Significance of immunohistochemistry in breast cancer. World J Clin Oncol 5(3):382-392.
[Crossref] [Google Scholar] [Pubmed]
- Shabanzadeh S, Vatandoust S, Hosseinifard SM, Sheikhzadeh N, Shahbazfar AA (2023) Dietary astaxanthin (Lucantin® Pink) mitigated oxidative stress induced by diazinon in rainbow trout (Oncorhynchus mykiss). Vet Res Forum 14(2):97-104.
[Crossref] [Google Scholar] [Pubmed]
- Raj GV, Moreno JG, Gomella LG (1998) Utilization of polymerase chain reaction technology in the detection of solid tumors. Cancer 82(8):1419-1442.
[Crossref] [Google Scholar] [Pubmed]
- Ghannam MG, Varacallo M (2023) Biochemistry, polymerase chain reaction.
[Google Scholar] [Pubmed]
- Ku JL, Jeon YK, Park JG (2011) Methylation-specific PCR. Methods Mol Biol 791:23-32.
[Crossref] [Google Scholar] [Pubmed]
- Quan PL, Sauzade M, Brouzes E (2018) dPCR: a technology review. Sensors (Basel) 18(4):1271.
[Crossref] [Google Scholar] [Pubmed]
- Licchesi JD, Herman JG (2009) Methylation-specific PCR. Methods Mol Biol 507:305-323.
[Crossref] [Google Scholar] [Pubmed]
- Cui C, Shu W, Li P (2016) Fluorescence In Situ hybridization: cell-based genetic diagnostic and research applications. Front Cell Dev Biol 4:89.
[Crossref] [Google Scholar] [Pubmed]
- Shakoori AR (2017) Fluorescence In Situ hybridization (FISH) and its applications. Chromosome structure and aberrations 343-367.
- Chrzanowska NM, Kowalewski J, Lewandowska MA (2020) Use of fluorescence In Situ hybridization (FISH) in diagnosis and tailored therapies in solid tumors. Molecules 25(8):1864.
[Crossref] [Google Scholar] [Pubmed]
- Bejjani BA, Shaffer LG (2006) Application of array-based comparative genomic hybridization to clinical diagnostics. J Mol Diagn 8(5):528-33.
[Crossref] [Google Scholar] [Pubmed]
- McCombie WR, McPherson JD, Mardis ER (2019) Next-generation sequencing technologies. Cold Spring Harb Perspect Med 9(11):a036798.
[Crossref] [Google Scholar] [Pubmed]
- Satam H, Joshi K, Mangrolia U, Waghoo S, Zaidi G, et al. (2023) Next-generation sequencing technology: current trends and advancements. Biology (Basel) 12(7):997.
[Crossref] [Google Scholar] [Pubmed]
- Alekseyev YO, Fazeli R, Yang S, Basran R, Maher T, et al. (2018) A next-generation sequencing primer—how does it work and what can it do?. Acad Pathol 5:2374289518766521.
[Crossref] [Google Scholar] [Pubmed]
- Qin D (2019) Next-generation sequencing and its clinical application. Cancer Biol Med 16(1):4-10.
[Crossref] [Google Scholar] [Pubmed]
- Totomoch-Serra A, Marquez MF, Cervantes-Barragán DE (2017) Sanger sequencing as a first-line approach for molecular diagnosis of Andersen-Tawil syndrome. F1000Res 6:1016.
[Crossref] [Google Scholar] [Pubmed]
- Nicolussi A, Belardinilli F, Mahdavian Y, Colicchia V, D’Inzeo S, et al. (2019) Next-generation sequencing of BRCA1 and BRCA2 genes for rapid detection of germline mutations in hereditary breast/ovarian cancer. PeerJ 7:e6661.
[Crossref] [Google Scholar] [Pubmed]
- Crossley BM, Bai J, Glaser A, Maes R, Porter E, et al. (2020) Guidelines for Sanger sequencing and molecular assay monitoring. J Vet Diagn Invest 32(6):767-775.
[Crossref] [Google Scholar] [Pubmed]
- Govindarajan R, Duraiyan J, Kaliyappan K, Palanisamy M (2012) Microarray and its applications. J Pharm Bioallied Sci 4(2):S310-312.
[Crossref][Google Scholar] [PubMed]
- Caputo V, Ciardiello F, Della Corte CM, Martini G, Troiani T, et al. (2023) Diagnostic value of liquid biopsy in the era of precision medicine: 10 years of clinical evidence in cancer. Explor Target Antitumor Ther 4(1):102.
- Nikanjam M, Kato S, Kurzrock R (2022) Liquid biopsy: current technology and clinical applications. J Hematol Oncol 15(1):131.
- Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, et al. (2013) Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 368(13):1199-1209.
- Castro-Giner F, Aceto N (2020) Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med 12(1):31.
- Messner CB, Demichev V, Wang Z, Hartl J, Kustatscher G, et al. (2023) Mass spectrometry‐based high‐throughput proteomics and its role in biomedical studies and systems biology. Proteomics 23(7-8):2200013.
- Dai X, Xiang L, Li T, Bai Z (2016) Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer 7(10):1281.
- Fu SW, Chen L, Man YG (2011) miRNA biomarkers in breast cancer detection and management. J Cancer 2:116.
- Alhajj M, Zubair M, Farhana A (2023) Enzyme linked immunosorbent assay. StatPearls.
- Green MR, Sambrook J (2019) Polymerase chain reaction. Cold Spring Harb Protoc (6):095-109.
- Alseekh S, Aharoni A, Brotman Y, Contrepois K, D’Auria J, et al. (2021) Mass spectrometry-based metabolomics: a guide for annotation, quantification and best reporting practices. Nat Methods 18(7):747-756.
- Seger C, Salzmann L (2020) After another decade: LC–MS/MS became routine in clinical diagnostics. Clin Biochem 82:2-11.
- Emwas AH, Roy R, McKay RT, Tenori L, Saccenti E, et al. (2019) NMR spectroscopy for metabolomics research. Metabolites 9(7):123.
- Dang K, Zhang W, Jiang S, Lin X, Qian A (2020) Application of lectin microarrays for biomarker discovery. ChemistryOpen 2020 Mar;9(3):285-300.
- Chen C, Gong X, Yang X, Shang X, Du Q, et al. (2019) The roles of estrogen and estrogen receptors in gastrointestinal disease. Oncol Lett 18(6):5673-5680.
- Schettini F, Prat A (2021) Dissecting the biological heterogeneity of HER2-positive breast cancer. Breast 59:339-350.
- Uxa S, Castillo-Binder P, Kohler R, Stangner K, Müller GA, et al. (2021) Ki-67 gene expression. Cell Death Differ 28(12):3357-3370.
- Sharma B, Kaur RP, Raut S, Munshi A (2018) BRCA1 mutation spectrum, functions, and therapeutic strategies: The story so far. Curr Probl Cancer 42(2):189-207.
- Andreassen PR, Seo J, Wiek C, Hanenberg H (2021) Understanding BRCA2 function as a tumor suppressor based on domain-specific activities in DNA damage responses. Genes (Basel) 12(7):1034.
- Cesewski E, Johnson BN (2020) Electrochemical biosensors for pathogen detection. Biosensors Bioelectronic 159:112214.
- Meldrum C, Doyle MA, Tothill RW (2011) Next-generation sequencing for cancer diagnostics: a practical perspective. Clin Biochem Rev 32(4):177.
- Arsenic R, Treue D, Lehmann A, Hummel M, Dietel M, et al. (2015) Comparison of targeted next-generation sequencing and Sanger sequencing for the detection of PIK3CA mutations in breast cancer. BMC Clinical Pathology 15:1-9.
- Tzanikou E, Lianidou E (2020) The potential of ctDNA analysis in breast cancer. Crit Rev Clin Lab Sci 57(1):54-72.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi