Research Article, J Chem Appl Chem Eng Vol: 1 Issue: 1
Assessment of De-fluoridation in Waste Water Using Activated Biochar: Thermodynamic and Kinetic Study
Swapnila Roy* and Papita Das
Department of Biological and Chemical Engineering, Hongik University, Sejong, South Korea
*Corresponding Author : Swapnila Roy
Department of Chemical Engineering, Jadavpur University, Kolkata, India
Tel: +91 3324572696
E-mail: swapnilaroy@gmail.com
Received: July 27, 2017 Accepted: September 08, 2017 Published: September 15, 2017
Citation: Roy S, Das P (2017) Assessment of De-fluoridation in Waste Water Using Activated Biochar: Thermodynamic and Kinetic Study. J Chem Appl Chem Eng 1:1 doi: 10.4172/2576-3954.1000102
Abstract
Thermally treated domestic food waste is utilized to prepare biochar, followed by chemical activation to obtain activated bio-char (Biochar-act) which is used as de-fluoridating agent in contaminated water. The structural property of the synthesized activated bio-char is studied in detail. From continuous batch process , it is shown that efficiency of bio-char with lower degree of carbonization has remarkable properties of de-fluoridation. Here temperature, contact time and adsorbent dose , used as process parameters, have strong influence on fluoride uptake process. The adsorption equilibrium data are well fitted to the Langmuir isotherm model. Comparatively the data provided by pseudo-second-order kinetic model correlated better experimentally than pseudo-first-order kinetic model. From thermodynamic point of view, it is experimentally proved that de-fluoridation onto activated bio-char is spontaneous and endothermic in nature. Here, a low-cost process is suggested using low-cost starting materials to convert bio-char upon heat treatment at 350�?C and then activated chemically by acid. The prepared adsorbent has higher de-fluoridation efficiency such as 91.24%. So it may be concluded that activated bio-char is economically and environmentally safe for de-fluoridation in waste water.
Keywords: De-fluoridation; Activated bio-char; Thermodynamic and kinetic study; Desorption study
Introduction
The most electronegative halogen is fluorine and its gaseous state is the strong oxidizing agent. Fluoride ion exists as F- ion. The toxicity of fluoride [1] is mainly observed when it exceeds the threshold limit of 1.5 mg/L [2]. The dental and skeletal fluorosis may occur by excessive consumption of fluoride. So it is necessary for removal of fluoride [3,4] by using different types of adsorbent such as charcoal, tamarind seed, agricultural waste materials, bermuda grass, ionic resin etc. There is difference between carbonized and chemically treated forms. Only continuous batch process (experimental procedure by varying three process parameters such as contact time, temperature and adsorbent dose) does not give fine optimization of de-fluoridation. So, thermodynamics and kinetics study are used for optimization of the de-fluoridation efficiency. The source of industrial fluoride are hydrofluoric Acid (HF), ammonium bi-fluoride(NH4HF). Fluoride has a very high affinity towards calcium (Ca) due to high electronegative character in periodic table. As fluoride is negative ion so it is naturally attracted by positive calcium ion. Fluorosis is resulted from the uptake of high amount of fluoride by children and adults, that’s why fluorosis [5] is spread in mild and high form. Conventionally, de-fluoridation from waste water is conducted by adding lime followed by precipitation of fluoride. Different method is utilized for de-fluoridation of water such as ion-exchange precipitation, reverse osmosis and electro coagulation. Activated carbon is the most important adsorbents because of excellent adsorptive capacity. There are different type of materials which are used for preparation of activated carbon such as walnut, wheat bran, saw dust, lemon shell etc. Mostly, activated carbon are utilized in waste water treatment, gas and water purification etc.
The present de-fluoridation study was performed with the aim to prepare chemically activated bio-char [6] prepared from urban food waste. The physical and chemical properties of the prepared activated bio-char [7-9] were determined and de-fluoridation efficiency is determined using different experimental procedure by adsorption as a function of contact time, adsorbent dose and temperature.
Materials and Methods
Preparation of adsorbent
Domestic food waste was available from regular household work. The food waste consisted of a variety of cooked food (like rice and chicken gravy), uncooked food (fruit peels, vegetable parts). The collected waste was initially weighed and bones, eggshells, plastic utensils, etc. were separated out. Then food waste was mixed homogeneously using blender and then stored at refrigerator temperature (at 40C). In this investigation all the chemicals were analytical grade reagent.
Preparation of biochar by hydrothermal carbonization (HTC)
HTC of food waste [10] was conducted in a 500 ml Parr stirred pressure batch reactor (Model 4575, Germany; Heater power: 1000 W). The reactor was run at 623 K with a constant residence time of 30 min. The reactor was sealed and heated to the desired reaction temperature with the help of an electric furnace [11-13]. After the desired residence time, the heater was turned off and the reactor was rapidly cooled to room temperature. Finally the black biochar was prepared and it was kept in the desiccator.
Chemical activation
In order to obtain the remarkable properties of de-fluoridation in water the bio-char was activated. Firstly, bio-char was purified using distilled water to remove chemical impurities and then it was kept for drying at 378 K. Then it was grinded and activated by using phosphoric acid (H3PO4) which was followed by carbonization in muffle furnace at 723 K. Then it was cooled to room temperature. After that it was washed with distilled water to make it neutral. Then final activated biochar was cooled and stored in a desiccator for further study.
Physicochemical properties of adsorbents
Various physicochemical properties [14,15] of activated bio-char were estimated using standard procedures. All the experiments were conducted in duplicate and results are represented in Table 1.
| Name of Sample | Bulk Density(g/cm3) | Porosity(unitless) | Moisture content(%) |
|---|---|---|---|
| Activated Bio-char | 0.7 | 0.58 | 9.7 |
Table 1: Physicochemical analysis.
Determination of bulk density: The dry centrifuge tube(10 ml) was cleaned and weighed (W1). This tube was filled with the activated bio-char in powder form and then it was weighed (W2). The difference in the weight indicates the weight of bio-char in tube. The bulk density was determined using the following equation:

Porosity determination: The porosity of bio-char was estimated using the formula:

The pore volume of activated bio-char was estimated using the formula:

Determination of moisture content: The empty crucible was dried at 383 K and then cooled in a desiccators and weighed (W1). Then the activated bio-char was weighed (W2) separately and then dried in an oven at 383 K. This weight was taken constantly at 30 minutes interval until the weight became constant. Then sample with crucible was cooled in desiccators and reweighed (W3). The weight difference of the sample is used to measure the moisture content (Xo) of prepared bio-char.

Measurement and characterization
The prepared activated bio-char was characterized by scanning electron microscopy (SEM), XRD (X-ray Diffraction) analysis and Fourier Transformed Infrared spectroscopy (FTIR).
Experimental
Batch adsorption procedure: The fluoride solution of desired concentration was prepared by further dilution of the stock solution with suitable volume of distilled water which was used in experimental study.
In this experiment, 100 ml fluoride solutions of concentration 50 mgL-1 were taken in 250 mL PTFE(Polytetrafluoroethylene)conical flasks. The definite amount of adsorbent was added to each solution. Then the flasks were stirred at 150 rpm in an incubator shaker at various temperatures. The effects of contact time, adsorbent dose and reaction temperature on the de-fluoridation were studied by using batch experiment.
Experimental set up: The batch experiments were performed in temperature controlled incubator shaker (INNOVA 4430, New Brunswick Scientific, Canada). After agitation for definite time intervals those samples were collected from the flasks for analysis of fluoride concentration in the solution. The dissolved fluoride in each conical flask was calculated by using ion-meter (Thermo Scientific Orion ion-meter, USA).
The percent removal (%) of fluoride was calculated by using the following equation:
 (1)
(1)
Where Ci is the initial fluoride concentration (mg L-1) and C0 is the final fluoride concentration in solution (mg L-1).
Determination of optimum conditions
Determination of optimum contact time: Contact time play a significant role in adsorption study. In order to study the effect of contact time, 100ml of fluoride solution of 100 mg/Land pH 2.0 ± 0.02, was mixed with 1.0 g activated bio-char, stirred at different contact times (20-100 min) and then filtered. These filtrates were analyzed for residual fluoride concentration using ion-meter.
Determination of optimum dosage of adsorbent: The optimum dosage of activated bio-char is added to the conical flask in different dosage varying from (200-2000 mg) which contains 100ml of 50mg/L fluoride solution and pH is maintained as 2.0 ± 0.02. The solution in the PTFE conical flask is subjected to stirring for optimum contact time and then filtered, and finally analyzed. The dosage which shows maximum de-fluoridation efficiency is selected as optimum dosage of adsorbent.
Determination of optimum temperature: The effect of temperature on fluoride adsorption was experimented by performing equilibrium adsorption within the range of temperature between 293- 363K. The temperature at which maximum de-fluoridation happened that is optimum temperature.
Adsorption isotherm of batch experiment:
Langmuir isotherm: In this case the following equation [16] is used as follows:
 (2)
(2)
Where Qe denotes amount of fluoride adsorbed at equilibrium (mg/L), Ce denotes concentration of fluoride in the aqueous phase at equilibrium (mg/L). KL and qm denotes Langmuir constants related to energy of adsorption and the adsorption capacity.
Freundlich isotherm: The Freundlich isotherm [17] constants are estimated using the following equation:
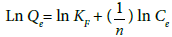 (3)
(3)
Where Qe denotes the amount of fluoride adsorbed at equilibrium, and KF and n are Freundlich constants indicates adsorption capacity and adsorption intensity respectively
Adsorption kinetics of batch experiment: The experiments of de-fluoridation were carried out at various temperatures to determine the optimum temperature for maximum adsorption efficiency and to determine the reaction rate constant. 100 ml of fluoride solution of concentration 50 mg/L was taken in PTFE conical flask and 1 g adsorbent is added to it. Then this mixture was agitated at 150 rpm for 1 hour. From this experiment, kinetic rate constant [18] at different temperatures is estimated.
Pseudo first order kinetics: The rate constant is estimated using the following equation:
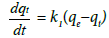 (4)
(4)
Where, qe= fluoride adsorbed at equilibrium/unit weight of adsorbent (mg/g), qt is the amount of fluoride adsorbed at any instant (mg/g) and k1 is the rate constant (min−1).
Integrating at these conditions as t=0 and qt=0 to t=t and qt=qt, the final equation is written as given below:
 (5)
(5)
Pseudo second order kinetics: The model equation is described as follows:
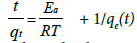 (6)
(6)
Where k2 denotes the pseudo-second-order rate constant of adsorption (g mg−1 min−1) and qe and qt are the amounts of fluoride adsorbed (mg/g) at equilibrium and at time respectively.
Activation energy:
From the obtained the rate constant, activation energy of the adsorption of fluoride is calculated using Arrhenius Equation (7) given as follows:
 (7)
(7)
Where Ea=activation energy (kJmol−1); R=gas constant (8.314 J mol−1 K−1); and A0=Arrhenius constant.
Adsorption thermodynamics: The thermodynamic parameters of de-fluoridation are estimated using the following formulas:
 (8)
(8)
Where, Kc=coefficient of distribution for the adsorption; Ca= fluoride adsorbed per unit mass of the adsorbent (mg L−1); Ce=equilibrium concentration of adsorb ate in aqueous phase (mg L−1).
 (9)
(9)
Where,ΔG0 (kJ mol−1)=change of Gibb’s free energy; R= universal gas constant(8.314 J/mol K); and T=absolute temperature (K); and
 (10)
(10)
WhereΔH0 (kJ mol−1)=change of enthalpy; ΔS0(J mol−1 K−1)=change of entropy.
Results and Discussion
Characterization of adsorbent
SEM(Scanning Electron Microscopy): From (Figure 1) it is revealed that the structure of bio-char contain major characteristics of the physical structure of the original feedstock. It is shown in the SEM images [JEOL-JSM-7600F] of the bio-chars that there is a remarkable difference in porosity (approximately 1m diameter) of structure and the amount of organic and inorganic matter coated to the surface.
FTIR(Fourier Transformed Infrared Spectroscopy): FT-IR spectra of the bio-char samples are given in the (Figure 2). Clear distinctions can be made between the different feedstock and pyrolysis process intensity. Comparing the FT-IR spectra of the bio-chars derived at different temperatures (400, 550, and 700°C), a reduction of the peak intensity of 1070 cm−1 (characteristic of C O stretching of carbohydrate-like substances) and 1470 cm−1(attributed to C O of phenolic, carboxylic, and alcohol groups)can be observed
XRD(X-ray Diffraction ) analysis: X-ray diffraction was carried out on bio-char and activated bio-char using a Diffracto meter (Bruker,D8 Advance). Two different types of char were granulated for powder diffraction using Cu Kα radiation (40 kV, 40 mA) from 5° to 65° (2θ) with 0.1 step size and 2 second measurement interval. The resulting peaks (Figure 3) were observed for two different samples.
Interaction effect:
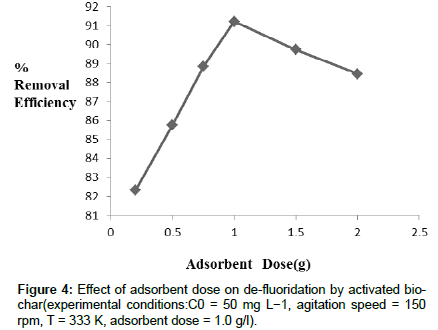
Effect of Adsorbent dose: Within the experimental range of adsorbent dose in between 0.2 g-2.0 g/l, percent removal of fluoride firstly increases (upto1.0g/ l ), then decreases slowly. The adsorbent dose in the range of 0.2-1.0g/l, de-fluoridation efficiency increases due to the number of ions increases on the adsorbent surface as the attractive force between adsorb ate ions and adsorbent. While increasing dosage of adsorbent higher than 1.0 g /l, it shows decrease in de-fluoridation on the adsorbent surface because surface of adsorbent is saturated by adsorb ate ions, and in that case the repulsive force between fluoride ions and adsorbent surface occurs. From Figure 4, it was observed that the removal efficiency of fluoride increases with increasing dosage of adsorbent (up to 1.0 g /l),then decreases slowly. So it can be inferred that activated bio-char can be used as effective adsorbent for de-fluoridation in water.
Effect of Contact time: It is observed from (Figure 5) the experimental results that on increasing the contact time at pH 2 and optimum dosage of adsorbent, de-fluoridation efficiency increases. As the contact time increases, higher the number of fluoride ions attached on the adsorbent surface. Chemically it is explained that the accumulation of fluoride ions on adsorbent surface increases due to attractive force between adsorb ate and adsorbent which results in increasing the de-fluoridation in solution. Within the experimental limit of contact time(20-100 min), after certain point (80 min), defluoridation efficiency decreases. The reason behind these phenomena is that maximum number of the fluoride ions attached on adsorbent surface when reaction time was 80 minutes. Beyond 80 min removal efficiency decreases. From Figure 5, it was observed that the removal efficiency of fluoride increased firstly and then decreases slowly, which was reflected in the plot.
Effect of temperature: In the above experiment, it is represented that with increasing temperature, the removal efficiency of fluoride increases sharply at 333K then it decreases. Following the adsorption process, 333 K is the feasible condition for batch de-fluoridation (Figure 6). Above 333K the de-fluoridation efficiency decreases. With increasing temperature, the attractive force between adsorbent and fluoride ions increases, resulting adsorption capacity of activated biochar increases. So the residual amount of fluoride ions decreases in the solution. Above 333 K, the amount of residual fluoride increases slowly. In (Figure 6) this phenomenon is reflected properly.
Adsorption isotherm study
From (Table 2) it is summarized the corresponding constants for all the isotherms. R2 value of Langmuir isotherm model (0.999) was higher than that of Freundlich (0.9863). It implies that Langmuir model (Figure 7) showed good agreement on de-fluoridation onto activated bio-char than Freundlich (Figure 8) in present work. This is also indicated that the surface of adsorbent is homogeneous for de-fluoridation. With increasing temperature adsorption capacity increased which implies that the process is endothermic in nature. In comparative study it is shown that activated bio-char has potentiality for de-fluoridation.
| Langmuir Isotherm | Estimated Value | Freundlich Isotherm | Estimated Value |
|---|---|---|---|
| qm(mg/g) | 21.562 | KF(mg/gm) | 20.521 |
| KL(L/mg) | 0.0273 | 1/n(L/mg)1/n | 0.141 |
| R2 | 0.9991 | R2 | 0.9863 |
Table 2: Parameter of Langmuir and Freundlich isotherm models for defluoridation by Activated Bio-char (condition: weight of adsorbent =1.0 mg/100ml, stirring = 150 rpm, temperature = 333 K, contact time = 80 min).
Adsorption kinetics
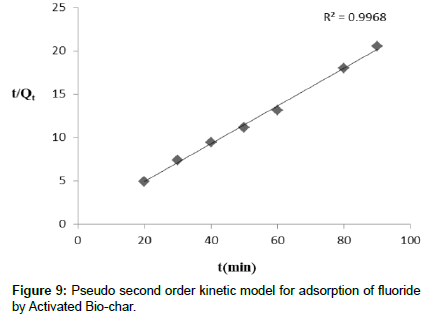
The present adsorption kinetics studies were carried out for defluoridation using activated bio-char prepared from food waste. The parameters of kinetic studies are discussed in this description. From this study, it is observed that pseudo second order kinetics study are well fitted than pseudo first order reaction. From the pseudo second order kinetic reaction it is indicated that adsorption capacity of activated bio-char is dependent on available binding site. The plot of t/qt Vs t (Figure 9) and ln Kc vs 1/T (Figure not shown)are represented. The value of k2 and qe were calculated from the intercept and slope plot of t/qt against t. Each kinetic model was analyzed by comparing the expected and calculated values of qe and correlation coefficient (R2). The value of R2 (Table 3) was 0.9968 and the corresponding k2 value was 0.0438, while the calculated qe(mg/g) was 24.17. The value of R2 for pseudo-second-order was greater than pseudo-first-order process. From these experimental values it is suggested that defluoridation onto activated bio-char followed pseudo second order kinetics.
| Adsorbents | Pseudo second order kinetics | ||
|---|---|---|---|
| k2(g mg-1) | qe(mg g-1) | R2 | |
| Activated Bio-char | 0.0438 | 24.17 | 0.9968 |
Table 3: Pseudo second order rate constants for Activated Bio-char at optimized conditions.
Thermodynamic study
In order to determine the feasibility of reaction, the thermodynamic parameters such as Gibbs free energy change (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) had to be estimated from equation which are shown in Table 4. The thermodynamic parameters are estimated using the equations (8-10). ΔH° and ΔS° were estimated by slope and intercept from the plot of ln Kc vs. 1/T. The values of ΔG° were negative at all temperatures, implies that the adsorption process is feasible and spontaneous nature of de-fluoridation onto activated bio-char. The decrease in the value of ΔG° with increasing temperature represents that affinity of fluoride on activated bio-char was higher at high temperature. The positive value of ΔH°(25.2215 kJ mol−1) indicated that the adsorption process was endothermic. If the value of ΔH° lies in between 80 and 200 kJ, then the adsorption process is chemisorption in nature, but here it is obtained as 25.2215kJ, denoting that de-fluoridation onto adsorbent followed physicochemical process. The positive value of ΔS° (92.24 J mol−1 K−1) indicated the affinity of fluoride towards activated bio-char and at solid-liquid interface increased during adsorption.
| Serial No. | T, K | ΔG0, kJ/mol | ΔH0, kJ/mol | ΔS0,J mol-1 K-1 |
|---|---|---|---|---|
| 1 | 293 | -9.86 | 25.2215 | 92.24 |
| 2 | 303 | -10.23 | ||
| 3 | 313 | -12.45 | ||
| 4 | 323 | -14.12 | ||
| 5 | 333 | -11.45 | ||
| 6 | 343 | -12.76 | ||
| 7 | 353 | -12.98 |
Table 4: Thermodynamic parameters for the adsorption of fluoride onto Activated bio-char.
Regeneration study: The regeneration study of adsorbent in defluoridation method is very significant. As the bio-char from food waste demonstrated higher de-fluoridation efficiency (91.24%), so its desorption study was determined by 5 adsorption–desorption cycles. The present adsorption-desorption study was carried out with 100 ml of 50 mg·L−1 of synthetic fluoride solution at the starting of each cycle. The study was investigated with 1% sodium hydroxide as desorbing agent. The adsorption capacities of each cycle were 90.92%, 87.46%, 84.13%, 80.01%, and 77.53%. These experimental results (Figure 10) represents that bio-char prepared from food waste can be reused for de-fluoridation in water.
Comparative adsorption capacity, isotherm of various adsorbents with Activated Bio-char synthesized in this study (Table 5).
| Sorbent | Maximum adsorbent capacity | Isotherm | Reference |
|---|---|---|---|
| Activated Carbon (Rice straw) | 18.9 mg·g−1 | Langmuir | [19] |
| Activated Carbon (MorringaIndica) | 0.2314 mg·g−1 | Langmuir | [20] |
| Activated carbon (Acacia farnesiana) | 2.622 mg·g−1 | Freundlich | [21] |
| Activated carbon (Pithacelobiumdulce) | 1.9333 mg·g−1 | Freundlich | [22] |
| Activated carbon (Arachishypogia) | 14.79 mg·g−1 | Freundlich | [23] |
| Activated carbon (Cynodondactylon) | 4.755 mg·g−1 | Langmuir | [24] |
| Activated carbon (Anacardiumoccidentale) | 1.95 mg·g−1 | Langmuir | [25] |
| Activated carbon (pecan nut shells) | 2.3 mg·g−1 | Langmuir | [26] |
| Graphene | 48.31 mg·g−1 | Langmuir | [27] |
| Activated bio-char from food waste | 49.47 mg·g−1 | Langmuir | Present study |
Table 5: Comparative adsorption capacity, isotherm of various adsorbents with Activated Bio-char.
Conclusion
The present investigation deals with the aim of de-fluoridation study by adsorption process onto activated bio-char from food waste. The adsorption studies were carried out as a function of temperature, contact time and adsorbent dose. The following conclusions may be drawn on the basis of the study:
It is proved that the adsorption equilibrium data are satisfactorily fitted to the Langmuir adsorption model rather than Freundlich isotherm model at different temperatures.
The obtained experimental results are well fitted to pseudo– second order kinetic model.
Thermodynamic parameters such as change in Gibbs free energy (ΔG0), enthalpy (ΔH0), and entropy (ΔS0) were determined from thermodynamic studies.
The nature of the adsorption mechanism is endothermic and spontaneous which is experimentally proved.
As food waste is easily available, therefore synthesized activated bio-char from urban food waste may be useful adsorbent for defluoridation in waste water.
Acknowledgment
This study was supported by Chemical Engineering Department, Jadavpur University, Kolkata, India and West Bengal Pollution Control Board, India. Authors are thankful for their support and service.
References
- Mohapatra M, Anand S, Mishra BK, Giles GE, Singh P (2009) Review of fluoride removal from drinking water. J Environ Manag 91: 67-77.
- WHO (2004) Guidelines for Drinking Water Quality, 3rd (edtn), Geneva.
- Chinoy NJ (1991) Effects of fluoride on physiology of animals and human beings. Indian J Environ Toxicol 1: 17-32.
- Yadav AK, Kaushik CP, Haritash AK, Singh B, Raghuvanshi SP, et al. (2007) Determination of exposure and probable ingestion of fluoride through tea, toothpaste, tobacco and pan masala. J Hazard Mater 142: 77-80.
- Harrison PTC (2005) Fluoride in water: A UK perspective. J Fluor Chem 126: 1448-1456.
- Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agronomy 105: 47-82.
- Singh B, Singh BP, Cowie AL (2010) Characterization and evaluation of biochars for their application as a soil amendment. Aust J Soil Res 48: 516-525.
- Brewer CE, Unger R, Schmidt-Rohr K, Brown RC (2011) Criteria to select biochars for field studies based on biochar chemical properties. Bioenerg Res 4: 312-323.
- Efremenko I, Sheintuch M (2006) Predicting solute adsorption on activated carbon: phenol. Langmuir 22: 3614-3621.
- Inyang M, Gao B, Yao Y, Xue Y, Zimmerman A, et al. (2012) Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour Technol 110: 50-56.
- Fuertes AB, Arbestain MC, Sevilla M, Macia-Agullo JA, Fiol S, et al. (2010) Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Aust J Soil Res 48: 618-626.
- Sevilla M, Macia-Agullo JA, Fuertes AB (2011) Hydrothermal carbonization of biomass as a route for the sequestration of CO2: chemical and structural properties of the carbonized products. Biomass Bioenergy 35: 3152-3159.
- Hu B, Wang K, Wu L, Yu SH, Antonietti M, et al. (2010) Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Mater 22: 1-16.
- Sun H, Hockaday WC, Masiello CA, Zygourakis K (2012) Multiple controls on the chemical and physical structure of biochars. Ind Eng Chem Res 51: 3587-3597.
- Ronsse F, Van Hecke S, Dickinson D, Prins W (2013) Production and characterization of slow pyrolysis biochar: influence of feedstock type and pyrolysis conditions. GCB Bioenergy 5: 104-115.
- Langmuir In (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40: 1361-1403.
- Freundlich H (1906) Uber die adsorption in losungen (adsorption in solution). Z Phys Chem 57: 385-490.
- Ghorai S, Pant KK (2005) Equilibrium, kinetics and breakthrough studies for adsorption of fluoride on activated alumina. Sep Purif Technol 42: 265-271.
- Daifullah AA, Yakout SM, Elreefy SA (2007) Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. J Hazard Mater 147: 633-643.
- Karthikeyan G, Siva Ilango S (2007) Fluoride sorption using Morringa Indica-based activated carbon. J Enviton Health Sci Eng 4: 21-28.
- Hanumantharao Y, Kishore M, Ravindhranath K (2011) Preparation and development of adsorbent carbon from Acacia farnesiana for defluoridation. Int J Plant Anim Environ Sci 1: 209-223.
- Emmanuel KA, Ramaraju KA, Rambabu G, Veerabhadra Rao A (2008) Removal of fluoride from drinking water with activated carbons prepared from HNO3 activation-A comparative study. Rasayan J Chem 1: 802-818.
- Alagumuthu G, Rajan M (2010) Kinetic and equilibrium studies on fluoride removal by zirconium (IV): Impregnated groundnut shell carbon. Hem Ind 64: 295-304.
- Alagumuthu G, Veeraputhiran V, Venkataraman R (2011) Fluoride sorption using Cynodondactylon based activated carbon. Hem Ind 65: 23-35.
- Alagumuthu G, Rajan M (2010) Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon. Chem Eng J 158: 451-457.
- Hernández-Montoya V, Ramírez-Montoya LA, Bonilla-Petriciolet A, Montes-Morán MA (2012) Optimizing the removal of fluoride from water using new carbons obtained by modification of nut shell with a calcium solution from egg shell. Biochem Eng J 62: 1-7.
- Li Y, Zhang P, Du Q, Peng X, Liu T, et al. (2011) Adsorption of fluoride from aqueous solution by graphene. J Colloid Interface Sci 363: 348-354.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi