Research Article, Clin Oncol Case Rep Vol: 7 Issue: 7
Anti-Neoplastic Activity of Tetrahydrocurcumin in Liver Cancer by Modulating FOXO1/Parkin Tumour Suppressor Genes through In-Silico and In-Vitro Approaches
Madhu Rani1, Rashmi Kumari1,2, Siya Ram2, M. Moshahid Alam Rizvi1*
1Department of Biosciences, Genome Biology Laboratory, Jamia Millia Islamia, New Delhi-110025, India
2Discipline of Life Sciences, School of Sciences, Indira Gandhi National Open University, New Delhi-110068, India
- *Corresponding Author:
- M. Moshahid Alam Rizvi
Department of Biosciences,
Faculty of Life Sciences,
Jamia Millia Islamia University,
New Delhi-110025,
India,
Tel: +91-9911661657;
E-mail: rizvijmi@gmail.com
Received: October 10, 2024, Manuscript No. COCR-24-149770
Editor assigned: October 14, 2024, PreQC No. COCR-24-149770 (PQ)
Reviewed: October 28, 2024, QC No. COCR-24-149770
Revised: December 10, 2024, Manuscript No. COCR-24-149770 (R)
Published: December 17, 2024, DOI: 10.4173/cocr.7(7). 359
Citation: Rani M. et al., (2024) Anti-Neoplastic Activity of Tetrahydrocurcumin in Liver Cancer by Modulating FOXO1/Parkin Tumour Suppressor Genes through In-Silico and In-Vitro Approaches. Clin Oncol Case Rep 7:7.
Abstract
Liver Cancer (LC) is the greatest prevalent malignancy and the 2nd factor for cancer death in worldwide. However, various conventional treatment strategies are unable to mitigate tumour recurrence and metastasis causes of poor prognosis and pose a major setback to improving the global survival rate of LC patients. As a consequence, there is a need to develop alternative strategies to improve the treatment of LC by utilizing phytocompounds. Tetrahydrocurcumin (THC) is a bio-oral available curcumin derivative derived from Curcuma longa. To assess the anti-cancer properties of THC by regulation of FOXO1 and Parkin, we have conducted several in-silico and in-vitro approaches. The results obtained in current studies, FOXO1 and Parkin gene expression as a tumour suppressor in LC were analysed by GEPIA 2 and UALCAN databases. THC had better pharmacokinetic characteristics and a higher binding affinity with FOXO1 and Parkin (-6.4 and -5.7 kcal/mol, respectively) than 5-fluorouracil. The IC50 value for THC against HepG2 LC cells was 88.89 µM by MTT assay, showing that THC significantly reduced the cellular replication of LC cells gradually in a concentration-dependent way, whereas DNA fragmentation assay inspected that THC induces apoptosis of HepG2 LC cells. Subsequently, THC also up regulates FOXO1 and down regulates Parkin protein levels as revealed by in-vitro ELISA method. The inference from these findings was that THC potently restored the tumour suppression activities of FOXO1 and Parkin that were lost during tumour development. However, the current study has proven the therapeutic potential of THC in the halting the proliferation and enhancing the cell death in LC cells by regulating the protein levels of FOXO1/Parkin. Hence, this pre-clinical assessment would be a way forward in developing a neo-adjuvant therapy for LC patients that might enhance their survival rate in a safer way.
Keywords: Liver cancer; Hepatocellular carcinoma; Tetrahydrocurcumin; Curcumin derivatives; ADME; Toxicity; Molecular docking; Cytotoxicity assay
Introduction
Cancer is a complicated disease triggered by gene changes, for example, proto-oncogenes, DNA repair genes, Tumour-Suppressor Genes (TSGs), and oncogenes. These changes disrupt cellular homeostasis and promote unregulated proliferation [1]. In the modern era, among all types of cancers, Liver Cancer (LC) raises the alarm on a worldwide scale. As per estimation of the International Agency for Research on Cancer’s, 2022 worldwide cancer statistics, LC grades sixth amid all malignancies but accounts for the 3rd most cancer-allied causalities (7.8%) trailing only lung (18.7%) and colorectal cancers (9.3%) worldwide [2]. More than 866,136 new cases of LC were reported in 2022. India ranks fourth in incidences of LC, while second in mortality from LC among all countries [3]. The load of incidents of LC is expected to be surged from 2020-2040 is 55%, resulting in the 1.4 million people being detected with LC in 2040. The estimated of causalities from LC will be about 1.3 million persons in 2040. This prediction suggests that more than 56.4% casualty from LC happened in 2020 [4]. Hepatocellular Carcinoma (HCC) is predominant kinds of LC that’s responsible for over 90% of LC cases. The crucial risk factors that associated with HCC progression are virus-related contagions for instance, hepatitis B and C [5]. Alcohol consumption, cigarette smoking, and non-alcoholic steatohepatitis linked to type 2 diabetes are other key risk factors for HCC development [6]. LC brings substantial concern and challenges for the physical and psychological strength of humans as well as social and economic improvement of a country. Therefore, effective therapeutic approaches for LC need to be taken carefully.
Presently, the main treatment options for primary liver cancers are surgical resection, radiotherapy, local ablation therapy, radiotherapy, and liver transplantation [7]. In the advanced stage of LC, various chemotherapeutic drugs are employed, including 5-fluorouracil (5-FU), sorafenib, lenvatinib, regorafenib and ramucirumabetic that target numerous cells signalling pathways like JAK/STAT, Hippo, Notch, Wnt and TGF-β which participated in HCC progression. The administration of these chemotherapeutic drugs is limited due to occurrence of numerous side effects for example chemo-resistance, tumour recurrence, metastasis, and weakening of immune system. These side effects worsened the survival rates of LC patients [8]. In a nutshell, available ablation personalized therapies and chemotherapy are less efficient, considering their efficacy, bioavailability and side effects in improving the life span of LC patients. Therefore, in consideration the side effects of conventional therapies that based on the coin of evidence, there is an imperative need for the advancement of novel, natural compounds that are more efficient therapeutic approaches for HCC for better prognostic features for instance enhanced inhibition of oxidative stress, viral infection, angiogenesis, inflammation, and metastatic action with lesser or no side effects.
In recent times, an array of herbal-resultant natural compounds having anticancer activity have been evaluated, including phenols, terpenoids, alkaloids, and flavonoids that can suppress tumour cell invasion, migration, angiogenesis, proliferation, and promote apoptosis. These compounds have been investigated as significant antitumour medications that selectively lysed malignant cells containing less toxic effects on typical cells [9,10].
Curcumin (CUR) is a crucial polyphenolic compound obtained primarily from Curcuma longa that has been traditionally utilized for treating a variety of ailments, including HCC [11]. CUR inhibits cancer by regulating enzymes, growth factors, transcription factors, inflammatory cytokines, and apoptotic proteins [12,13]. In contrast, its inadequate systemic bioavailability and solubility hinder its therapeutic usefulness, possibly due to its extensive metabolism [14]. As a consequence, there is a critical requirement to explore new therapeutic approaches that possess great effectiveness, enhanced bioavailability, and little side effects for better management of LC.
Tetrahydrocurcumin (THC) is a vital colourless major metabolite of CUR in the form of glucuronide conjugate and possesses stronger antioxidative activity than CUR and has been documented to possess a variety of biological roles and efficient effects on various diseases like neurological disorders, inflammatory diseases, and metabolic syndromes, including, cancer [15]. Numerous studies have shown that THC reduces neoplastic activity in different malignancies, including LC, by modulating cell signalling molecules/pathways. For example, THC regulates the CYP1A1/NF-κB signalling cascade, triggering cell death and suppressing movement in MCF-7 Breast Cancer (BC) cells [16]. THC also helped in the chemo-sensitivity of BC in-vitro model (BC cells) and in-vivo xenograft BC tumour model towards albuminbound paclitaxel drug via ameliorating the level of SPARC (secreted protein acidic and rich in cysteine) by the usage of an agent which is demethylating like 5-Aza-Cdr (5-Aza-2’-deoxycytidine) [17]. Song et al., have revealed that THC significantly induced autophagy by repressing the PI3K/Akt/mTOR cascade and reduced lung tumour in non-small cell lung carcinoma cells [18]. The synergistic treatment of THC with radiation in glioma cells have slumped the cell viability and enhanced apoptosis as compared to radiation group alone. This combined treatment potently promoted the G0/G1 cell cycle arrest as well as diminished the S-phase via suppression of PCNA and cyclin D1 [19]. THC inhibits cervical cancer cell-induced angiogenesis by lowering, hypoxia-inducing factor-α and vascular endothelial growth factor levels in nude mice [20]. Additionally, THC's anti-proliferative and cytotoxic properties have also been observed in MCF-7 cells. Furthermore, THC mediates apoptosis and halts the cell cycle by activating caspases 3 and 9, increases in intracellular ROS and Bax levels and sharp decreases in PARP and Bcl-2 expression and it also results in the demise the potential of mitochondrial membrane. These findings suggested that THC significantly inhibits BC cells [21]. THC is far more efficient than CUR in triggering apoptosis in H22 LC cells by modulating the mitochondrial apoptosis pathway. THC increased p53 and Bax extents while decreasing mdm2 and Bcl-2, so accelerating apoptosis and cytochrome C release. THC also increases the breakdown of caspase 3 and 9, inhibiting H22-induced apoptosis ascites cancer cells [22]. It was also revealed that the THC potently enhanced the oxidative stress, hepatic steatosis, and inflammation in NASH rats promoted by a high-fat diet and inhibited the accumulation of lipids in steatosis L02 cells and HepG2 cells. The treatment of THC promoted the nuclear translocation of TFEB by suppressing mTORC1, resulting in upregulation of lysosomal biogenesis [23]. THC might possess a bioactive antimalignant sort of CUR, thus rendering it an intriguing, feasible avenue of therapy for HCC. Therefore, molecular insights into THC role in LC is still remain to be explored.
In mammals, the Fork Head Box (FOX) O transcription factors like FOXO1, FOXO3, FOXO4, and FOXO6 are four crucial members that perform a chief part in cellular adaptation to varied stress circumstances and translate numerous environmental stimuli into vibrant type of gene expression that affect an extensive array of physiological and pathological situations, for instance cancer and ageing. FOXOs are also regulating the insulin/IGF and growth factor-activated Receptor Kinase (RTK)-Phosphoinositide-3-Kinase (PI3K) signalling cascade [24]. It has been reported that FOXO1 is mainly expressed in vital organs like liver, pancreas and many tissues like fat and muscles. The deregulation of FOXO1 protein has been seen in innumerable tumour categories, like LC and its alteration affects diverse cellular processes like apoptosis, cell cycle arrest, oxidative stress, DNA damage and repair, glucose metabolism and carcinogenesis. On the basis of various scientific evidences, loss or mutation of FOXO1 gene function is the crucial reason for the development of cancer types [25]. It was recently reported that FOXO1 inhibited the HCC progression by reducing the expression of IL-6 from macrophages [26]. In 5-FU resistant patients of LC, miR-590-5p reduced the level of FOXO1 and promoted LC growth and chemo-resistance. The silencing of miR-590-5p resulted in accelerated level of FOXO1 that further led to re-sensitization of LC cells towards 5- FU [27]. Therefore, FOXO1 TSG emerged as an appropriate target for future therapeutic discovery to curb LC. On the other side, Parkin/ PARK-2 is a multi-protein E3 ubiquitin ligase, an established TSG that observed to be dysregulated in various human malignancies [28]. Parkin activity is strongly controlled by both inter- and intra-molecular interactions. Parkin functions are associated with mitochondrial homeostasis, protein turnover, metabolism, stress response, xenophagy, and various other biological mechanisms that govern the growth and viability of the cell [29]. Parkin transcription might be modulated through N-myc, Max, p53, and ATF4, as well as numerous environmental stimuli that include nutrition, growth signals, and mitochondrial and ER stresses [30]. A vast number of scientific researchers have demonstrated that Parkin is one of the largest genes (1.5 Mb) that play a substantial part in targeted therapy, drug development, and combating cancer [31].
In light of the above facts, we speculate that THC might inhibit liver carcinogenesis by upregulating the FOXO1 and Parkin genes. Despite this, numerous studies have found that THC has anti-cancer properties via altering many cell signalling molecules in various malignancies, including LC. Nobody yet studied the activities of THC in the suppression of LC by regulation of these TSGs. Therefore, we contemplate that THC must mitigate LC through the modulation of FOXO1 and Parkin. The activation of FOXO1 and Parkin is critical in combating LC through oral supplementation with natural compounds. However, the effect of THC in the envisaging of LC through the control of FOXO and Parkin in the cell signalling pathway is yet to be elucidated. Hence, both in-silico and in-vitro strategies were performed to evaluate these findings. Initially, we analysed the expression of FOXO1 and Parkin by employing online available GEPIA 2 and UALCAN databases. Later, we examined the pharmacokinetic profile and molecular docking to study the drug similarity and bio-oral availability and drug discovery with regards to THC, as well as explore its binding affinity with the FOXO1 and Parkin proteins. Additionally, preclinical studies, including the MTT assay, DNA fragmentation assay, and ELISA, have also been performed to confirm the anti-neoplastic activity of THC against LC. The present findings have the potential to establish THC as an efficient, natural, safe therapeutic regimen for the treatment and management of LC (Figure 1).
Materials and Methods
Materials
FBS (Foetal Bovine Serum) and DMEM (Dulbecco's Modified Eagle's Medium) and were procured from Gibco (Thermo Fisher Scientific USA), DMSO (Dimethyl sulfoxide), 1X Penstrep antibiotic solution, Trypsin-EDTA, MTT ([3-(4,5-dimethylthiazoyl-2-yl) 2,5- diphenyl tetrazolium bromide]), RNase, Proteinase K, Agarose powder were acquired from Sigma-Aldrich and Sisco Research Laboratories Pvt. Ltd. (SRL), anti-FOXO1 rabbit monoclonal antibody (1:1000; ab52857), anti-PARK-2 rabbit polyclonal antibody (1:1000; cat#ab15954) were purchased from Abcam. The goat anti-rabbit secondary antibody- HRP (Horseradish Peroxidase) (1:10,000), was supplied by Thermo Fisher Scientific. The chemotherapeutic drug 5- FU (F6627-1G) was supplied by Sigma Aldrich. Other chemicals were supplied by local vendors.
Cell lines and culture conditions
HepG2 LC cell and HEK-293 normal cell lines were implemented in this study and bought from the NCCS (National Centre for Cell Science) in Pune, India. The cells were propagated in a DMEM medium enriched with 1% penicillin/streptomycin and 10%FBS. According to standard protocols, the cells were cultured in a 5%carbon dioxide atmosphere at 37°C in a NuAire incubator (manufactured by NuAire Laboratory Equipment). Once cells attained confluency of 80-90% then cells were transferred every 2-3 days using a solution comprehending 0.25% trypsin-EDTA via an inverted phase contrast microscope (LMI).
In-silico analysis of FOXO1 and Parkin proteins expression in liver cancer
The expression levels of FOXO1 and Parkin were investigated using GEPIA 2 and UALCAN (The University of ALabama at Birmingham CANcer) an open, free internet web server application [32-35]. Using GEPIA 2 and UALCAN under expression, we examined the profile of both genes independently in Liver Hepatocellular Carcinoma (LIHC). After that, we selected match TCGA normal and GTEx data and generated the expression of these target genes by using box plots for normal and tumour cases via GEPIA 2 and UALCAN databases.
In-silico prediction of pharmacokinetic and toxicity profile
Assessment of THC's pharmacokinetic profile, including Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET), is a significant characteristic in the development and discovery of drugs. SwissADME [36], a freely available web tool, was used in this study to identify the physicochemical properties, pharmacokinetics, and drug-likeness of THC, whereas toxicity was assessed using an online web tool server, ProTox II [37,38]. The ProTox II predicts the toxicity class, LD50, and other types of organ toxicity for THC structures. The ADMET characteristics were retrieved by loading the THC structure into the website's interface using the canonical SMILES format and created ADMET profiles.
Molecular docking studies
Preparation of FOXO1 and Parkin protein and THC ligand: The crystal structure of both FOXO1 (PDB ID: 3CO6) and Parkin (PDB ID: 4I1H) were fetched from protein data bank [39] and prepared employing BIOVIA Discovery Studio (BDS) 2022 [28,40], as the software to delete water molecules, other ligands, cofactors, extra chains and het atoms. Molecular docking study using the pdb file of the target protein, which solely contained the protein chain and all polar hydrogen atoms. The 3D chemical structure of THC was derived from the PubChem database and kept in sdf format [41].
Molecular docking: Molecular docking was executed by implementing the Pyrx-Python prescription 0.8 software [42], and the binding mode and interaction of FOXO1 and Parkin proteins with THC were assessed. The software allowed the loading of the protein (macromolecule) and drug THC (ligand). The ligand bindings were designed to be capable of enduring rotation. To determine the docking region on the target protein, a 3D grid box was created, enclosing the entire protein in the X, Y, and Z dimensions. The ideal conformation will be identified by the model that positions itself in the best possible way while having the lowest energy. After that, both proteins were docked separately with THC-ligand.
In-vitro studies
Maintenance of cell lines: NCCS, Pune, India has provided the HEK-293 normal cell and HepG2 LC cell lines. Cells were grown in DMEM which was also nourished with 10% FBS and 1X Penstrep antibiotic solution. Further, cells have been incubated at 37°C in a carbon dioxide incubator maintaining 5% CO2.
MTT assay: HEK-293 normal cells and HepG2 LC cells were grown about the concentration of 5000 cells per well in a 96-well microtitre plate with 100 μl of complete DMEM medium. Subsequently, the cells were permitted to adhere overnight. Moreover, washed the cells with PBS and subjected to various concentrations ranging from 0 μM to 150 μM of the THC for 24 hours at 37°C. Later, the media will be removed after 3 hours. A cytotoxicity assessment was performed using the MTT assay for the evaluation of the impact of THC on these cells. Furthermore, MTT solution (20 μl) which had a dose of 5 mg/ml, and DMSO (200 μl) were put to every well of 96- well plate to aid in the dissolution of the formazan crystals. The absorbance measurement was taken after 10 minutes using a spectrophotometer (Bio-Rad) at the wavelength of 570 nm. All readings were performed in triplicates [43]. The percentage of relative cell viability will be calculated by both the untreated cells (control) and treated cells (THC in different doses):
[A]Sample-Blank/[A] Control-Blank × 100
DNA fragmentation assay: The DNA ladder assay was performed as per protocol described before [44]. About 1 × 105 cells were grown/ well in 6-well plates and left it for incubation about 16 hrs. Further, the cells were administered with different combinations like untreated cells (negative control), 5-FU (50 μM) (positive control), THC (88.89 μM), THC+5-FU drug for 24 hrs and left them for incubation about 37°C and 5% CO2 for 48 hrs. Afterwards, cells has been harvested, supplemented with proteinase K (1 mg/mL) and RNAase (200 g/mL) and left it for incubation for 1 hr at 37°C. The DNA samples were monitored by horizontal electrophoresis and morphologic detection of apoptosis from DNA samples was visualized under UVtransilluminator and photographed in Gel doc instrument (Bio-Rad).
In-vitro ELISA from cell lysate: The 96-Well plate was coated with 200 μg cell lysates of LC with different combinations like untreated cells (Control), 5-FU (50 μM), THC (88.89 μM), THC+5- FU drug, blank (PBS) in NP-40 lysis buffer. Further, primary antibodies of FOXO1 and Parkin (1:1000 diluted) were placed in each well and left it at 4°C for 2 hrs. Further, 100 μl of goat anti-rabbit secondary antibodies-HRP-conjugated (1:10000 diluted) were placed in each well and left at RT for 2 hrs. ELISA was developed with the help of 100 μl of 1 mg/ml p-nitro-phenyl phosphate solution, made in an alkaline phosphate buffer (pH 9.5) for 30 minutes. After stopping the reaction by adding 0.1 mM EDTA, reading of samples was measured in an ELISA reader instrument (Bio-Rad) at 405 nm [44].
Statistical analysis
The entire statistical data was measured by applying the student ttest to determine variations between groups. The data were displayed as the means ± Standard Deviation (SD) of three consecutive replicates. P-values ≤ 0.05 was defined as statistically significant.
Results
Analysis of FOXO1 and Parkin gene expression in liver cancer
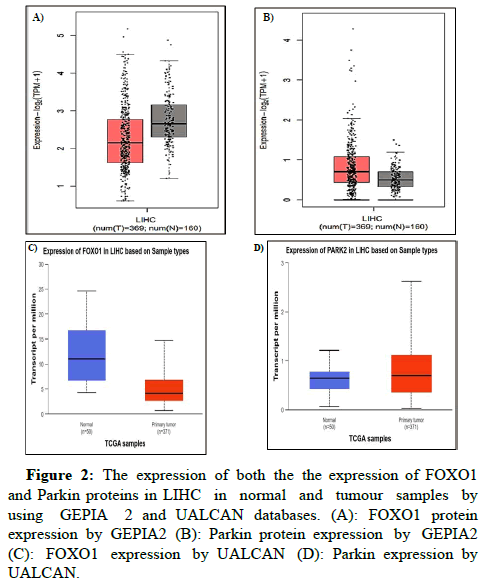
To ascertain the role of FOXO1 and Parkin in LC, we have analysed the expression of both the FOXO1 and Parkin protein in LIHC in normal and tumour samples by using GEPIA 2 and UALCAN databases, which are TCGA clinical annotations (Figures 2A-D).
Figure 2: The expression of both the the expression of FOXO1 and Parkin proteins in LIHC in normal and tumour samples by using GEPIA 2 and UALCAN databases. (A): FOXO1 protein expression by GEPIA2 (B): Parkin protein expression by GEPIA2 (C): FOXO1 expression by UALCAN (D): Parkin expression by UALCAN.
In the case of the FOXO1 gene in LIHC, the red colour boxplot has shown reduced expression in 369 different tumour samples, whereas grey colour boxplots displayed the induced expression of the FOXO1 gene in 16 normal tissues. The expression is displayed in the form of log2 transcription per million (log2 (TPM+1)) in the Y-axis. As a comparison to normal vs. primary tumour, 3.38618022510673E-13 was found to have statistical significance by the UALCAN database, where FOXO1 expression was lower in 371 primaries and higher in 50 normal samples.
On the other side, Parkin gene expression log2 (TPM+1) has found increased expression in 369 tumour samples and decreased expression in 160 normal tissues by GEPIA 2 whereas the UALCAN also revealed among 371 primary tumour and 50 normal samples where Parkin has shown upregulation expression in primary tumour and vice versa. Combined with this study, we have also observed that similar results have been found by employing both online databases GEPIA 2 and UALCAN. In addition, 1.85450000023124E-07 was examined by the UALCAN database in normal versus tumour comparison samples.
Analysis of drug-likeness, pharmacokinetics, pharmacodynamic and toxicity of THC
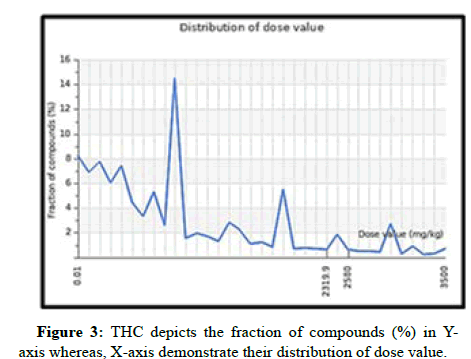
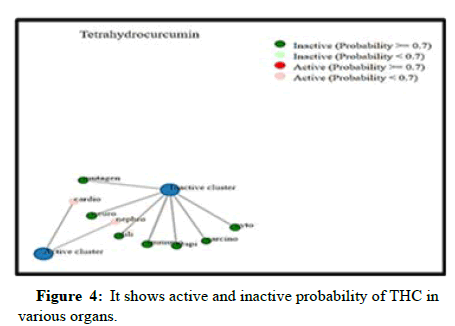
The ADME features of THC have been anticipated, as indicated in Table 1. Table 1 explicitly demonstrates that THC compounds met the parameters stipulated by Lipinski's rule of five and Veber's rule. THC possesses a molecular weight below 500 Da which is 372.4. The THC LD50 dose value was also found to be 2560 mg/kg it is considered to be a safe compound and it was not acutely toxic (Figure 3). THC's octanol-water partition coefficient (LogP) was likewise less than 5. Notably, THC also contains a better bioavailability score of 0.55, which means 55% of the drug, was absorbed into the circulatory system i.e., THC has oral bioavailability and shows drug-likeness. Topological Polar Surface Area (TPSA) was the opposite of bioavailability (46=47). For optimal bioavailability, the value should be less than 140. Hence, THC carries 93.1, which defines it as having good bioavailability along with high gastrointestinal absorption features. Also, the non-rotatable H-bond was equal to 10 for THC as per Veber’s rule. Not only does THC have ADME attributes, but it also has toxicity class 5, which means it may be detrimental if swallowed and has little to no toxic activity for human organs, with the exception of hepatotoxicity (Table 1), which was also shown in green (inactive) and red colour (active) by Figure 4.
| ADME profiles | ||||||||
| Compounds | Lipinski rules | Lipinski’s violations | Veber’s rules | |||||
|---|---|---|---|---|---|---|---|---|
| MW | HBA | HBD | Log P | nRB | TPSA | |||
| <500 | <10 | <5 | ≤ 5 | ≤ 1 | ≤ 10 | ≤ 140 | ||
| THC | 372.4 | 6 | 2 | 3.21 | 0 | 10 | 93.1 | |
| Toxicity profiles | ||||||||
| Toxicity class | LD50 (mg/kg) | Neurotoxicity | Hepatotoxicity | Carcinogenicity | Mutagenicity | Immunotoxicity | Cardiotoxicity | |
| 5 | 2580 | No | No | No | No | No | Yes | |
Table 1: ADME and toxicity profiles details of THC for bio-oral availability and drug likeness parameters.
Molecular docking interaction analysis of protein FOXO1 and Parkin with THC compound
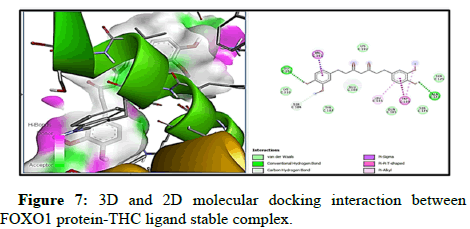
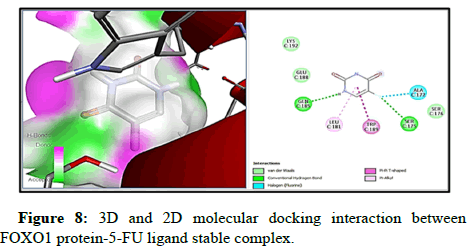
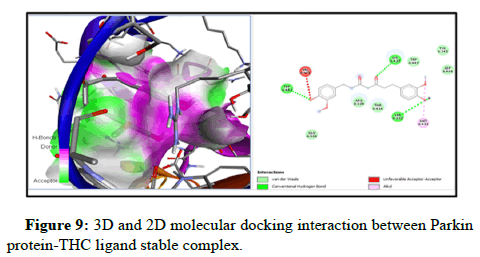
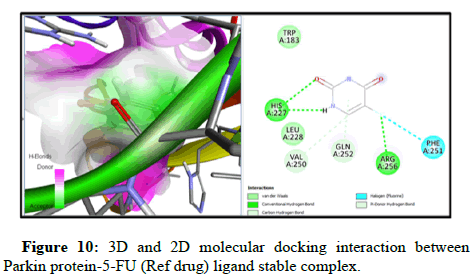
Docking of FOXO1 and Parkin proteins with compounds THC as well as with the reference drug 5-FU was run individually on Pyrx software. All minimum energy docked models were kept in pdb format. BDS was applied to comprehend the molecular interaction with the ligands and both proteins, and saved in the superlative visual pose that exhibited the hydrogen bond (H-bond) in FOXO1 and Parkin proteins with the THC ligands. To achieve a better understanding of interactions beyond the H-bond that involve pi- the donor bond, conventional H-bond, van der Wal, pi-alkyl bond, carbon-H-bond, and alkyl bond, (represented in various colours). Molecular docking with FOXO1 and Parkin protein with the THC compounds give rise to a stable docked complex because binding affinities are larger than -5 kcal/mol. However, as per analysis the binding affinity of FOXO1 protein with THC ligand is found to be higher and contains lower binding energy (-6.4 kcal/mol) than reference drug 5-FU (-4.3 kcal/ mol) and Root Mean Square Density (RMSD) was observed to be the zero for FOXO1 (Table 2 and Figure 3). Furthermore, the two-H-bond interaction has been examined between interacting residue Ala172, and Gly201, with FOXO1 protein, and 5-FU with THC comprising Gln185, Ser 175 Amino Acid (AA) interacting residue having H-bond. On the other side, Parkin has the lowest binding energy along with the highest binding affinity (-5.7 kcal/mol) with THC than drug 5-FU (-4.6 kcal/mol). Three H-bonds interacting residue Trp403, Lys427, and Ser233 have been found between Parkin and THC docking (Table 2, Figures 5-10). In addition, two H-bond His 227 and Arg256 interacting residues were scrutinized between Parkin and 5-FU drug. Thus far, THC has been identified as a medication with liver cancerpreventing abilities based on its reduced binding energy and higher stability of the complex.
| FOXO1 (3CO6)-THC docking details | |||||
| CID | Compound name | Binding energy (Kcal/mol) | RMSD (upper and lower bound) | Amino acid interacting residues | No. of hydrogen bond |
|---|---|---|---|---|---|
| 124072 | Tetrahydrocurcumin | -6.4 | 0 | Gly201, Ala 172 | 2 |
| 3385 | 5-fluorouracil | -4.1 | 0 | Gln185, Ser 175 | 2 |
| Parkin (4I1H)-THC docking details | |||||
| CID | Compound name | Binding energy (Kcal/mol) | RMSD (upper and lower bound) | Interacting residues | No. of hydrogen bond |
| 124072 | Tetrahydrocurcumin | -5.7 | 0 | Trp403, Lys427, Ser233 | 3 |
| 3385 | 5-fluorouracil | -4.6 | 0 | His 227, Arg256 | 2 |
Table 2: It describes the molecular docking interaction of protein FOXO1 and Parkin with THC and 5-fluorouracil reference drugs.
THC inhibits proliferation of cells in LC
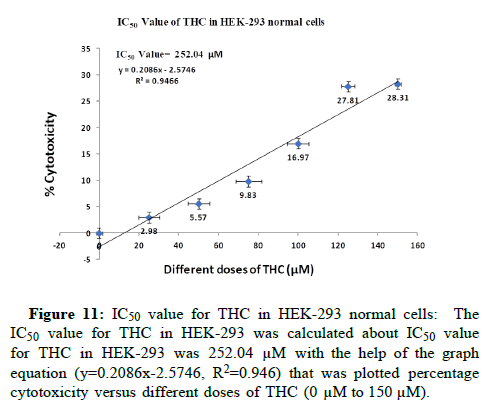
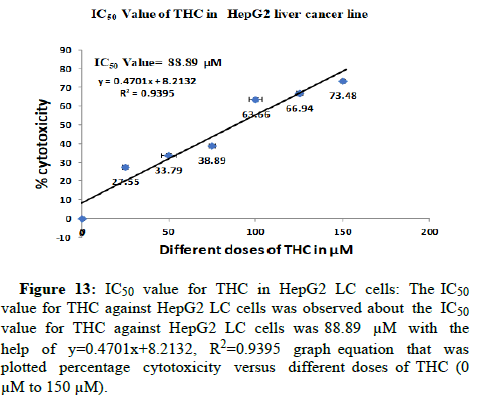
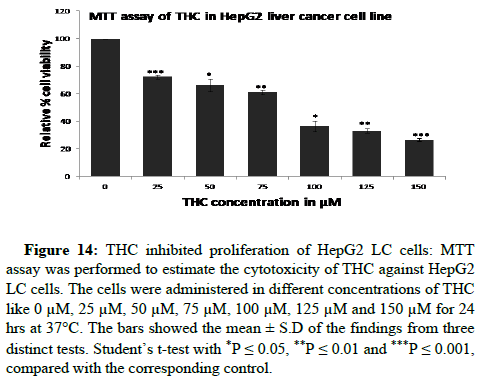
The cells have treated in different doses of THC like 0 μM, 25 μM, 50 μM, 75 μM, 100 μM, 125 μM and 150 μM for 24 hrs at 37°C for study the cytotoxic effect of THC in HEK-293 normal cell line and HepG2 LC cell lines. Firstly, we have checked the biocompatibility of THC in HEK-293 normal cells. THC has been observed as non-toxic to HEK-293 normal cells till 100 μM by the determination of IC50 value. We have calculated the IC50 value for THC in HEK-293 was 252.04 μM with the help of the graph equation (y=0.2086x-2.5746, R2=0.946) that was plotted percentage cytotoxicity versus different doses of THC (0 μM to 150 μM). THC did not inhibit the proliferation of normal cells (Figures 11 and 12). The biocompatibility of THC has been found in HEK-293 normal cells which further resulted in the exploration of the anti-proliferation activity of THC against HepG2 LC cells. Therefore, the anti-proliferation feature of THC has been determined in HepG2 LC cells. Further, we have revealed the IC50 value for THC against HepG2 LC cells was 88.89 μM with the help of y=0.4701x+8.2132, R2=0.9395 graph equation that was plotted percentage cytotoxicity versus different doses of THC (0 μM to 150 μM). Moreover, it has been observed that THC abrogated the expansion of HepG2 LC cells gradually in a dosagedependent way (Figures 13 and 14). These results have shown that THC substantially repressed the cell growth of LC cells as agrees with previous studies that were conducted on BC cells. They were reported that the THC selectively repressed the expansion of MCF-7 BC cells in a time and dosage-dependent way [16].
Figure 11: IC50 value for THC in HEK-293 normal cells: The IC50 value for THC in HEK-293 was calculated about IC50 value for THC in HEK-293 was 252.04 μM with the help of the graph equation (y=0.2086x-2.5746, R2=0.946) that was plotted percentage cytotoxicity versus different doses of THC (0 μM to 150 μM).
Figure 12: THC is non-toxic to HEK-293 normal cells: MTT assay was performed for estimating the cytotoxicity of THC in normal cells. Cells were treated in different doses of THC like 0 μM, 25 μM, 50 μM, 75 μM, 100 μM, 125 μM and 150 μM for 24 hrs at 37°C. The bars showed the mean ± S.D of the findings from three distinct tests. Student’s t-test with *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001, as compared with corresponding control.
Figure 13: IC50 value for THC in HepG2 LC cells: The IC50 value for THC against HepG2 LC cells was observed about the IC50 value for THC against HepG2 LC cells was 88.89 μM with the help of y=0.4701x+8.2132, R2=0.9395 graph equation that was plotted percentage cytotoxicity versus different doses of THC (0 μM to 150 μM).
Figure 14: THC inhibited proliferation of HepG2 LC cells: MTT assay was performed to estimate the cytotoxicity of THC against HepG2 LC cells. The cells were administered in different concentrations of THC like 0 μM, 25 μM, 50 μM, 75 μM, 100 μM, 125 μM and 150 μM for 24 hrs at 37°C. The bars showed the mean ± S.D of the findings from three distinct tests. Student’s t-test with *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001, compared with the corresponding control.
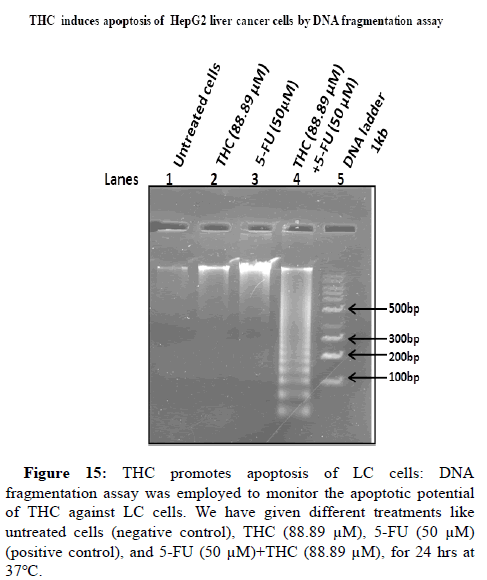
THC induces apoptosis of LC cells
THC might trigger apoptosis by inhibiting expansion of LC cells. Therefore, next, it has been studied the effect of THC on cell death in LC cells by DNA fragmentation assay. For this, LC cells were administered with different combinations like untreated cells (negative control), THC (88.89 μM), 5-FU (50 μM) (positive control), 5-FU (50 μM)+THC (88.89 μM), for 24 hrs at 37°C. We have observed that the THC induces apoptosis of LC cells (Lane 2, Figure 15) as compared to untreated cells (Lane 1, Figure 15). In the presence of chemotherapeutic drug 5-FU, the further increases the process of apoptosis as compared to THC (Lane 2, Figure 15). Whereas, the coadministration of the THC and 5-FU in LC cells resulted in the further elevation in the cell death of HepG2 LC cells like the smear pattern of genomic DNA in Lane 4, Figure 15.
Lane 5 shows a 1 kb DNA ladder for comparison analysis. Therefore, THC acts as a novel adjuvant therapeutic regimen to potentiate apoptosis of LCcells. These results have recommended that THC might utilize as a better therapeutic approach to tackle the side effects of standard chemotherapeutic drugs.
Figure 15: THC promotes apoptosis of LC cells: DNA fragmentation assay was employed to monitor the apoptotic potential of THC against LC cells. We have given different treatments like untreated cells (negative control), THC (88.89 μM), 5-FU (50 μM) (positive control), and 5-FU (50 μM)+THC (88.89 μM), for 24 hrs at 37°C.
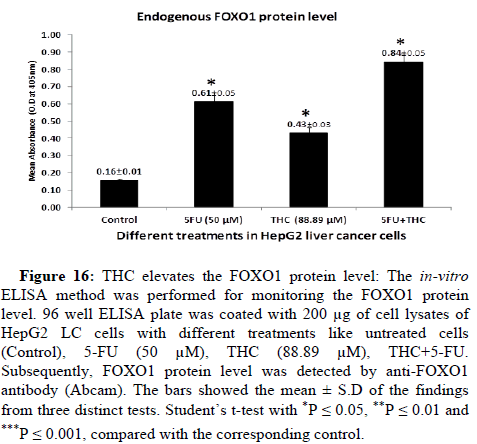
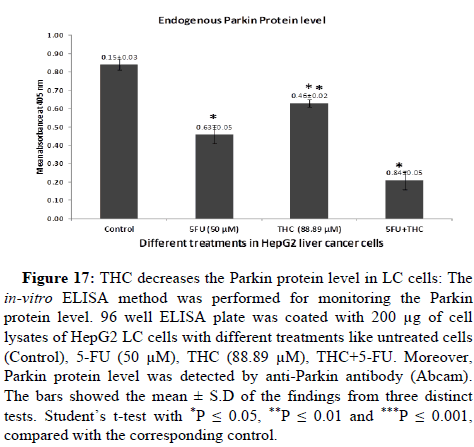
THC elevates FOXO1 protein level and reduces Parkin protein level
It has been reported that the FOXO1 protein level was found low whereas level of Parkin protein was on the upper side in LC [24,45]. FOXO1 and Parkin proteins regulate many crucial processes of cells like cell death, cell cycle arrest, oxidative stress and carcinogenesis. Therefore, we moved on to the next concern over whether THC regulates protein levels or not. To investigate this, in-vitro ELISA was employed for the estimation the expression of FOXO1 and Parkin proteins in the presence or absence of THC. To determine the endogenous protein levels of FOXO1 and Parkin, we have treated the HepG2 LC cells with different treatments like untreated cells (Control), 5-FU (50 μM), THC (88.89 μM), THC+5-FU drug. The 200 μg of cell lysates per well on the ELISA plate was coated. The FOXO1 and Parkin protein levels were detected by anti-FOXO1 and anti-Parkin antibodies (Abcam). We have observed that the THC significantly upregulates the FOXO1 protein level whereas downregulates the expression of Parkin protein as compared to untreated cells i.e., control (Figures 16 and 17). These results have indicated that the THC potently increases the FOXO1 protein level and decreases the Parkin protein level which might aid in their tumour suppression activity against LC in a safer way with lesser side effects as compared to conventional chemotherapy.
Figure 16: THC elevates the FOXO1 protein level: The in-vitro ELISA method was performed for monitoring the FOXO1 protein level. 96 well ELISA plate was coated with 200 μg of cell lysates of HepG2 LC cells with different treatments like untreated cells (Control), 5-FU (50 μM), THC (88.89 μM), THC+5-FU. Subsequently, FOXO1 protein level was detected by anti-FOXO1 antibody (Abcam). The bars showed the mean ± S.D of the findings from three distinct tests. Student’s t-test with *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001, compared with the corresponding control.
Figure 17: THC decreases the Parkin protein level in LC cells: The in-vitro ELISA method was performed for monitoring the Parkin protein level. 96 well ELISA plate was coated with 200 μg of cell lysates of HepG2 LC cells with different treatments like untreated cells (Control), 5-FU (50 μM), THC (88.89 μM), THC+5-FU. Moreover, Parkin protein level was detected by anti-Parkin antibody (Abcam). The bars showed the mean ± S.D of the findings from three distinct tests. Student’s t-test with *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001, compared with the corresponding control.
Discussion
LC is responsible for high mortality due to the frequent number of cases growing worldwide [2]. Besides, latest therapeutic developments in the treatment of LC, but acquisition of chemoresistance and its huge side effects are revealed that is a major challenge for their limited use as it worsens the survival rate of LC patients [8]. THC is one of the CUR metabolites extracted from Curcuma longa and is known for its various health-beneficial properties including anti-cancer activity and also exhibits limited or no side effects as compared to pre-existing chemotherapeutic drugs [46]. The anti-neoplastic activity of THC is well recognized by the regulation of various cell signalling molecules like growth factors, cytokines, transcription factors and tumour suppressor proteins that modulate the cellular proliferation and apoptotic activities in cancer cells which might result in mortality or suppression of proliferation of cancer cells [15]. In the current study, the anti-neoplastic properties of THC against LC by modulation of FOXO1 and Parkin were studied both via in-silico and in-vitro experimental strategies.
The occasional emergence of chemo-resistance harshly compromised the anti-neoplastic activities of available chemotherapeutic drugs that affected the cancer treatment. The functional loss/mutation in TSGs resulted in the development of chemo-resistance, and promotion of anti-apoptotic and proliferation processes. A better understanding of molecular insights about TSGs might lead to the restoration of their functions of tumour suppression [47]. The FOXO1 is a crucial transcription factor that acts as a TSG which modulates proliferation and apoptosis which are vital processes for tumour initiation and tumour progression [24]. The anti-tumour property of FOXO1 has been recently reported by suppressing the expansion, migration and invasion in YD-9 oral cancer cells [48]. The FOXO1 also inhibited Gastrointestinal Stromal Tumours (GISTs) expansion and stimulates cell death by downregulation of the MAPK/PI3K signalling cascade [49]. As per a very recent study, Parkin behaves as a tumour suppressor which diminishes the expansion and migration of bladder cancer cells by ubiquitinating the catalase protein [50]. The cell growth of HCT-15 colorectal cancer cells and A549 lung cancer cells were also inhibited by Parkin via regulation of DNA damage, activation of p53, reduction in cycle B1 and promotion of cell cycle arrest [51].
In light of the growing threat towards LC, it is necessary to seek out compounds with anticancer potential and binding affinities for the proteins FOXO1 and Parkin. Due to this dire need, various clinical findings have revealed that the notion of repurposing existing chemotherapeutic medications is a reliable method for developing a distinct, efficient treatment paradigm. This situation offered a chance to investigate alternative therapeutic options, with an emphasis on the possible application of medicinal plants [8,9]. The main purpose of this investigation is to seek out THC compounds that can effectively interact with FOXO1 and Parkin to govern the emergence of LC in a person, consequently decreasing or avoiding liver LC metastasis. It is noteworthy that earlier research sought natural anticancer medications with pharmacological features in plants, which used in the literature to treat and prevent a variety of severe diseases including cancer.
In our present investigations, GEPIA 2 and UALCAN, freely available online databases, pharmacokinetic profiles, and molecular docking were first used to investigate the anticancer potential of THC compounds [16] with FOXO1 and Parkin protein for LC. Boxplot representation via GEPIA2 and UALCAN offers the best identification of both FOXO1 and Parkin TSG expression in LIHC in different sample types (normal and tumour). Drug similarity, bioavailability, pharmacodynamics and toxicity profile predictions are critical for establishing and recommending a medicine for human health. Since a result, THC meets all of the necessary criteria for using the drug as a safer way of human anticancer drug because THC follows both Lipinski’s and Veber’s rules as demonstrated by several studies [52]. Additionally, the compound with the higher binding affinity and lowest binding energy with FOXO1 and Parkin proteins is identified to play a crucial function. The findings demonstrate that THC exhibited the highest molecular interactions with FOXO1 and Parkin protein-6.4 kcal/mol and -5.7 kcal/mol whereas 5-FU which is a man-made commercial chemotherapeutic drug shows lower interactions -4.1 kcal/mol and -4.6 kcal/mol with FOXO1 and Parkin protein. Accordingly, selection revealed that THC compound could be an effective anti-cancerous drug with FOXO1 and Parkin protein against LC proliferation and provide the search for new and established therapy for this cancer.
In the current study, we have also shown that THC reduces LC by in-vitro system. These findings results have revealed that the THC effectively inhibited the expansion of HepG2 LC cells and promoted cell death in LC cells consistent with results observed by a very recent study [16]. We have also confirmed the biocompatibility of THC in HEK-293 normal cells and also found that the THC is non-toxic to HEK-293 normal cells by the determination of IC50 value is about for THC in HEK-293 was 252.04 μM. Moreover, we have found the IC50 value for THC against HepG2 LC cells was 88.89 μM which repressed the expansion of LC cells gradually in a dosage-dependent way. These results have shown that THC significantly suppressed the expansion of LC cells as it is similar to previous studies that were conducted on BC cells. They reported that the THC selectively repressed the cell growth of MCF-7 BC cells in a time and dosage-dependent way [16].
With the help of DNA fragmentation assay, we have confirmed that the THC also facilitates the apoptosis process by inhibiting the expansion of LC cells. For this apoptotic study, we treated the LC cells with THC alone (88.89 μM), 5-FU alone (50 μM), and 5-FU (50 μM) +THC (88.89 μM), for 24 hrs at 37°C. We have observed that the THC significantly induces apoptosis against LC as compared to control cells. Although 5-FU, further increases the process of apoptosis as compared to THC side effects of chemotherapy and drug resistance are well reported by many researchers [8]. Therefore, THC might act as a better therapeutic regimen to control LC in a safer way to overcome the side effects of chemotherapy.
As per cited literature, FOXO1 and Parkin proteins are modulating vital processes like apoptosis, cell cycle arrest, proliferation, oxidative stress and carcinogenesis. The FOXO1 and Parkin protein levels are found to be altered in various cancers [24,53,54]. Therefore, the restoration of their protein levels will aid in the suppression of tumours.
Therefore, we have determined the influence of THC on these protein levels by in-vitro ELISA method in HepG2 LC cells. We have observed that THC significantly upregulates the FOXO1 protein level whereas down regulates the expression of the Parkin protein which might be useful in the inhibition of LC. However, this pre-clinical assessment would be useful for the development of a better and novel adjuvant therapy in a safer way against LC over available conventional treatments.
Conclusion
The present study has recommended that the THC is an efficient phytocompound in the management of LC. The pharmacokinetic and molecular docking scores expanded the knowledge of FOXO1 and Parkin's molecular interactions with THC. In addition, numerous in-vitro evaluations, including MTT assay, ELISA, and DNA fragmentation assay in this study, validated and implied THC as a better chemotherapeutic drug in LC. These studies have observed that THC significantly reduces the cellular expansion in HepG2 LC cells as well as also promotes apoptosis. Further, THC also increases the FOXO1 protein level and decreases the parkin protein level, which might aid in the tumour suppression activity of these proteins. However, this conclusion must be corroborated by more advancement in silico studies, preclinical studies, and clinical studies in future. So, in this regard, future in-vivo experiments are needed to determine the molecular mechanism that is relevant to THC-promoted the inhibition of LC by regulating FOXO1/ Parkin TSGs. Our studies might aid in the development of THC as a better therapeutic approach against LC in a safer way with fewer side effects as compared to conventional chemotherapy.
Credit Author Statement
Madhu Rani: Conceptualization, methodology, visualization, investigation, validation, data analysis, writing-original draft and writing-review editing. Rashmi Kumari: Investigation, methodology, validation, data analysis, writing-original draft and writing-review editing. Siya Ram: Visualization, Reviewing and Editing, M. Moshahid Alam Rizvi: Supervision, Resources, conceptualization, and critically reviewing draft.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
Not applicable.
Acknowledgements
The financial assistance (Grant No.BL/18-19/0058) was provided to Dr Madhu Rani by UGC under the research scheme for Dr. D. S. Kothari Postdoctoral fellowship is highly acknowledged.
References
- Kumari R, Ram S, Rizvi MM, Rani M (2024) Molecular insights of exonal polymorphism in cancer risk. Ann Oncol Case Rep 4: 1018.
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, et al. (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74: 229-263.
[Crossref] [Google Scholar] [PubMed]
- World Cancer Research Fund (2022) Liver cancer statistics.
- Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, et al. (2022) Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 77: 1598-606.
[Crossref] [Google Scholar] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, Singal A, Pikarsky E, et al. (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7: 7.
- Yang M, Zhang H, Zhang J, Yao X (2023) Risk factors and prevention of liver cancer: A bibliometric and visual analysis. Medicine 102: e35740.
[Crossref] [Google Scholar] [PubMed]
- Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A (2022) Hepatocellular carcinoma. Lancet 400: 1345-1362.
[PubMed]
- Singh AK, Singh SV, Kumar R, Kumar S, Senapati S, et al. (2023) Current therapeutic modalities and chemopreventive role of natural products in liver cancer: Progress and promise. World J Hepatol 15: 1.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Li J, Xia L (2023) Plant-derived natural products and combination therapy in liver cancer. Front Oncol 13: 1116532.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Zhang W, Xu L, Zhou H, Yuan M, et al. (2022) Recent progress in understanding the action of natural compounds at novel therapeutic drug targets for the treatment of liver cancer. Front Oncol 11: 795548.
[Crossref] [Google Scholar] [PubMed]
- Gull N, Arshad F, Naikoo GA, Hassan IU, Pedram MZ, et al. (2023) Recent advances in anticancer activity of novel plant extracts and compounds from Curcuma longa in hepatocellular carcinoma. J Gastrointest Cancer 54: 368-390.
[Crossref] [Google Scholar] [PubMed]
- Darvesh AS, Aggarwal BB, Bishayee A (2012) Curcumin and liver cancer: A review. Curr Pharm Biotechnol 13: 218-228.
[Crossref] [Google Scholar] [PubMed]
- Giordano A, Tommonaro G (2019) Curcumin and cancer. Nutrients 11: 2376.
[Crossref] [Google Scholar] [PubMed]
- Ratnatilaka Na Bhuket P, El-Magboub A, Haworth IS, Rojsitthisak P (2017) Enhancement of curcumin bioavailability via the prodrug approach: Challenges and prospects. Eur J Drug Metab Pharmacokinet 42:341-53.
[Crossref] [Google Scholar] [PubMed]
- Zhou M, Li R, Hua H, Dai Y, Yin Z, et al. (2024) The role of tetrahydrocurcumin in disease prevention and treatment. Food Funct 15: 6798-824.
[Crossref] [Google Scholar] [PubMed]
- Zeng A, Yu X, Chen B, Hao L, Chen P, et al. (2023) Tetrahydrocurcumin regulates the tumor immune microenvironment to inhibit breast cancer proliferation and metastasis via the CYP1A1/NF-κB signaling pathway. Cancer Cell Int 23: 12.
[Crossref] [Google Scholar] [PubMed]
- Qiu D, Li X, Liu Y, Bao X, Ma L (2022) Tetrahydrocurcumin cc hemosensitizes breast cancer to albumin-bound paclitaxel by enhancing SPARC expression through demethylation. J Oncol 2022: 7961537.
[Crossref] [Google Scholar] [PubMed]
- Song G, Lu H, Chen F, Wang Y, Fan W, et al. (2018) Tetrahydrocurcumin-induced autophagy via suppression of PI3K/Akt/mTOR in non-small cell lung carcinoma cells. Mol Med Rep 17: 5964-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Peng L, Liu A, Ji J, Zhao L, et al. (2018) The enhanced effect of tetrahydrocurcumin on radiosensitivity of glioma cells. J Pharm Pharmacol 70: 749-59.
[Crossref] [Google Scholar] [PubMed]
- Yoysungnoen B, Bhattarakosol P, Patumraj S, Changtam C (2015) Effects of tetrahydrocurcumin on hypoxia-inducible factor-1α and vascular endothelial growth factor expression in cervical cancer cell-induced angiogenesis in nude mice. Biomed Res Int 2015: 391748.
[Crossref] [Google Scholar] [PubMed]
- Han X, Deng S, Wang N, Liu Y, Yang X (2016) Inhibitory effects and molecular mechanisms of tetrahydrocurcumin against human breast cancer MCF-7 cells. Food Nutr Res 60: 30616.
[Crossref] [Google Scholar] [PubMed]
- Liu W, Zhang Z, Lin G, Luo D, Chen H, et al. (2017) Tetrahydrocurcumin is more effective than curcumin in inducing the apoptosis of H22 cells via regulation of a mitochondrial apoptosis pathway in ascites tumor-bearing mice. Food Funct 8: 3120-9.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Guan F, Huang H, Chen H, Liu Y, et al. (2024). Tetrahydrocurcumin ameliorates hepatic steatosis by restoring hepatocytes lipophagy through mTORC1-TFEB pathway in nonalcoholic steatohepatitis. Biomed Pharmacother 178: 117297.
[Crossref] [Google Scholar] [PubMed]
- Rani M, Kumari R, Singh SP, Devi A, Bansal P, et al. (2023) MicroRNAs as master regulators of FOXO transcription factors in cancer management. Life Sci 321: 121535.
[Crossref] [Google Scholar] [PubMed]
- Yang S, Pang L, Dai W, Wu S, Ren T, et al. (2021) Role of forkhead box O proteins in hepatocellular carcinoma biology and progression. Front Oncol 11: 667730.
[Crossref] [Google Scholar] [PubMed]
- Cui X, Zhao H, Wei S, Du Q, Dong K, et al. (2023) Hepatocellular carcinoma-derived FOXO1 inhibits tumor progression by suppressing IL-6 secretion from macrophages. Neoplasia 40: 100900.
[Crossref] [Google Scholar] [PubMed]
- Jia G, Tang Y, Deng G, Fang D, Xie J, et al. (2019) miR-590-5p promotes liver cancer growth and chemotherapy resistance through directly targeting FOXO1. Am J Transl Res 11: 2181.
[Google Scholar] [PubMed]
- Kumari R, Shakya AK, Rizvi MM, Ram S (2024) Anti-cancer potential of bioactive compounds from Moringa oleifera against PARK2 in oral cancer protein: In silico approach: Anti-cancer potential of bioactive compounds from Moringa oleifera. J Ayurveda Integr Med 12.
- Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, et al. (2013). The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 501: 512-6.
[Crossref] [Google Scholar] [PubMed]
- Xu L, Lin DC, Yin D, Koeffler HP (2014) An emerging role of PARK2 in cancer. J Mol Med 92: 31-42.
[Crossref] [Google Scholar] [PubMed]
- Wang F, Denison S, Lai JP, Philips LA, Montoya D, et al. (2004) Parkin gene alterations in hepatocellular carcinoma. Genes Chromosomes Cancer 40: 85-96.
[Crossref] [Google Scholar] [PubMed]
- Tang Z, Li C, Kang B, Gao G, Li C, et al. (2017) GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45: W98-102.
[Crossref] [Google Scholar] [PubMed]
- Tang Z, Chen T, Li C, Kang B (2019) GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res.
- The University of Alabama at Birmingham cancer data analysis portal (2022).
- Xie J, Zhu Z, Cao Y, Ruan S, Wang M, et al. (2021) Solute carrier transporter superfamily member SLC16A1 is a potential prognostic biomarker and associated with immune infiltration in skin cutaneous melanoma. Channels 15: 483-95.
[Crossref] [Google Scholar] [PubMed]
- SwissADME. Molecular modeling group of the SIB. Swiss Institute of Bioinformatics.
- ProTox 3.0. Prediction of toxicity of chemicals. Last updated: May 2024.
- Arulanandam CD, Hwang JS, Rathinam AJ, Dahms HU (2022). Evaluating different web applications to assess the toxicity of plasticizers. Sci Rep 12: 19684.
[Crossref] [Google Scholar] [PubMed]
- RCSB protein data bank (RCSB.org).
- Dassault systemes. Discovery studio visualizer.
- PubChem. National Library of Medicine.
- PyRx-Python prescription. Sourceforge.
- Virtual Screening ToolMeena R, Kesari KK, Rani M, Paulraj R (2012) Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human breast cancer cells (MCF-7). J Nanoparticle Res 14: 1-1.
- Meena R, Rani M, Pal R, Rajamani P (2012) Nano-TiO2-induced apoptosis by oxidative stress-mediated DNA damage and activation of p53 in human embryonic kidney cells. Appl Biochem Biotechnol 167: 791-808.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Lin C, Song J, Chen H, Chen X, et al. (2019) Parkin facilitates proteasome inhibitor-induced apoptosis via suppression of NF-κB activity in hepatocellular carcinoma. Cell Death Dis 10: 719.
[Crossref] [Google Scholar] [PubMed]
- Lai CS, Ho CT, Pan MH (2020). The cancer chemopreventive and therapeutic potential of tetrahydrocurcumin. Biomolecules 10: 831.
[Crossref] [Google Scholar] [PubMed]
- Gao L, Wu ZX, Assaraf YG, Chen ZS, Wang L (2021) Overcoming anti-cancer drug resistance via restoration of tumor suppressor gene function. Drug Resist Updat 57: 100770.
[Crossref] [Google Scholar] [PubMed]
- Kim YG, Seong C, Cho KA, Lee SM, Kim TJ, et al. (2023) Anti-tumor properties of FoxO1 in YD-9 oral squamous cell carcinoma cells. Oncol Rep 49: 1-0.
[Crossref] [Google Scholar] [PubMed]
- Wang T, Zhao H, Gao H, Zhu C, Xu Y, et al. (2018) Expression and phosphorylation of FOXO1 influences cell proliferation and apoptosis in the gastrointestinal stromal tumor cell line GIST-T1. Exp Ther Med 15: 3197-202.
[Crossref] [Google Scholar] [PubMed]
- Zhang R, Jiang W, Wang G, Zhang Y, Liu W, et al. (2024) Parkin inhibits proliferation and migration of bladder cancer via ubiquitinating Catalase. Commun Biol 7:245.
[Crossref] [Google Scholar] [PubMed]
- Jung BC, Kim SH, Cho Y, Kim YS (2023) Tumor suppressor Parkin induces p53-mediated cell cycle arrest in human lung and colorectal cancer cells. BMB Rep 56: 557.
[Crossref] [Google Scholar] [PubMed]
- Mahal A, Al-Janabi M, Eyupoglu V, Alkhouri A, Chtita S, et al. (2024) Molecular docking, drug-likeness and DFT study of some modified tetrahydrocurcumins as potential anticancer agents. Saudi Pharm J 32: 101889.
[Crossref] [Google Scholar] [PubMed]
- Ejma M, Madetko N, Brzecka A, Guranski K, Alster P, et al. (2020) The links between Parkinson’s disease and cancer. Biomed 8: 416.
[Crossref] [Google Scholar] [PubMed]
- Bernardini JP, Lazarou M, Dewson G (2017) Parkin and mitophagy in cancer. Oncogene 36: 1315-27.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi