Research Article, J Physiother Rehabil Vol: 3 Issue: 2
An Experimental Study on Effectiveness of Spontaneous Respiratory Modulation and Aerobic Exercise in Subjects with Grade-1 Hypertension
Afeef TV1, Heera S1, Rahul Krishnan Kutty1* and Praveena D2
1Institute of Paramedical Health Sciences, Kannur Medical College, Prestige Educational Trust, Anjarakandy, Kerala, India
2Co-operative College of Health Sciences, Nettur PO, Thalasserry, Kerala, India
*Corresponding Author : Rahul Krishnan Kutty
Professor, Institute of Paramedical Health Sciences, Kannur Medical College, Prestige Educational Tust, Anjarakandy, Kerala, India
Tel: +91-9819563135
E-mail: physioraul@ gmail.com
Received: March 04, 2019 Accepted: April 23, 2019 Published: May 03, 2019
Citation: Afeef TV, Heera S, Kutty RK, Praveena D (2019) An Experimental Study on Effectiveness of Spontaneous Respiratory Modulation and Aerobic Exercise in Subjects with Grade-1 Hypertension. J Physiother Rehabil 3:2.
Abstract
Background: Objective of the study was to compare the effect of spontaneous respiratory modulation and aerobic exercise in Grade 1 hypertensive patients. Study Design is an Experimental study, study was conducted at Co-operative Institute of Health Sciences, Thalasseri, India, A total numbers of 30 subjects were included for the study according to inclusion and exclusion criteria of the study.
Intervention: The participants were divided into two groups, experimental group (=15) and control group (n=15). The control group received aerobic exercise on five days per week for a month, while the experimental group was assigned to do spontaneous respiratory modulation on two days per week and aerobic exercise on five days per week for a month.
Outcome measures: Systolic blood pressure and Diastolic blood pressure was evaluated using sphygmomanometer.
Results: Significant improvements in all outcome parameters were observed in response to the intervention. Between group analysis showed a statistically significant difference in SBP (P=0.000) and DBP (P=0.008)
Conclusion: The study concluded that spontaneous respiratory modulation and aerobic exercise training is an effective approach to improve blood pressure in grade 1 hypertensive patients.
Keywords: Spontaneous respiratory modulation; Aerobic exercise; Hypertension; Physiotherapy; Exercise protocol
Introduction
High blood pressure has been observed as a major health problem that prevails in India affecting the urban and rural population [1,2]. Persistent elevation of arterial blood pressure above 140 mm Hg systolic or 90 mm Hg diastolic is defined as hypertension.
WHO/ISH has divided hypertension into three grades: Grade 1 (Systolic BP 140-159 mm Hg, Diastolic BP 90-99 mm Hg), Grade 2 (Systolic BP 160-179 mm Hg, Diastolic BP 100-109 mm Hg), Grade 3 (Systolic BP ≥ 180, Diastolic BP ≥110) [3]. Hypertension is classified as primary or essential hypertension and secondary hypertension. Though primary hypertension is strongly associated with lifestyle, it has no specific origin. It is treated with changes in diet, increased physical activity, and medication as it has been responsible for 90 to 95% of diagnosed hypertension. The main cause of Secondary Hypertension is the pre-existing medical condition such as congestive heart, kidney and liver failures, or damage to the endocrine system [4], responsible for 5 to 10% of diagnosed hypertension.
The term “Hypertension” states that Hypertension is a common disease that contributes importantly to the high cardiovascular morbidity and mortality observed in industrialized countries. The proper diagnosis and management of this disorder affords considerable reduction of the risk of developing cardiac, cerebral, and renal complications. Approximately 95% of patients with high blood pressure exhibit the so-called essential or primary form of hypertension [5].
“Trends in hypertension epidemiology in India” stated that cardiovascular disease caused 2.3 million deaths in India in 1990 and this is projected to double by the year 2020. Hypertension is directly responsible for 57% of all stroke death and 24% of all coronary heart disease death in India. As per this study on an average there are 31.5 million hypertensive subjects in rural and 34 million in urban populations and 70% of these would be ‘stage I hypertension’. Recent reports show that borderline hypertension and stage I hypertension carry a significant cardiovascular risk and there is a need to reduce this blood pressure. For this, population based cost effective hypertension control strategies should be developed [6].
Hypertension affects 23.10% men and 22.60% women over 25 years old in India. Increased blood pressure is a high-risk condition that causes approximately 51% deaths from stroke and 45% from coronary artery disease. It was directly responsible for 7.5 million deaths in 2004 that is about 12.8% of the total global deaths [7]. A survey of 26,000 adults in South India showed a hypertension prevalence of 20% (men 23% and women 17%) but 67% of those with hypertension was unaware of their diagnosis. Most of the people still remain undetected and it’s controlling also inadequate. These situation calls for urgent prevention and control measures [8].
A recent survey study on hypertension and stroke in Asia, which expressed about the prevalence, control and strategies in developing countries for prevention of hypertension and stroke, States that In India, China, Philippines, Thailand, Sri Lanka, Iran, Pakistan, Nepal, there has been a rapid increase in stroke mortality and prevalence of hypertension. According to the new criteria the prevalence of hypertension varies between 15%-35% among urban adults and it is two to three times lower among rural subjects. Hypertension and stroke occur at a relatively younger age and the risk of its increase is at lower levels of BMI of 23-25 kg/m2 among Asians. It has been observed that overweight, sedentary behaviour, alcohol, higher social class, salt intake, diabetes mellitus and smoking are main risk factors of hypertension [9].
Progression of hypertension is strongly associated with functional and structural cardiac and vascular abnormalities that damage the heart, kidneys, brain, vasculature, and other organs and lead to premature morbidity and death [10]. 70% of these patients would be Stage I hypertensive. Each 10 mm of Hg increase in blood pressure doubles the risk of death in hypertensive patients [11]. Recent reports show that borderline hypertension (systolic BP 130-139 mm Hg and/or diastolic BP 85-89 mm Hg) and Stage I hypertension carry a significant cardiovascular risk [12]. 91% of hypertension cases precede the development of Congestive Heart Failure (CHF) with high blood pressure increase having the risk of developing CHF by two to three times [13].
Lowering blood pressure with antihypertensive drugs can reduce the risk [14]. The side effects and cost of antihypertensive drugs have stimulated the search for a non-pharmacological approach to control BP either as a first line or adjunctive treatment. Several studies have demonstrated that lifestyle modifications such as physical exercise, salt restriction and weight reduction can lower BP [14,15].
Baroreflex sensitivity can be improved by slow breathing and it decreases blood pressure in healthy [16] and hypertensive subjects [17]. Therefore, respiratory modulation may be of therapeutic value in controlling hypertension. According to a study, such improvement is associated with a change in patient's breathing pattern, which would initiate at a lower frequency and higher amplitude [18]. The breathing exercises that is practised regularly increases parasympathetic tone, decreasing the sympathetic activity, and as a result it improves the cardiovascular and respiratory functions and decreases the effect of stress and strain on the body and improves the physical and mental health [19,20].
In hypertensive and normotensive person’s blood pressure can be reduced by the aerobic exercises. For the prevention and treatment of high blood pressure aerobic physical activity can be considered as an important component of lifestyle modification [17]. To promote and maintain health, all healthy adults aged 18-65 years, need moderateintensity aerobic physical activity for a minimum of 30 minutes on five days each in a week or vigorous-intensity aerobic activity for a minimum of 20 minutes on three days each week [21].
Subjects with hypertension are known to have a two-fold higher risk of developing coronary artery disease (CAD), four times higher risk of congestive heart failure and seven times higher risk of cerebrovascular disease and stroke compared to normotensive subjects [22].
Reducing blood pressure can decrease cardiovascular risk and this can be achieved by lifestyle measures in mild cases and should be the initial approach to hypertension management in all cases. This includes dietary interventions weight reduction, tobacco cessation, and physical activity [1].
There are many studies on effect of aerobic exercise in hypertension. The effect of aerobic training in reducing clinical blood pressure in hypertensive subjects is well proven and accepted. There are only few studies which explain about effect of spontaneous respiratory modulation in hypertensive patient. Therefore, the purpose of the study is to find out effectiveness of spontaneous respiratory modulation and aerobic exercise and to compare the effect of spontaneous respiratory modulation and aerobic exercise in subjects with Grade 1 hypertension.
Materials and Methods
A total 30 subjects were selected based on inclusion criteria.
Grade 2 hypertension and Grade 3 hypertension, Secondary hypertension; patients with recent cardiovascular and Pulmonary disease/disorders were excluded from the study. Patients reported of Cardiac arrhythmias, patient on Antihypertensive drugs, Subjects with major neurologic disease, recent fracture and surgery of spine upper and lower extremity, any Psychogenic disorders like Physical fatigue, depression and frustration were also excluded. Non-alcoholic, non-smoker and Cooperative patients were only included in the study.
The total duration of the study was 3 months and duration of exercise for each individual subjects was 4 weeks.
Grade 1 Hypertension and both genders with age group 45-55 years were included in the study. Subjects were explained about the research work and a written informs consent was collected. Selected subjects were informed to sign a consent stating the voluntary participation in this study. An institutional ethical clearance was obtained. The study was conducted at Co-operative Institute of Health Science, Thalasseri, India.
Later the subjects were divided into two groups, Control Group and Experimental Group based on purposive random sampling method. Control Group was given aerobic exercise and Experimental Group was given spontaneous respiratory modulation and aerobic exercise. The procedures were explained to them and exercises were demonstrated with commands. Prior to the training pre-test systolic and diastolic blood pressure was measured using sphygmomanometer. After pre-test measurement training was given as per protocol. Posttest measurements were taken after the end of 4th week training.
Group I received an exercise protocol which included an Aerobic Exercise regime. It includes Warm up phase (10 minutes), 5 minute of light (low intensity) physical activity such as walking, jogging on the spot. Pumping arms or makes large but controlled circular movements with your arms to help warm the muscles of your upper body. Static stretching was performed after muscles warm up state.
A Static stretching for 5 minutes for patients were also performed (stretching a muscle and holding it in this position without discomfort for 10-30 seconds).
Aerobic phase (30 minutes) includes Static cycling, Intensity: Moderate 12-13 RPE Duration: 30 minute session 5 days per week for a month. Cool down phase (10 minutes) Low intensity exercise for 5 minutes and Static stretching for 5 minutes.
Whereas Group II receives Spontaneous Respiratory Modulation: Training protocol for breathing control was applied using, first, diaphragmatic, intercostal, and upper chest breathing pattern to make the patient aware of his respiratory movements. Patients were asked to lie supine, with knees flexed and feet flat on the floor, and raise their hands to the area of the chest related to each breathing pattern: diaphragm, intercostal muscles, and clavicular region. At each breathing pattern, patients were instructed to feel and identify the rib cage motion and its amplitude. Subsequently, they were instructed to decrease their respiratory rate gradually while increasing respiratory amplitude. This procedure was performed ten times for each breathing pattern.
The patients then sat down and performed the slow breathing technique, intended to promote quieter breathing by increasing respiratory amplitude and reducing respiratory rate while the three breathing patterns described above are simultaneously performed. This technique was done in 30-minute sessions held twice a week during one month.
Other than the above mentioned procedure, an aerobic phase (30 minutes) and Cool down phase (10 minutes) were performed. Aerobic phase (30 minutes) includes a static cycling Intensity: Moderate 12-13 RPE, Duration: 30 minute session 5 days per week for a month and a Cool down phase (10 minutes) includes low intensity exercise for 5 minutes static stretching for 5 minutes.
Statistical analysis
All the data were collected and cleaned and checked in SPSS 16 version for any missing or wrong entry. Kolmogorov-Smirnov test was done to find out the normality in the groups. Paired' t-test was performed as parametric test to find out the intra group significance and for inter group significance Mann-Whitney 'U' test was used as a non-parametric test.
Results and Interpretation
All the data were collected and entered in SPSS 16 version, the demographical data of the two groups are illustrated below (Table 1).
| Variables | Controlled Group (n=15) | Experimental Group (n=15) | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | Std. Deviation | Minimum | Maximum | Mean | Std. Deviation | |
| Age | 45 | 55 | 49.80 | 3.234 | 46 | 54 | 50.13 | 2.669 |
| Height | 153 | 169 | 161.60 | 5.514 | 155 | 167 | 162.40 | 4.517 |
| Weight | 61 | 70 | 65.07 | 2.939 | 60 | 69 | 64.27 | 3.105 |
| BMI | 23.42 | 26.56 | 24.9340 | 0.88831 | 21.77 | 28.30 | 24.3987 | 1.45631 |
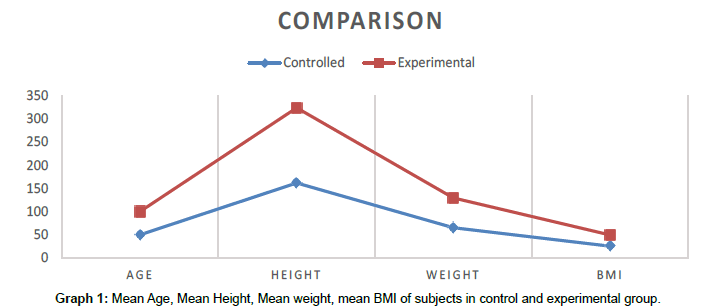
Table 1: Mean of age, height, weight and BMI of control group and experimental group.
In Control group consist of 15 subjects with mean age 49.80 ± 3.234 (SD), Mean Height of 161.60 ± 5.234 (SD), Mean Weight of 64.53 ± 3.441 (SD) and Mean BMI 24.9340 ± 0.88831 (SD) and in Experimental group consist of 15 subjects with mean age 49.73 ± 2.789 (SD), Mean Height of 161.87 ± 4.190 (SD), Mean Weight of 64.47 ± 3.378 (SD) and Mean BMI 24.3987 ± 1.45631 (SD) (Graph 1).
Analysis of scale used in the study was checked and statistical analysis was performed by SPSS 16.0 software and the results are illustrated below.
Analysis of scale 1
From above table, it is evident that ‘t’ test was used to analyse the intra group significance of SBP of control group (Table 2).
| Kolmogorov-Smirnov | Shapiro-Wilk | |||||
|---|---|---|---|---|---|---|
| Statistic | df | Sig | Statistic | df | Sig. | |
| SBP Pre Test | .100 | 15 | .200 | .970 | 15 | .861 |
| SBP Post Test | .133 | 15 | .200 | .963 | 15 | .747 |
Table 2: Test of normality for intra-group significance of SBP control group.
The above table shows the paired ‘t’ test scores of SBP of control group (Table 3).
| Control group | Mean | Std Deviation | Std error mean | 95% confidence interval of difference | T | df | Sig | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| SBP | Pre test | 149.27 | 5.861 | 1.513 | 3.455 | 4.278 | 20.149 | 14 | .000 |
| Post test | 145.40 | 6.345 | 1.638 | ||||||
Table 3: Analysis pre-test and post-test scores of SBP of control group.
Interpretation: The Graph 2 shows that mean pre-test SBP value of control group is 149.27 and the post-test value is 145.4 so there is significance difference in pre and post-test value in control group and from the graph it is clear that the systolic blood pressure has decreased significantly in post-test. It also represents mean pretest SBP value of experimental group is 150.4 and the post-test value is 145.33 so there is significance difference in pre and posttest value in experimental group and from the graph it is clear that the systolic blood pressure has decreased significantly in post-test.
From above table, it is evident that ‘t’ test can be used to analyse the intra group significance of SBP of experimental group (Table 4).
| Kolmogorov-Smirnov | Shapiro-Wilk | |||||
|---|---|---|---|---|---|---|
| Statistic | df | Sig | Statistic | df | Sig. | |
| SBP Pre test | .144 | 15 | 200 | .950 | 15 | .527 |
| SBP Post test | .132 | 15 | 200 | .969 | 15 | .839 |
Table 4: Test of normality for intra-group significance of SBP experimental group.
The above table shows the paired ‘t’ test scores of SBP of experimental group (Table 5).
| Experimental group | Mean | Std Deviation | Std error mean | 95% confidence interval of difference | T | df | Sig | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| SBP | Pre test | 150.40 | 5.180 | 1.337 | 4.677 | 5.456 | 27.884 | 14 | .000 |
| Post test | 145.33 | 5.381 | 1.389 | ||||||
Table 5: Analysis pre-test and post test scores of SBP of experimental group.
From above table, it is evident that the inter-group significance of SBP can be analysed by Mann-Whitney U Test (Table 6).
| Kolmogorov-Smirnov | Shapiro-Wilk | |||||
|---|---|---|---|---|---|---|
| Statistic | df | Sig | Statistic | df | Sig. | |
| Control SBP | .238 | 15 | .022 | .817 | 15 | .006 |
| Experimental SBP | .271 | 15 | .004 | .815 | 15 | .006 |
Table 6: Test of normality for inter-group significance of SBP.
Interpretation: From above table through Mann-Whitney U-test, it is evident that significant value .000 which is less than probability value p=0.05; which indicates that there is significant difference of SBP in the control and experimental group. Hence, pretest and post-test value difference of experimental group shows grater improvement than that in control group (Table 7).
| VAR00004 | N | Mean rank | Sum of ranks |
|---|---|---|---|
| PRESBP-POSTSBP 1 2 Total |
15 15 30 |
20.90 10.10 |
313.50 151.50 |
| Mann-Whitney U Wilcoxon W Z Asymp.Sig (2-tailed) Exact Sig [2*(1-tailed Sig.)] |
31.500 151.500 -3.526 .000 .000a |
||
Table 7: Analysis of pre-test and post-test value difference of SBP of control and experimental group.
Analysis of scale 2
From above table, it is evident that ‘t’ test can be used to analyse the intra group significance of DBP of control group (Table 8).
| Kolmogorov-Smirnov | Shapiro-Wilk | |||||
| Statistic | df | Sig | Statistic | df | Sig. | |
| DBP Pre test | .126 | 15 | .200* | .938 | 15 | .352 |
| DBP Post test | .139 | 15 | .200* | .949 | 15 | .503 |
Table 8: Test of normality for intra-group significance of DBP control group.
The above table shows the paired ‘t’ test scores of DBP of control group (Table 9).
| Control group | Mean | Std Deviation | Std error mean | 95% confidence interval of difference | T | df | Sig | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| DBP | Pre test | 94.80 | 3.052 | .788 | 1.847 | 2.419 | 16.000 | 14 | .000 |
| Post test | 92.67 | 3.436 | .887 | ||||||
Table 9: Analysis pre-test and post test scores of DBP of control group.
From above table, it is evident that ‘t’ test can be used to analyse the intra group significance of DBP of experimental group (Table 10).
| Kolmogorov-Smirnov | Shapiro-Wilk | |||||
| Statistic | df | Sig | Statistic | df | Sig. | |
| SBP Pre test | .149 | 15 | 200* | .931 | 15 | .285 |
| SBP Post test | .109 | 15 | 200* | .983 | 15 | .985 |
Table 10: Test of normality for intra-group significance of DBP experimental group.
The above table shows the paired ‘t’ test scores of DBP of experimental group (Table 11).
| Experimental group | Mean | Std Deviation | Std error mean | 95% confidence interval of difference | T | df | Sig | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| DBP | Pre test | 95.60 | 2.667 | .689 | 2.344 | 3.123 | 15.043 | 14 | .000 |
| Post test | 92.87 | 3.114 | .804 | ||||||
Table 11: Analysis pre-test and post test scores of DBP of experimental group.
From above table, it is evident that the inter-group significance of DBP can be analysed by Mann-Whitney U Test (Table 12).
|
|
Kolmogorov-Smirnov | Shapiro-Wilk | ||||
| Statistic | df | Sig | Statistic | df | Sig. | |
| Control DBP | .402 | 15 | .000 | .694 | 15 | .000 |
| Experimental DBP | .381 | 15 | .000 | .771 | 15 | .002 |
Table 12: Test of normality for inter-group significance of DBP.
Interpretation: From above table through Mann-Whitney U-test, it is evident that significant value .008 which is less than probability value p=0.05; which indicates that there is significant difference of DBP in the control and experimental group. Hence, pretest and post-test value difference of experimental group shows grater improvement than that in control group (Table 13).
| VAR00005 | N | Mean rank | Sum of ranks |
|---|---|---|---|
| Pre DBP-Post DBP 1 2 Total |
15 15 30 |
19.33 11.67 |
290.00 175.00 |
| Mann-Whitney U Wilcoxon W Z Asymp.Sig (2-tailed) Exact Sig [2*(1-tailed Sig.)] |
55.000 175.000 -2.638 .008 .016a |
||
Table 13: Analysis of pre-test and post-test value difference of DBP of control and experimental group.
Discussion
The study performed was opted an experimental pre-test, posttest approach to find out the effect of spontaneous respiratory modulation with aerobic exercise in Grade-1 hypertension.
In this study, 30 stage 1 hypertensive patients satisfying the inclusion criteria were selected and they are divided into two groups experimental group (n=15) and control group (n=15). Both the group were assessed on the first day and the last day of treatment. Systolic and Diastolic blood pressure reference were taken and noted. Those values were taken as Pre and Post blood pressure value which was measured by using sphygmomanometer.
The result of the study showed that there is improvement in both the groups, and the experimental group training was found to be more beneficial than the control group training on improving blood pressure in Grade 1 hypertensive subjects.
The effect of aerobic training in reducing clinical blood pressure in hypertensive subjects is well proven and accepted. Hypertension has a multifactorial etiology and, therefore, several mechanisms may be involved in the hypotensive effects of aerobic training. Nevertheless, a meta-analysis [23] has concluded that aerobic training reduces blood pressure due to a reduction in peripheral vascular resistance.
During dynamic exercise, cardiac output increases dramatically to ensure adequate perfusion to the working musculature. This increase is achieved by a withdrawal of parasympathetic tone (causing an increased heart rate and contractility), an increase in sympathetic activity (directly and indirectly increasing heart rate and contractility) and pronounced vasoconstriction of the venous vasculature (causing a greater venous return and therefore stroke volume). In parallel, the need for increased blood flow and oxygen delivery to the exercising muscle is achieved through regional vasodilatation of those arterioles supplying blood to the exercising tissue in combination with a vasoconstriction of arterioles, which perfuse non-essential tissues. Contraction of the active muscle mass also assists in returning blood towards the heart. This ‘muscle pump’ effect further increases venous return and stroke volume. Increased cardiac output and vasoconstriction in non-exercising vascular beds increases systolic blood pressure (SBP), but the significant vasodilation at the exercising muscle beds helps to buffer this increase and results in a minimal rise in diastolic blood pressure (DBP). As exercise continues at the same intensity, blood pressure is often found to diminish from the peak values achieved early in exercise. This may be attributed to a redistribution of blood to the periphery for heat dissipation, and a resultant reduction in cardiac filling [24].
During endurance exercise (i.e. cycling, running), SBP is tightly coupled to the exercise intensity and can often reach values of over 200 mm Hg [25]. Although it is usually reported that DBP changes little throughout changes in the exercise intensity, Palatinis [26] has suggested that changes in DBP are more variable and can range from a slight decrease, due to the vasodilatation of the muscle vasculature, to an increase of 10 to 20 mm Hg, presumably from the occlusion of blood flow caused by the forceful contractions of the exercising muscle. Following exercise, blood pressure rapidly returns to normal.
The results of our study are supported by studies in the literature showing that an acute slow and regular breathing pattern may beneficially affect reflex control of the cardiovascular system and modulates BP, probably via stimulation of slowly adapting pulmonary stretch receptors [27-29]. More specifically, slowing down breathing rate increases baroreceptor sensitivity [30]. According to Joseph et al. [17], the decrease in blood pressure during slow breathing, more precisely less than ten breaths per minute (BPM), is associated with improved baroreflex sensitivity, which states a change in autonomic balance resulting from an absolute or relative reduction in sympathetic activity or increase in parasympathetic tone.
Slow breathing at 6 cycle/minute affects all respiratory rate interval fluctuations and cause them to merge at the rate of respiration and to increase greatly in amplitude. This increase in RR interval fluctuations (relative to blood pressure changes) has the effect of enhancing the baroreflex efficiency [31,32] and in turn, might have contributed to lower blood pressure. It reduces sympathetic activity by enhancing central inhibitory rhythms [33] as result it decrease the blood pressure while enhancing the baroreflex. Added to that, the increase in Vt (Tidal Volume) (deriving from the slowing in breathing rate) activates the Hering-Breuer reflex [34] which in turn reduces the chemoreflex sensitivity and thus might enhance the baroreflex by reducing blood pressure and sympathetic activity [34-37]. Whatever may the mechanism, it is certain that changes in sympathetic activity and in baroreflex sensitivity are interrelated [38,39].
In hypertension, the sympathetic hyperactivity has been found associated with a generalized enhancement of the excitatory pathways, leading not only to sympathetic vasoconstriction, but also chemoreflex activation [40,41]. Therefore, one can expect that a modification in the respiratory control would affect also the control of the cardiovascular system, as the breathing is also under voluntary control and so it is possible to induce such changes by voluntary breathing modification. This may induce long-term cardiovascular and respiratory effects as a result of independent volitional control.
On statistical analysis, the mean pre-treatment value of SBP was 150.40 and 149.27 for experimental and control group respectively and mean post treatment value of SBP was 145.33 and 145.40 for experimental and control group respectively. Both experimental and control group shows that decrease of SBP 5.07 and 3.87 for respectively. This reveals that there is an improvement of Systolic blood pressure in experimental group when compare to control group.
The result showed that the experimental group was more significant. This favourable improvement of blood pressure indicates that Spontaneous respiratory modulation and aerobic exercise can be advisable in Grade 1 hypertension.
Conclusion
The study was an experimental approach- pre-test, post-test with control group design, aimed to evaluate the effect of spontaneous respiratory modulation and aerobic exercise in Grade 1 hypertensive subject. Population included Grade 1 hypertension with age group 45 to 55 years. Thirty samples were selected based on inclusion and exclusion criteria. All the subjects were divided into two groups, experimental group and control group based on purposive random sampling method.
Experimental group was given spontaneous respiratory modulation and aerobic exercise and control group was given aerobic exercise only.
The outcome measures used for study were Systolic blood pressure and Diastolic blood pressure. The measurements were taken prior to commencement of treatment (pre-test) and after 4 weeks (post-test).
The result of statistical analysis showed significant improving in the systolic and diastolic blood presser in Grade 1 hypertension.
Spontaneous respiratory modulation and aerobic exercise appears to be a useful adjunctive for improving blood pressure in s Grade 1 hypertensive patients. And it showed the potential to be a simple and inexpensive method to reduce blood pressure and it can be advisable in Grade 1 hypertension.
Recommendations
1. To establish the accuracy of the study, a large sample size can be taken and Use of a different outcome measure could make the study more valuable.
2. Activities, stressful events, and food consumption should be evaluated and Blinding of the procedures could improve the reliability of the outcome.
3. To make the results more valid and more generalized, a long term study may be carried out in all age group people would be suggested.
Conflicts of Interest
No conflicts of interest reported among authors.
References
- Gupta R, Guptha S (2010) Strategies for initial management of hypertension. Indian J Med Res 132: 531-542.
- Gupta R, al-Odat NA, Gupta VP (1996) Hypertension epidemiology in India: Meta-analysis of 50 year prevalence rates and blood pressure trends. J Hum Hypertens 10: 465-472.
- World Health Organization, International Society of Hypertension Writing Group (2003) World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 21: 1983-1992.
- Anderson, Douglas M (2002) Mosby's medical, nursing, and allied health dictionary (6th edtn). St. Louis, MO: Mosby.
- Waeber B, Brunner HR, Burnier M, Cohn JN (2007) Hypertension. Cardiovasc Med: 1833-1870.
- Gupta R (2004) Trends in hypertension epidemiology in India. J Hum Hypertens 18: 73-78.
- World Health Statistics (2012) World Health Statistics. World Health Organization
- Mohan V, Deepa M, Farooq S, Datta M, Deepa R (2007) Prevalence, awareness and control of hypertension in Chennai–The Chennai Urban Rural Epidemiology Study (CURES-52). J Assoc Physicians India 55: 326-332.
- Singh RB, Suh IL, Singh VP, Chaithiraphan S, Laothavorn P, et al. (2000) Hypertension and stroke in Asia: Prevalence, control and strategies in developing countries for prevention. J Hum Hypertens 14: 749-763.
- Jeffrey S (2005) ASH writing group propose a new definition of hypertension. Medscape.
- Keys A (1980) Seven countries: A multivariate analysis of death and coronary heart disease. Harvard University Press.
- Sembulingam K, Sembulingam P (2006) Essentials of medical physiology (4th edtn). Jaypee Brothers Medical Publishers.
- American Heart Association (2004) Heart disease and stroke statistics: 2004 update. American Heart Association, Dallas, Texas, USA.
- The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (1997) Arch Intern Med 157: 2413-2446.
- Non-pharmacological approaches to the control of high blood pressure (1986) Final report of the Subcommittee on Non-pharmacological Therapy of the 1984 Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 8: 444-467.
- Radaelli A, Raco R, Perfetti P, Viola A, Azzellino A, et al. (2004) Effects of slow, controlled breathing on baroreceptor control of heart rate and blood pressure in healthy men. J Hypertens 22: 1361-1370.
- Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, et al. (2005) Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension 46: 714-718.
- Schein MH, Gavish B, Herz M, Rosner-Kahana D, Naveh P, et al. (2001) Treating hypertension with a device that slows and regularises breathing: A randomised, double-blind controlled study. J Hum Hypertens 15: 271-278.
- Bhargava R, Gogate MG, Mascarenhas JF (1988) Autonomic responses to breath holding and its variations following pranayama. Indian J Physiol Pharmacol 32: 257-264.
- Mohan M, Saravanane C, Surange SG, Thombre DP, Chakrabarty AS (1986) Effect of yoga type breathing on heart rate and cardiac axis of normal subjects. Indian J Physiol Pharmacol 30: 334-340.
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, et al. (2004) American College of Sports Medicine position stand, exercise and hypertension. Med Sci Sports Exerc 36: 533-553.
- Stamler J (1991) Blood pressure and high blood pressure: Aspects of risk. Hypertension 18: I95-I107.
- Fagard RH (2006) Exercise is good for your blood pressure: Effects of endurance training and resistance training. Clin Exp Pharmacol Physiol 33: 853-856.
- MacDougall JD, Reddan WG, Layton CR, Dempsey JA (1974) Effects of metabolic hyperthermia on performance during heavy prolonged exercise. J Appl Physiol 36: 538-544.
- MacDougall JD (1994) Blood pressure responses to resistive, static and dynamic exercise. Cardiovascular Response to Exercise: 155-173.
- Palatini P (1994) Exercise haemodynamics in the normotensive and the hypertensive subject. Clin Sci 87: 275-287.
- Daly MD (1986) Interactions between respiration and circulation. Handbook of Physiology: The Respiratory System, Control of Breathing: 529-594.
- Novak V, Novak P, de Champlain J, Nadeau R (1994) Altered cardiorespiratory transfer in hypertension. Hypertension 23: 104-113.
- Cooke WH, Cox JF, Diedrich AM, Taylor JA, Beightol LA, et al. (1998) Controlled breathing protocols probe human autonomic cardiovascular rhythms. Am J Physiol 274: H709-H718.
- Pitzalis MV, Mastropasqua F, Massari F, Passantino A, Colombo R, et al. (1998) Effect of respiratory rate on the relationships between RR interval and systolic blood pressure fluctuations: A frequency-dependent phenomenon. Cardiovasc Res 38: 332-339.
- Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, et al. (2002) Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 105: 143-145.
- Lehrer P, Sasaki Y, Saito Y (1999) Zazen and cardiac variability. Psychosom Med 61: 812-821.
- Montano N, Cogliati C, Porta A, Pagani M, Malliani A, et al. (1998) Central vagotonic effects of atropine spectral modulate spectral oscillations of sympathetic nerve activity. Circulation 98: 1394-1399.
- Bernardi L, Gabutti A, Porta C, Spicuzza L (2001) Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J Hypertens 19: 2221-2229.
- Goso Y, Asanoi H, Ishise H, Kameyama T, Hirai T, et al. (2001) Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circulation 104: 418-423.
- Spicuzza L, Gabutti A, Porta C, Montano N, Bernardi L (2000) Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet 356: 1495-1496.
- Francis DP, Coats AJ, Ponikowski PI (2000) Chemoreflex-baroreflex interactions in cardiovascular disease. Sleep Apnea: Implications in Cardiovascular and Cerebrovascular Disease: 33-60.
- Somers VK, Mark AL, Abboud FM (1991) Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87: 1953-1975.
- Pagani M, Pizzinelli P, Bergamaschi M, Malliani A (1982) A positive feedback sympathetic pressor reflex during stretch of the thoracic aorta in conscious dogs. Circ Res 50: 125-132.
- Somers VK, Mark AL, Abboud FM (1988) Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension 11: 608-612.
- Trzebski A, Tafil M, Zoltowski M, Przybylski J (1982) Increased sensitivity of the arterial chemoreceptor drive in young men with mild hypertension. Cardiovasc Res 16: 163-172.
- Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, et al. (1998) Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 97: 943-945.
- Van De, Borne P, Mezzetti S, Montano N, Narkiewicz K, et al. (2000) Hyperventilation alters arterial baroreflex control of heart rate and muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 279: H536 –H541.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi