Review Article, J Nucl Ene Sci Power Generat Technol Vol: 11 Issue: 6
An Alternative Method for Nuclear Waste Management with Biosorption
Prasanna Mishra1*, Yogesh Sharma1, Varun Kumar Singh2 and Durgesh Wadhwa3
1Department of Physics, SGT University, Gurugram, Haryana, Tunsia, India
2Department of Electrical and Electronic Engineering, Teerthanker Mahaveer University, Mandi Gobindgarh, Punjab
3Department of Electrical and Electronic Engineering, Sanskriti University, Mathura, Uttar Pradesh, India
*Corresponding Author: Prasanna MishraDepartment of Physics, SGT University, Gurugram, Haryana, Tunsia, India; E-mail: prasannamishra234@gmail.com
Received date: 09 February, 2022, Manuscript No. JNPGT-22-46571; Editor assigned date: 11 February, 2022, PreQC No. JNPGT-22-46571 (PQ); Reviewed date: 25 February, 2022, QC No. JNPGT-22-46571; Revised date: 13 April 2022, Manuscript No. JNPGT-22-46571 (R); Published date: 04 May 2022, DOI: 10.4172/2325-9809.1000292
Citation: Prasanna M, Yogesh S, Varun KS, Durgesh W (2022) An Alternative Method for Nuclear Waste Management with Biosorption. J Nucl Ene Sci Power Generat Technol 11:6.
Abstract
In the present era each home and industry needs power, yet the most concerning issue is that the greatest population of the world is confronting power issues in view of energy emergencies. The petroleum derivatives are restricted in sum. There are different kinds of energy present in the earth, for example, wind energy, biomass energy and sun oriented energy. Sun oriented energy or solar energy is the most widely recognized energy which is used in the different industries. This paper presents a review dependent on sun oriented energy in fixed authority. In this review, it examined the different kinds of fixed authority, sun oriented energy stockpiling, applications in different fields, favourable position and weakness of sun powered energy. In future, sun powered energy is exceptionally requesting wellspring of energy since it is environmentally friendly power and financially savvy. It supports national energy autonomy in light of the fact that sunlight based energy is used where it can be created. Further this solar energy makes the nearby positions for the new energy economy.
Keywords: Bio sorbents; Solar energy; Stationary collector
Introduction
Many concerns about the final transport of elevated nuclear wastes have arisen as a result of recent advances inside the nuclear energy industry. Long-lived radionuclides emitted by nuclear power stations as well as mining operations are recognized to represent a significant environmental hazard. There's always the risk of radionuclides leaking from geological deposits and mines contaminating adjacent bodies of water including ground water [1]. Several regarding such radioactive particles being higher energies emitters, as well as long-term exposure to them would cause cancer in humans. Nuclear waste treatment is being used to solve all of these problems. It is mainly concerned with the recovery of valuable radionuclides such as uranium, plutonium, thorium, as well as americium from nuclear waste solutions, as well as other long-lived fissionable materials. After treating it in ceramic or glass, it is safely disposed of in geological repositories. For something like the extraction of radioactive elements from radioactive wastes solution, a variety of separation methods have been used, including ion exchange, solvothermal, as well as adsorption.
Actinides are often separated consuming Tri-Butyl Phosphates (TBP) within organically available solvents such as hexane, kerosene, or dodecane [2]. Unfortunately, TBP being damaged by radiation after a few separation cycles, since breakdown products such as dibutylphosphoric acid (HDBP) and hydro monobutylphosphoric acids (H2MBP) co-extract additional metal complexes, reducing the procedure' specificity. Furthermore, TBP's non-biodegradability as well as the process's high amount of low-level animal manure needs additional effort because of its treatment and disposal. Many liquid-liquid separation techniques have been developed in recent years based on biodegradable as well as radiolytically durable ligands as well as environmental solvents such as ionic liquids; even though this technology might be still unviable owing towards poor separating features [3]. Adsorption may be used to pre-concentrate or separate actinides in nuclear waste solutions, as shown by the use of a variety of solid-phase adsorbents, both synthetic and natural, for nuclear waste treatment. Those solid-phase biosorbents have demonstrated exceptional radiation resistance as well as excellent adsorption capability.
Before evaluating the effectiveness of adsorbents for separation, cost is an essential consideration. Biomass as well as biofuels biosorbents are now the most cost-effective for separation because they are widely accessible and considerably less expensive than manufactured adsorbents such ion-exchanger resin or molecular sieves. Whereas the adsorption performance of an uncooked adsorbent material is lower to that of a manufactured adsorbent, it can be enhanced by chemical modifying raw biosorbents. This one has been attempted to modify the surfaces for adsorbent materials with functionalities including such phosphorus [4]. These features not only increase the adsorption efficiency by a factor of ten, but they also make biosorbents more selective. Methanol, formaldehyde, CaCl2, acetic acid, citric acid, and cetyltrimethyl ammonium bromide treated biomass, as well as biofuels adsorbed onto sodium polyacrylate, carboxy-methyl-cellulose, agar, sodium alginate, clinoptilolite, as well as other adsorbents, are all promising for nuclear waste treatment [5]. In the literature, there is noteworthy work on the biosorbents of fission products utilizing biofuels carbon-based adsorbents, magnetic activated carbons, as well as biofuel composite with clay particles, graphene, or silica gel.
Literature Review
Biosorbents from various sources
Green algae biosorbents and how they're made: Algae is mostly utilized in the biomedical and pharmaceutical sectors to manufacture additive products. Along with its larger efficiency as well as wide availability, microalgae feedstock has now been utilised effectively adsorption toxic metal ions as well as other harmful pollutants through industrial wastewater. Through use of algae inside the decontamination of hazardous material having lately become studied. Glucose, carrageenan, but also chitosan is among the constituents of distinct kinds of biomass production (red, brownish, as well as green). Certain components are required for particular metal cations interaction along with containment activities. Several distinct algae species, whether terrestrial and freshwater in origin, have been utilised for something like the absorption of fission products as well as their diverse functional groupings [6]
Bacterial biosorbents are a kind of bacterial biosorbent: Bacterial were single celled microbiological organisms that are currently prevalent in soil and water. From a toxicological and separation standpoint, many studies on the accumulation of heavy metals but also biological treatment of dyes and heavy metals from effluent discharge have really been published [7]. The carboxyl, carbonate, amine, as well as hydroxyls throughout the bacterial cell membrane were important for metal absorption in microbial species. Biosorption of radioactive f-metals using microorganisms has been expanded, as well as a number of promising taxa for nuclear waste treatment have been discovered, including Geobacillus.
Biosorbents from fungi and various ways of modification: Fungi are eukaryotic creatures with chitin as a component of their cell walls. The anionic, thiadiazole, phosphoric, nitrate, cysteine, as well as hydroxyl functions in the fungal cell wall are important for metal sequestration. The majority of the fungal sorbents beneath investigation are less expensive, simpler for grow, generally non-pathogenic, making them ideal for practical use. The biosorption capacity of fungus including such Aspergillus as well as Gibberella in both natural as well as modified forms has really been investigated for radionuclides.
Biosorbents derived from plants and various ways of modifications: Plants-created adsorbents being cost-effective, as well as plentiful, and they being mostly made up primarily lignin and cellulose, making them perfect for separating radionuclides form radioactive material treatments or polluted groundwater. The surface of agro-based biosorbents contains a variety of biological functionality including such ketone, acetaldehyde, aromatic compounds, carboxyl group, hydrocarbons, as well as phenolic groups [8]. Pollen, seed, leaf, root, timber, peel, gum, straw, bunches, fibre, agricultural residues, as well as shell have all been used in biosorbent studies for radioactive removal utilizing plant material as well as agricultural residues. Other flower remnants, like that as sawdust, and domestic trash, such as tea leaves, are already being used to control radioactive accepted manuscript wastes.
Animal-Derived Biosorbents and various ways of modifications: Animal-derived biosorbents (scores were obtained, peelings, bones, chitosan, and so much more) have been extensively researched for removing heavy metals from effluent discharge. Similarly, significant studies employing biosorbents with animal origin for something like the sorption process of f-metals have been reported and are worth noting here. The adsorption kinetics of uranyl ion has only been investigated in shrimp shells, starfish, as well as EDTA treated L. cylindrical. Monosaccharide unit’s polymer made from the carapace bones of shrimp as well as other invertebrates after an alkaline treatment. Chitosan may serve as a metal binding site because it contains three kinds of functional groups: amino/acetamido, fundamental hydroxy, as well as supplementary hydrogen peroxide (H2O2) [9]. Magnetic composites have been successfully used for complex formation of ionic species, grafting of functions, and simpler emulsification. For radiological waste management, chitosan, microbiota chitin, functionalized chitosan, chitin gels, chitin composites, as well as magnetic chitin have all been used.
Carbon-Based adsorbents produced from biomass: The use of carbon-based adsorbent materials (graphene oxide as well as carbon nanotube) for radioactive material remediation is currently being researched. Though these adsorbents outperform traditional energy activated carbon including chemical activation in terms of performance, they are not economically feasible on a wide scale. Activated charcoal activation bio-chars, carbon nanotube, as well as carbon Nano microspheres have been produced utilizing industrial residues as well as microbial biomass as precursors like efficient to the cost method again.
Bio-sorbents for the management of nuclear waste
Uranium biosorption: Because it serves mostly as a source of energy as well as an elevated pollutant, uranium is perhaps the most essential element in the development of atomic energy plants. Leaching, unregulated mining activities, but the use of nuclear materials including both military and civil objectives are all contributing to the growing uranium concentrations inside the ecoregions. Along with its long half-life, wide distribution, as well as carcinogenicity, uranium poses a serious threat to human health, as well as the World Health Organization (WHO) therefore determined uranium in drinking water acceptable limits. For the long-term growth of the nuclear energy program, uranium removal from groundwater, radioactive material disposal, as well as saltwater have been emphasized. For uranium recovery, methods such as solvent extraction, chemical vapor deposition, ion exchange, as well as adsorbent were used. Adsorption is the most practical of these methods because of its simplicity, ease of use, and broad application. The method is more cost-effective when biosorbents are used for adsorption. Various bioreactors including microbiological, fungal, algal, plant, as well as animal origin have already been explored for separation/pre-concentration for uranium utilizing acidity along with saline fluids. In the papers, the effects of zeta potential on uranium biosorption have already been widely investigated. This study is useful since it illuminates the complexation process.
Two different complexation mechanisms are known for something like the heavy metal adsorption over onto surface of an adsorbent: internal (chemical exchanges) as well as outer-sphere. If the adsorption process is hampered by rising ion concentration, the adsorption kinetics proceeds the outer-sphere complex formation procedure. When increased ionic strength, there is no change or an increase throughout the adsorption characteristics for something like the innermost sphere complexation mechanism [10]. This reduction in performance may also be ascribed towards the higher level of competition of Na+ ions with U (VI) for active sites (because NaCl, NaNO3, NaClO4, and other electrolytes were employed in the experiments). The sorption process is generally controlled through electrostatic contact as well as ion-exchange, according to this research [11].With increased salinity throughout the solution, such "salting out" effects caused within decreased CO2 solubility, which inhibited uranium desorption again from surface of adsorbent by maintaining soluble thorium bicarbonate molecules [12]. With increasing ionic strength, oxy-HTCMs showed even more complex behavior, with a reduction in adsorption capacity followed by a rise with increasing NaNO3 concentrations. The increase in adsorption capacity was caused by the compressing of the electrostatic interaction as the diffusion coefficient increased. It's also possible that raising the concentration of Na+ in the solution disturbed the uranyl ion's hydration sphere, allowing for easier absorption of U (VI) upon oxy-HTCMs.
Thorium biosorption: Researchers are interested in using uranium as something of a possible radioactive fuel source since it can be converted from non-fissile to fissile 233 U. The Thorium’s Recoveries through Extractions (THOREX) method being within the early stages of developments it will need a lot of tweaking before it can be used on a large basis. The decay byproducts of h are very hazardous, and their seepage into groundwater puts ecosystems at danger. As a result, recovering Th (IV) from radioactive material solutions and removing it from polluted groundwater is critical. Biomass-derived adsorbents, like synthetic adsorbents, are equally effective in these situations.
Americium and plutonium biosorption: The advantages and disadvantages of plutonium recovery techniques include the possible use of 239 Pu as a Mixture Oxides (MOX) fuels within faster breeder’s apparatuses and the non-peaceful usage of enhanced thorium in the manufacture of nuclear weapons. 241 are increasingly employed for neutrons optoelectronic devices, smoke’s detectors, as well as the manufacture of these other heavier actinides because of its excellent purity. A lot of work is being done on separating plutonium and americium using adsorption as well as solvent’s extractions [13]. This article reviews some noteworthy studies on the use of biosorption in the recovery of americium as well as plutonium via spent nuclear wastes solutions. Biosorbents like Bacillus have carboxylic, glycolic, carboxyl, amide, as well as phosphate structural features in their cell walls. These functional groups deprotonated at pH>3, resulting in an improvement in adsorption efficiency owing to a favourable electrostatic interaction between dissociated organic compounds as well as +charged plutonium ions.
Americium and plutonium biosorption: The potential utilization 239 Pu like a Mixed Oxide (MOX) fuels within breeder reactors as well as the non-peaceful utilization enriched plutonium in the construction for atomic bombs are both benefits as well as downsides of plutonium recovery techniques [14]. Because of its high purity, 241 is widely used during neutron solid solutions, carbon monoxide detectors, and the production of some the higher actinides. Utilizing adsorption as well as supercritical fluid extraction, considerable great deal of effort is being done to separate plutonium as well as americium. This page summarizes several notable researches upon that application of biosorption towards the recovering of plutonium as well as americium from expended radioactive material fluids.
Other radionuclides biosorption: Additional long-lasting radionuclides for example Am, Pa, 152+154 Eu as well as Np, in addition to Th, U, and Pu, must be removed via higher-level radioactive wastes into reduce its activities. Np has been linked to bone, liver, and lung cancer [15]. Although separating of these radioactive elements using synthesized adsorbents as well as solvent systems has shown promising results, cost as well as radiolytic problems commonly limit their commercial use. Biosorption has really been suggested as an alternate separation technique in this respect.
Isotherms of adsorption
For something like an adsorption process in balance, this concentration of adsorbent surface inside the bulk as well as at the solution interface is very well defined once at specific temperature. These adsorption efficiency (qmax) like an adsorbent at a given temperature T is indeed a consequence of sorbent concentrations (C), which also is given with qmax=f. (C). Different isotherm strategies have been introduced based on reasonable assumptions in order to get a better comprehension of either the adsorption process.
Kinetics of adsorption
Mass transport, particle diffusion, as well as biochemical processes all plays a role in adsorption kinetics. Within instance of something like adsorption mechanism, mass movement for metallic ions from such a solution phase towards the external surface of a sorbent is the initial stage, subjected to chemical processes or intracellular migration through into channels/pores for adsorption over onto interface of something like a biosorbent. Various kinetic parameters of dynamic data modelling were thoroughly studied in the field, and they are explained briefly here.
Pseud-1st-ordered kinetic models of lagergren
These rates regarding surface response governs these one-site occupancy adsorption characteristics throughout this paradigm [16].
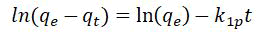
Pseud-2nd-ordered kinetic model
Such models are functional if rates regarding different adsorption’s processes (generally, ionic exchanging reactions) controlling these total adsorptions kinetics [16].
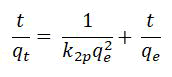
Elovich kinetic model
Such models are utilized for describing these adsorption’s kinetics regarding the ionic exchanges of the systems [17].
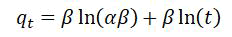
Intra-particles diffusing models
Such models are utilized towards describing such behaviors regarding solute’s diffusion as well as binds the internal poreschannels/of a biosorbents [18].
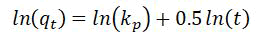
The adsorption kinetics is influenced by the dispersal of biosorption in an aqueous environment. More importantly, metal uptake pathways constitute this same adsorbent process' rate-limiting component, therefore underscores why different adsorbent really do have a broad variety of balance time frames (0.5-72 h). The equilibration period for the majority of the biosorbents was between 1-4 hours. The carbonyl groups complex formation with metallic ions onto the membrane of an adsorbent material is primarily responsible for the rapid dynamism of the sorption process. The sluggish dynamics, on the other hand, are caused by weak intermolecular forces as well as intracellular dispersion [19]. After 72 hours of contact time with CTTP beads, effective U (VI) absorption reached equilibrium. The rapid dynamics of the process appeared originally driven through physical adsorption, however after 90 minutes of classroom instruction, due to the predominance of molecular diffusion, a lethargic dynamical within the period of 2-6 h as well as a significantly slower dynamics following 6 h was observed. The frequency-reconfigurable kinetic study of U (VI) desorption against grape branches as well as malt wasting root indicated a rapid return to homeostatic at a higher pressure, implying the necessity for change in enthalpy within the adsorption mechanism.
The adsorption capability reduced even as temperature climbed, showing that the both physi-sorption as well as chemisorption may indeed be effective with temperatures lower. Since these slow dynamics of something like the process induced via van Der waals Interactions or intra-particle dispersion leads to uncertainty inside the qe estimate in very many circumstances of adsorbent-adsorbate interactions, the qe values are estimated in the pseudo-first-order kinetic model. As a result, the pseudo-first-order kinetic data would be only useful during the first hour of interaction. By comparing empirically measured qe values with those derived through data modelling, the validity of this reaction model for something like an adsorption system may be ensured. This same pseudo-second-order reaction mechanism, and from the other hand, may be utilized to efficiently describe incorporate a number (complex formation as well as ionic transfer) processes. This model's most remarkable characteristic i.e. it explains the adsorptions progressions in which chemisorption’s becomes unimaginably slow and the quantity of metallic cations adsorbs would be less as compared to the maximum proportion [20].
Thermodynamic research
Temperature-dependent metrics such as increase throughout renewable power (G°), change in enthalpy (H°), as well as increase in susceptibility (S°) are crucial in understanding the biosorption process' intrinsic energetic changes. The following formulae may be used to compute these parameters:
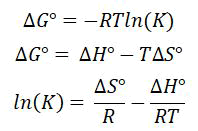
Power is necessary to remove the hydrogen atoms contained together in metal ion's hydrated circle throughout order for it to seems to onto to the membrane of an adsorbent material (fully or partially). A significant quantity of energy is also released as the completely/partly dehydrating metallic ionic being sorbet over these surfaces regarding the biosorbents. The adsorption mechanism is exergonic (H°>0) if the energy needed for metal-ligand dehydrating surpasses overall energy generated from metal ion desorption, otherwise it is exergonic (H° 0).
Discussion
Recovery and reusability
Sustainable extraction of specific radionuclides through packed adsorbent materials should indeed be given significant attention in during process high radioactive fuel. The ionic strength desorption research may be useful in optimizing the recovery procedure throughout this case. PH-dependent research findings have always shown unexpected results throughout the majority of something like the previous works, with a preliminary addition of lime leading to a rise throughout adsorption capacity that promptly fell until a certain pH value, suggesting how both large and powerful buffer solutions were used as extracts for something like the recovery of specific target radionuclides. For something like the quantifiable peeling of radionuclides, several acidic and basic solutions were employed. In certain instances, a rise in pH was related to increase in adsorption efficacy, indicating that sorbet radionuclide recovery may be successfully accomplished by employing a muriatic acid like an extracts. Chelating ligands such as EDTA, Na2CO3, as well as sodium carbonate proved able to eliminate or more 95 percent of the radionuclides from the sorbet. Many various combinations of acidic, ions, as well as basic were tried and deemed promising for back-extraction research in order to achieve optimum back-extraction effectiveness. One of the key factors that determine whether or not a sorbent may be used on a wide scale has always been its adaptability. It is self-evident that after a certain frequency of adsorbent cycles, a biosorbent's adsorption performance reaches a point where it loses its use. The utilization of adsorbent materials was tested for a maximum of 3 to a total of 6 sessions in the majority of the investigations, and adsorption capabilities were reported.
Mechanisms of adsorption
The absorption of f-metal particles out on to the biosorbent surface is clearly influenced by the preponderance of metal cations organisms at a certain solution pH as well as the adsorbent's negligible potential (pHzpc). It moreover includes an explanation about just the dominant cyanuric species inside the liquid solution, as well as the pHzpc of adsorbent materials as well as the solution pH at which excellent adsorption capability to biosorbents were observed inside the situation that uranium biosorbent. pHmax measurements have been observed to be decreased or comparable to corresponding pHzpc values in the majority of instances. The cyanuric compounds are neutrality or electrostatic attraction across the pH range. At pH pHzpc, an adsorbent's surfaces become electrostatic attraction, whereas at pH>pHzpc, it is considered impolite. At pHzpc, the electrostatic repulsion between uranyl molecules and high adsorption substrates is unfavorable, which makes the adsorption mechanism harder. When the pH is higher above pHzpc, nevertheless, a change in surface polarity results in a favorable electrostatic attraction between the electrolyte interface and the electrode surface uranyl molecule, culminating inside the best adsorption characteristics at a certain pH. After pHmax (typically pH>6), a sharp drop in adsorption efficacy was seen in several instances. This phenomenon may be explained by the decomposition of cyanuric ions, whereby produces neutral UO2 (OH)2. H2O as well as anionic UO2 (OH)3 species, resulting in sedimentation as well as electrostatic interactions, accordingly.
Mechanisms Of desorption
The desorption process is determined more by adsorbent's pH-dependent adsorption characteristics. Two types of pH-dependent behaviours have been seen in the majority of the research covered here:
- A curve that resembles an asymmetric Lorentzian function, in which the adsorption capacity increases, peaks, and then progressively decreases
- A curve that resembles a sigmoid function, in which the adsorption capacity saturates at a particular pH value.
Because ionic or electromagnetic interactions predominate in the adsorption process, Le Chatelier's theory strongly indicates that metal ion desorption may be accomplished by decreasing the pH. Chelating ligands such as EDTA, bicarbonate, as well as citrate may be utilized to desorb when the complexation process is the cause of adsorption.
Conclusion
The abandonment of radiological f-elements employing adsorbent materials of microbial, fungus, algae, vegetal, as well as animal’s provenance was emphasized in this study. Overall majority of raw adsorbent materials remain inexpensive as well as plentiful, although relatively high adsorption ability makes compounds useless. The concentration of organic as well as artificial characteristics towards the interface of whole biosorption resulted in higher throughout adsorbent dosage, making it a formidable competitor against established synthetic adsorbent materials throughout terms of cost-to-performance and good biocompatibility. These isotherm models, kinematics, as well as thermodynamic parameters of the biosorbent have all been extensively studied. Since a deeper comprehension of sorption process processes, a thorough study was conducted. Furthermore, a comparison of both the desorption capabilities from different characteristics as well as adjusted biosorption regarding radioisotope decontamination has already been carried out. The purpose of this review seems to be to demonstrate how sorbents may be utilized successfully in radioactive management of waste. Considering contemporary advances in the development on biosorbents, a transformation between scientific experiments towards large-scale application is anticipated in the not-too-distant ahead. Ignoring the fact that adsorption process may be the most cost-effective method for processing radioactive wastes solutions, its commercial application is hampered by concerns about biotransformation resilience and discrimination.
The radioactive material solutions seem to be a complex program that integrates variable quantities of transition metals, poisonous chemicals, and f-metals, among other things. Almost all of these radioactive particles are excellent generators of this and particles, as well as radiation. These irradiations as well as nanoparticles have enough energy to disrupt carbon-carbon bonds, which deteriorates the interface of adsorbent materials as well as reduces their adsorption characteristics. Only a few studies on biotransformation endurance as well as discrimination have really been made accessible in this literature study. Furthermore, the adsorption efficiency of biosorption is only of practical value in some of the other activities. Those three components (adsorption capacity, biotransformation endurance, as well as discrimination) are pillared on which biosorption's potential use in nuclear waste management is based, and recent papers have demonstrated some success in addressing these problems. Research will also focus on developing resource surface functionalization methods as well as looking towards radiolytically durable biosorption, among other things.
References
- Farnan I, Cho H, Weber WJ (2007) Quantification of actinide α-radiation damage in minerals and ceramics. Nature 445:190-193.
[Crossref] [Google Scholar] [PubMed]
- Mathur JN, Murali MS, Natarajan PR, Badheka LP, Banerji A, et al. (1993) Partitioning of actinides from high-level waste streams of PUREX process using mixtures of CMPO and TBP in dodecane. Waste Mng 13:317-325.
- Gupta NK, Sengupta A, Biswas S (2017) Quaternary ammonium based task specific ionic liquid for the efficient and selective extraction of neptunium. Radiochimica Acta 105: 689-697.
- Rajasulochana P, Preethy V (2016) Comparison on efficiency of various techniques in treatment of waste and sewage water-A comprehensive review. Res-Effi Technol 2:175-184.
- Hou D, Chen F, Yang SK, Yan XM, Long W, et al. (2016) Study on uranium (VI) biosorption of marine-derived fungus treated by cetyltrimethyl ammonium bromide. J Radiol Nuclear Chem 307:1147-1154.
- Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Bio adv 26: 266-291.
- Holyst R (1991) Landau-Peierls instability x-ray diffraction patterns and surface freezing in thin smectic films. Phys Rev 44: 3692.
- Pooja Rani, Jindal VK (2012) Designing band gap of graphene by B and N dopantatoms. RSC Adv 3: 802-812.
- Nigar S, Zhou Z, Wang H, Imtiaz M (2017) Modulating the electronicand magnetic propertiesof graphene. RSC Adv 7: 514546-51580.
- Boehm HP, Clauss A, Fischer GO, Hofmann U (1962) Das Adsorptionsverhalten sehr dunner Kohlenstoff-Folien. Zeit fur Anorg Allg Chemie 316: 3-4.
- Wei DC, Liu YQ, Wang Y, Zhang HL, Huang LP, et al. (2009) Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett 9: 1752-1758.
[Crossref] [Google Scholar] [PubMed]
- Castro AH, Guinea F, Peres NMR, Novoselov KS, Geim AK, et al. (2009) The electronic properties of graphene. Rev Modern Phys 81: 109-162.
- Yang XB, Liu GX, Balandin AA, Mohanram K (2010) Triple-mode singletransistor graphene amplifier and its applications. ACS Nano 4: 532-553.
- Huang B, Yan QM, Zhou G, Wu J, Gu BL, et al. (2007) Making a field effect transistor on a single graphene nanoribbon by selective doping. App Phy Lett 91: 4-9.
- Romero HE, Joshi P, Gupta AK, Gutierrez HR, Cole MW, et al. (2009) Adsorption of ammonia on graphene. Nanotechnology 20: 3-4.
- Bonaccorso F, Sun Z, Hasan T, Ferrari AC (2010) Graphene photonics and optoelectronics. Nature Photonics 4: 611-622.
- Sengupta A, Gupta NK (2017) MWCNTs based sorbents for nuclear waste management: A review. J Environ Chem Eng 5: 5099-5114.
- Gupta NK, Sengupta A, Rane VG, Kadam RM (2017) Amide-mediated enhancement of sorption efficiency of trivalent f-elements on functionalized carbon nanotube: evidence of physisorption. Sep Sci Technol 52: 2049-2061.
- ŠabanoviÄ? E, MuhiÄ?-Šarac T, NuhanoviÄ? M, MemiÄ? M (2019) Biosorption of uranium (VI) from aqueous solution by Citrus limon peels: kinetics, equlibrium and batch studies. J Radioanal Nucl Chem 319: 425-435.
- NuhanoviÄ? M, Grebo M, DraganoviÄ? S, MemiÄ? M, SmjeÄanin N (2019) Uranium (VI) biosorption by sugar beet pulp: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 322: 2065-2078.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 

