Research Article, J Chem Appl Chem Eng Vol: 2 Issue: 1
Adsorption of Cu (II) from Aqueous Solution by Salacca Peel Based Activated Carbons
Arenst Andreas Arie*, Hans Kristianto and Febe Apecsiana
Department of Chemical Engineering, Faculty of Industrial Technology, Parahyangan Catholic University, Ciumbuleuit 94 Bandung, Indonesia
*Corresponding Author : Arenst Andreas Arie
Department of Chemical Engineering, Faculty of Industrial Technology, Parahyangan Catholic University, Ciumbuleuit 94 Bandung Indonesia
Tel: 62222032658
E-mail: arenst@unpar.ac.id
Received: February 16, 2018 Accepted: March 03, 2018 Published: March 10, 2018
Citation: Arie AA, Kristianto H, Apecsiana F (2018) Adsorption of Cu (II) from Aqueous Solution by Salacca Peel Based Activated Carbons. J Chem Appl Chem Eng 2:1. doi: 10.4172/2576-3954.1000113
Abstract
In n 687.67 mg Cu(II)/g activated carbon. The kinetic study showed that the copper adsorption on the SPAC followed the pseudo-second order model. The results of this study indicated that SPAC was an effective adsorbent for removing copper ions from aqueous solutions.
Keywords: Activated carbons; Salacca peel; Adsorption; Langmuir
Introduction
Copper (II) ion is often found in the industrial wastewater usually produced by the mining, farming, metal plating and manufacturing operations in relatively high amount [1]. According to the World Health Organization (WHO), copper is regarded as one of the most toxic metals [2]. It is a hazardous, carcinogenic and mutagenic substance due to the high accumulation abilities in living organism including human beings [3,4]. From the Environmental Protection Agency (EPA), it is regulated that the maximum amount of Cu(II) in the waste water cannot exceeded 1.3 mg/L [5]. Above that maximum limit, Cu(II) can cause several diseases such as nausea, lung disorders, liver and kidney failure [6]. There have been a lot of methods are introduced to treat wastewater containing such heavy metal ions [7]. One of the most well-known methods among others is adsorption [8]. Adsorption process has been commonly used to remove heavy metal ions and considered as the most viable alternative for removing Cu(II) from waste waters [9]. Hence, the urgent needs for effective and efficient adsorbents have come to a find for new, abundant, low-cost and renewable material [10].
Undoubtedly, activated carbons have been known as excellent materials removal of toxic substances from wastewaters [11]. However, the drawbacks main for their application are on their high production cost, adsorbent regeneration after use and environmental problems. Hence related to those facts, the utilization of biomass wastes as carbon precursors for the synthesis of activated carbons has become a hot topic in research in the area of adsorbent material technology. Recently, many types of biomass wastes has been used for the synthesis of activated carbons which have been found to be potential and low cost precursors as adsorbents and would solve the disposal problem of biomass waste [11-25].
Salacca fruit or also known as snake fruit reddish-brown scaly skin is a typical tropical fruit cultivated in Indonesia especially in Bali, Lombok, Maluku and Sulawesi [26]. The fruit inside consists of three lobes with the two larger ones, or even all three, containing a large non-edible seed [27]. After consumption of the fruit, salacca peel has usually discarded as waste and until now, there has not been utilized as raw materials for useful products [28]. In order to make better use of this biomass waste, it is proposed to convert salacca peel to activated carbons. Our previous works focused on the application of SPAC as adsorbent material for removal of methylene blue [29].
The aim of this work was to investigate the potential of SPAC as adsorbent for the removal of Cu(II) from synthetic aqueous solutions. Batch adsorption studies were carried out to investigate the effects of various parameters such initial pH solution, initial metal ion concentration, dosage of SPAC and adsorption temperature on the adsorption performance of Cu(II). The adsorption kinetics was analyzed using pseudo-first-order and pseudo-second-order models. Equilibrium data were modeled using two different equations such as Langmuir and Freundlich model.
Experimental
Preparation of SPAC
The carbonaceous precursor selected was salacca peel. The peels were crushed and sieved to particle sizes of 2.0-2.8 mm. The powders were initially subjected to the pre-carbonization process at temperature of 500°C for 1 h. After this process, the salacca peel powders were impregnated with solution of KOH with mass concentration of 20 %-w and impregnation ratios (weight of KOH to weight of salacca peel) of 4:1. The salacca peel powders were impregnated for 20 h. The slurries were then heated at 80°C for 24 h to dryness. Next, samples were pyrolyzed under N2 flow at 800°C for 1 h. Prepared activated carbons were washed with 0.1 M HCl and then with distilled water until the pH of the washing water was 7 (neutral).
Structural and textural characterization of SPAC
Characterization in terms of specific surface area, pore volume, and pore diameter of the obtained activated carbons was determined by N2 adsorption at with surface area and pore size analyzer using the Brunnaeur-Emmet-Teller (BET) method. The micropore volume was calculated by using t-Plot micropore volume. The pore size distribution was determined by using Barrett–Joyner–Halenda (BJH) model. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) were used for observing the morphology and determining elemental composition of SPAC.
Adsorption of Cu(II) using SPAC
Adsorption experiments were conducted in a set of 500-mL Erlenmeyer flasks containing 30 mg of SPAC and 500 mL of copper ion solutions with initial concentrations of 100 ppm. The initial pH was adjusted to 2.0, 3.5 and 5.0 with 1 mol/L HCl. The flasks were placed in a shaker at a room temperature (25°C) and agitation rate of 100 rpm. The samples were filtered, and the residual concentration of copper ion was analyzed by UV Visible spectrophotometry (UV-Vis). Amonium solution (NH4OH) was used as complex agent to analyze Cu(II) ions.
To study the effect of the initial metal ion concentration, 500 mL of Cu(II) at various concentrations (100, 150, 200, 250 and 300 ppm) were mixed with 30 mg of SPAC at pH 5 in the Erlenmeyer flasks. These mixtures were kept at room temperature with continuous shaking at 100 rpm. All samples were filtered and analyzed by UV-Vis spectrophotometer.
The effect of the SPAC dosage was then investigated by mixing 500 mL of Cu(II) at concentration of 100 ppm with 30, 60 and 90 mg of SPAC at pH 5 in the Erlenmeyer flasks. The mixtures were maintained at room temperature and continuous shaking at 100 rpm. All samples were filtered and analyzed by UV-Vis spectroscopy.
In order to study adsorption kinetics, batch adsorption experiments were conducted by mixing 30 mg of SPAC with 100 mL of 100, 150, 200, 250 and 300 ppm Cu(II) solutions. All the samples were stirred at 100 at room temperature and pH 5. For all the adsorption experiments, they are conducted triplicate and the average values were used for the analysis. The adsorption performances were measured through the removal efficiency (%) and adsorption capacity which can be calculated using the following equations:
 (1)
(1)
 (2)
(2)
Where C0 and Ce (mg/L) are the initial and equilibrium liquidphase concentrations of Cu(II) ion, respectively. V(L) is the total solution volume, and m(g) is the mass SPAC-adsorbent.
Results and Discussion
Characterization of SPAC
Figure 1(a) shows the SEM- morphology observation of SPAC, it can be seen that the porous structure was found on the surface carbon samples presents an adequate morphology for copper ions adsorption. Figure 1(b) shows the EDX spectra of obtained sample, the sample contains mainly carbons, oxygen and silicon, as presented in Table 1.
| Elements | C | O | Si |
|---|---|---|---|
| Weight (%) | 83.08 | 10.71 | 6.21 |
| Atomic (%) | 88.6 | 8.57 | 2.83 |
Table 1: Elemental Composition of SPAC provided by EDX Spectra.
The textural characteristics of activated carbon are determined from the standard N2-adsorption techniques. The BET surface area is 2526 m2/g, the total pore volume is 1.45 cm3/g and the average pore size is 2.30 nm. It shows that activated carbon has a very large specific surface area. Figure 2 shows the N2 adsorption desorption profiles of SPAC. It can be concluded the profiles follows the Type IV according to the International Union of Pure and Applied Chemistry (IUPAC) classification of adsorption isotherms [30]. This is a typical structure of mesoporous material and it is beneficial for adsorption purposes [31,32].
Adsorption of Cu(II) from aqueous solution by SPAC
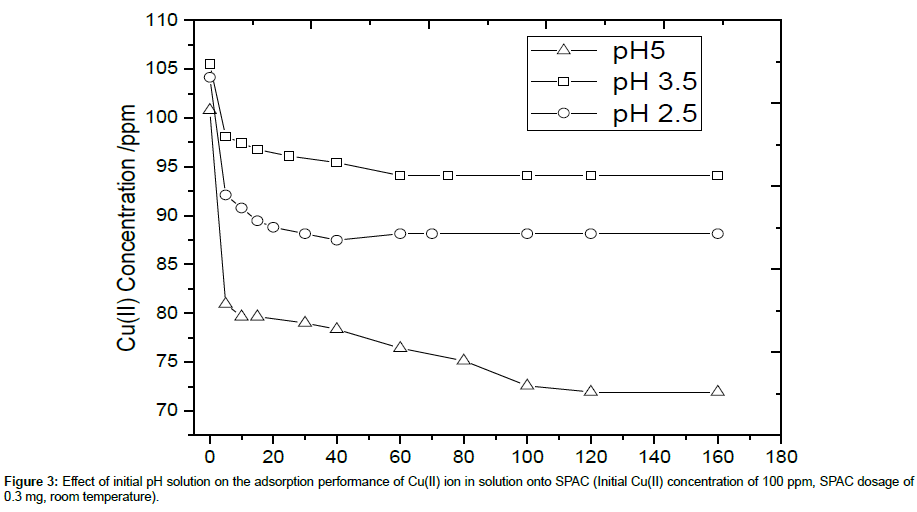
The pH of the Cu(II) solution can be one factor influencing the adsorption process. The pH value can determine the ionization degree of the Cu(II) ions and the surface property of the SPAC. Figure 3 shows the effect of initial pH of Cu(II) solution on the adsorption performance of SPAC. For all cases, it can be seen that adsorption rate of Cu(II) ion increases sharply at short contact time and slowed gradually as equilibrium was approached. It could be due to the availability of initial large number of vacant surface active sites for adsorption, the adsorption rate is very fast. As equilibrium was approached, the filling of vacant sites becomes difficult due to the repulsive forces between the Cu(II) ion adsorbed on solid surface and Cu(II) ion from solution.
It can be observed also that the adsorption capability and percent removal efficiency of SPAC is increased as the initial pH is increased from 2.5 to 5. It is due to the fact that the most determining mechanism during the adsorption is caused by the ion exchange. At lower pH value, the presence of H+ in higher content may cause the direction of reversible ion exchange equilibrium return to the sorbent materials. For lower value of pH (2.5 and 3.5), the equilibrium was reached within 60 minutes, while for pH of 5, it was reached longer, within 100 minutes. Based on the highest percent removal and adsorption capacity, pH 5 was then selected for the next adsorption experiments by varying initial Cu(II) concentration, SPAC dosage and adsorption temperature.
The adsorption performance of SPAC as a function of the initial concentrations of Cu(II) within is presented in Figure 4. The solution concentration of Cu(II) was varied in the range 100-300 ppm and the initial pH was fixed at 5.0. As seen in Figure 4, the adsorption capacity and percent removal efficiency increase rapidly at an initial stage of sorption and no significant increase was seen beyond this time which shows saturation of the active sites (which are available for specific metal ions) in the SPAC. In addition, the results show that the percent removal increased as the inital Cu(II) concentration was reduced from 300 to 100 ppm due to the electrostatic forces between adsorbate and SPAC adsorbent. It was also observed that under the experimental conditions,there was no significant adsorption after 80 minutes of stirring for all variation of initial Cu(II) concentration.
To examine the effect of SPAC dosage on the sorption intake of Cu(II), three different values were taken by varying the amount of SPAC while keeping the volume of the metal solution constant at 500 ml as shown in Figure 5. It can be observed that the amount of Cu(II) metal ions adsorbed on the surface of SPAC increases with the increasing SPAC dosage due to the more available of active sites or surface area for adsorption of Cu(II) ions. The highest percent removal of Cu(II) was about 70% at SPAC dosage of 90 mg. Hence, the determination of the weight of adsorbent is important when using SPAC for Cu(II) removal (Figure 5).
In order to investigate the kinetic mechanism of Cu (II) ions sorption, two models were used in this study. The adsorption capacity data, as shown in Figure 6, was used to study the adsorption kinetic of Cu(II) onto the SPAC adsorbent.
The linear pseudo-first-order model of Lagergren [33] is given as follows :
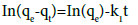 (3)
(3)
where, qe and qt are the amounts of copper ions absorbed onto the adsorbent (mg/g) at equilibrium and at t, respectively. k1 is the rate constant of first order adsorption (min−1). The linear plot for calculating parameters in the pseudo first order model is given in Figure 7.
The pseudo-second-order kinetic model developed by Ho and Mckay [34] is based on the experimental information of solid-phase sorption. The linear pseudo-second-order model can be expressed as follows:
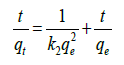 (4)
(4)
where, k2 is the rate constant of second-order adsorption (g/mg min−1). The adsorption kinetic models were shown in Table 2. The rate constant (k1 and k2), correlation coefficient R2, and equilibrium adsorption density of qe could be derived from the linear fitting of the data. Fig 8 shows the linear plot for determining the pseudo second order model (Figure 8).
| C0 / ppm |
Pseudo First Order Model | Pseudo Second Order Model | ||||
|---|---|---|---|---|---|---|
| k1 (minute-1) |
qe (mg Cu2+/g SPAC) |
R2 | k2 (g/mg min−1) |
qe (mg Cu2+/g SPAC) |
R2 | |
| 100 | 0.0259 | 253.14 | 0.8104 | 0.00024 | 499.41 | 0.9331 |
| 150 | 0.0594 | 381.62 | 0.8530 | 0.00032 | 616.87 | 0.9986 |
| 200 | 0.0417 | 266.91 | 0.8376 | 0.00043 | 673.45 | 0.9992 |
| 250 | 0.0285 | 259.16 | 0.8573 | 0.00032 | 684.51 | 0.9982 |
| 300 | 0.0280 | 462.45 | 0.9650 | 0.00009 | 663.04 | 0.9878 |
Table 2: Adsorption kinetic parameters.
The validity of the exploited models is verified by the correlation coefficient (R2). For all variation of initial Cu(II) concentration, it can be seen from Table 2 that the R2 values of the pseudo-secondorder model are higher than those of pseudo-first-order model. This result confirmed that the adsorption of copper ion onto SPAC better fits to pseudo-second-order kinetic model [35,36]. It implies that the predominant process is chemisorption, which involves in a sharing of electrons between the adsorbate and the surface of the adsorbent. Furthermore, most of the capacities calculated from the second order model are approaching the actual capacity of SPAC which is around 680 mgCu(II)/g SPAC (Table 2).
The capacity of an adsorbent can be represented by its equilibrium adsorption isotherm. The Langmuir and Freundlich adsorption models are commonly used to study the adsorption behavior of materials and the correlation among adsorption parameters. Accordingly, equilibrium data, as presented in Fig 9, were fitted by the Langmuir and Freundlich models (Figure 9).
The Langmuir isotherm equation is represented by the following equation:
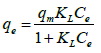 (5)
(5)
Where Ce is the equilibrium concentration of Cu(II) ions (mg/L), qe is the amount of Cu(II) ions adsorbed (mg/g), qm is the maximum adsorption capacity of Cu(II) ions (mg/g), and KL is the Langmuir adsorption equilibrium constant (L/mg) related to the affinity of the binding sites. The linear plot for Langmuir model is shown in Figure 10.
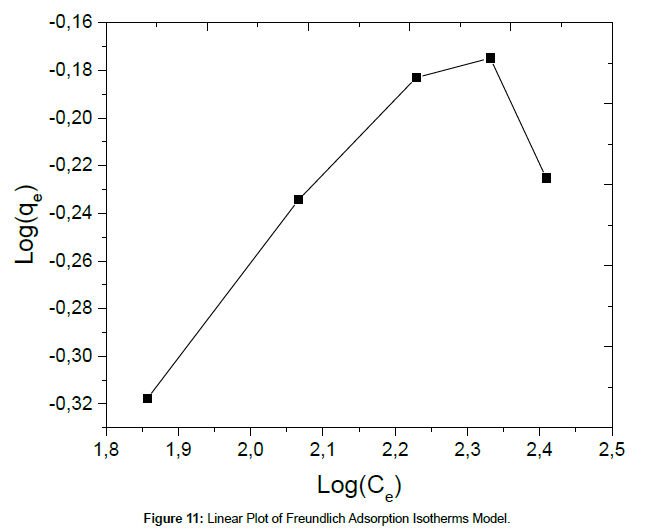
The Freundlich isotherm equation is described by the following equation:
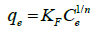 (6)
(6)
where KF and n are the Freundlich adsorption isotherm constants, which are indicators of adsorption capacity and adsorption intensity, respectively. The linear plot for Freundlich model is given in Figure 11.
The corresponding values of Langmuir and Freundlich isotherms for Cu(II) ions adsorption on SPAC were shown in Table 3. The results indicate that the Langmuir isotherm fits better than the Freundlich isotherm, which may be due to the homogeneous distribution of active sites onto SPAC. The maximum adsorption capacity obtained from the Langmuir isotherm is 687.67 mg/g. The results show that activated carbon salacca peel should be a promising adsorbent for removal of Cu(II) ion in aqueous solution. Table 4 present the result comparison, related to the adsorption of Cu(II) from the aqueous solution, between this work and those found in the literature. It can be seen that the adsorption capacity obtained in this work is relatively higher than those achieved in the previous references.
| Langmuir model qm (mg/g) | KL (L/mg) | R2 | Freundlich model KF (mg/g) | n | R2 |
|---|---|---|---|---|---|
| 687.7 | 0.0473 | 0.9632 | 0.2084 | 4.798 | 0.6536 |
Table 3: Isothermal Adsorption Parameter Models.
| Raw materials for Activated Carbons | BET Surface Area (m2/g) | Maximum Adsorption Capacity (mg/g) | Optimum pH | Fitted Adsorption Isotherm Model | Reference |
|---|---|---|---|---|---|
| Green Vegetable | - | 75 | 2.0 - 4.5 | Langmuir | [21] |
| Banana Peel | 63.5 | 14.3 | 6.5 | Langmur | [35] |
| Grape Bagasse | 1455 | 43.47 | 5 | Langmuir | [12] |
| Pomegranate Wood | - | 1.683 | 6 | Langmuir | [13] |
| Typha Latifolia | 130.41 | 34.48 | 6 | Langmuir | [14] |
| Corn Cob | 88 | 38.61 | 7 | Langmuir | [17] |
| Mangosteen Peel | 1106 | 21.74 | 6.7 | Langmuir | [24] |
| Ulva Fasciata | 543.45 | 13.77 | 6 | Langmuir | [23] |
| Cassava Peel | 1567 | 55 | 6 | Langmuir | [36] |
| Red Algae | 1014 | 49.5 | 6 | Langmuir | [25] |
| Salacca Peel (This Work) | 2526 | 687.67 | 5 | Langmuir | - |
Table 4: Comparison of adsorption capacity of SPAC with other biomass based activated carbons for Cu(II) removal.
Conclusions
In this study, activated carbon was successfully synthesized from salacca peel wastes by the KOH activation process. The synthesized carbons were characterized by BET, SEM and EDX. An application study was then conducted on the adsorption performance of salacca peel based activated carbons for the removal of Cu(II) metal ions from an aqueous solution. BET surface analysis showed that the synthesized carbons is a mesoporous solid with a high surface area of 2526 m2/g and from the SEM images, it can be seen that welldeveloped poreswere found on the surface of the activated carbons.
The adsorption equilibrium data was represented well by the Langmuir model results fit with maximum adsorption capacity of 687.67 mg Cu(II)/g activated carbon. The large surface area and mesoporous nature of activated carbons derived from salacca peel contribute to the high adsorption. A kinetic study suggested that the adsorption properties of the activated carbon followed the pseudosecond- order kinetic model. Based on the experimental results, it can be said that the synthesized activated carbons derived from salacca peel have promising potential to be used as an efficient, effective, low cost and green adsorbent for removal of Cu(II) metal ions from aqueous solutions.
References
- Bilal M, Shah JA, Tayyab A, Gardazi SMB, Tahir AA, et al.(2013) Waste biomass adsorbents for copper removal from industrial wastewater-A review. J Hazard Mater 263: 323-333.
- Zheng W1, Li XM, Yang Q, Zeng GM, Shen XX, et al.(2007) Adsorption of Cd(II) and Cu(II) from aqueous solution by carbonate hydroxylapatite derived from eggshell waste. J Hazard Mater 147: 534-539.
- Verghese PS (2015) Investigation of toxic heavy metals in drinking water of Agra city, India. Orient J Chem 31: 1835-1839.
- Harvey PJ, Handley HK, Taylor MP (2016) Widespread copper and lead contamination of household drinking water, New South Wales, Australia. Environ Res 151: 275-285.
- D. Quality (2004) Copper in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality.
- Izah SC, Chakrabarty N, Srivastav AL (2016) A review on heavy metal concentration in potable water sources in nigeria: human health effects and mitigating measures. Expo Heal 8: 285-304.
- Zhao M, Xu Y, Zhang C, Rong H, Zeng G (2016) New trends in removing heavy metals from wastewater. Appl Microbiol Biotechnol 100: 6509-6518.
- Islam S, Ang BC, Gharehkhani S, Afifi ABM (2016) Adsorption capability of activated carbon synthesized from coconut shell. Carbon Lett 20: 1-9.
- Pathan SA, Pandita NS (2016) Nanoporous carbon synthesized from grass for removal and recovery of hexavalent chromium. Carbon Lett 20: 10-18.
- Ramrakhiani L, Ghosh S, Majumdar S (2016) Surface modification of naturally available biomass for enhancement of heavy metal removal efficiency, upscaling prospects, and management aspects of spent biosorbents: a review. Appl Biochem Biotechnol 180: 41-78.
- Marsh H, Rodríguez-Reinoso F (2006) Characterization of Activated Carbon.
- Demiral H, Güngör C (2015) Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J Clean Prod 124.
- Ghaedi AM, Ghaedi M, Vafaei A, Gupta VK, keshavarz M,et al.(2015) Adsorption of copper (II) using modified activated carbon prepared from Pomegranate wood: Optimization by bee algorithm and response surface methodology. J Mol Liq 206: 195-206.
- Song J, Zhang R, Li K, Li B, Tang C (2015) Adsorption of copper and zinc on activated carbon prepared from Typha latifolia L. Clean (Weinh) 43: 79-85.
- Treviño-Cordero H, Juárez-Aguilar LG, Mendoza-Castillo DI, Hernández-Montoya V, Bonilla-Petriciolet A, et al. (2013) Synthesis and adsorption properties of activated carbons from biomass of Prunus domestica and Jacaranda mimosifolia for the removal of heavy metals and dyes from water. Ind Crops Prod 42: 315-323.
- Dávila-Guzmán NE, Cerino-Córdova J, Soto-Regalado F, Rangel-Mendez E, Díaz-Flores JR,et al.(2013) Copper biosorption by spent coffee ground: equilibrium, kinetics, and mechanism. Clean (Weinh) 41: 557-564.
- Hou XX, Deng QF, Ren TZ, Yuan ZY (2013) Adsorption of Cu2+ and methyl orange from aqueous solutions by activated carbons of corncob-derived char wastes. Environ Sci Pollut Res 20: 8521-8534.
- Uçar S, Erdem M, Tay T, Karagöz S (2014) Removal of lead (II) and nickel (II) ions from aqueous solution using activated carbon prepared from rapeseed oil cake by Na2CO3 activation.Clean Technol Environ Policy 17: 747-756.
- VukÄ√?¬ćeviÄ√?¬? M, Pejic B, Kalijadis A, PajiÄ√?¬?-LijakoviÄ√?¬? I, Kostic MM, et al.(2014) Carbon materials from waste short hemp fibers as a sorbent for heavy metal ions - Mathematical modeling of sorbent structure and ions transport. Chem Eng J 235: 284-292.
- Hadjittofi L, Prodromou M, Pashalidis I (2014) Activated biochar derived from cactus fibres-preparation, characterization and application on Cu(II) removal from aqueous solutions.Bioresour Technol 159: 460-464.
- Sabela MI, Kunene K, Kanchi S, Xhakaza N, Bathinapatla A, et al.(2016) Removal of copper (II) from wastewater using green vegetable waste derived activated carbon: An approach to equilibrium and kinetic study. Arab J Chem.
- Ni H, Xiong Z, Ye T, Zhang Z, Ma X, et al.(2012) Biosorption of copper (II) from aqueous solutions using volcanic rock matrix-immobilized Pseudomonas putida cells with surface-displayed cyanobacterial metallothioneins. Chem Eng J 204-206: 264-271.
- Suresh Jeyakumar RP, Chandrasekaran V (2012) Comparative studies on the removal of copper (II) by Ulva fasciata activated carbon and commercially activated carbon. Polish J Chem Technol 14: 88-94.
- Chen Y, Huang M, Chen W, Huang B (2012) Adsorption of Cu(II) from aqueous solution using activated carbon derived from mangosteen peel. BioResources 7: 4965-4975.
- El-Sikaily, El Nemr A, Khaled A (2011) Copper sorption onto dried red alga Pterocladia capillacea and its activated carbon. Chem Eng J 168: 707-714.
- Haruenkit R, Poovarodom S, Leontowicz H, Leontowicz M, Sajewicz M, et al. (2007) Comparative study of health properties and nutritional value of durian, mangosteen, and snake fruit: Experiments in vitro and in vivo. J Agric Food Chem 55: 5842-5849.
- Wijanarti S, Putra AB, Nishi K, Harmayani E, Sugahara T (2016) Immunostimulatory activity of snake fruit peel extract on murine macrophage-like J774.1 cells. Cytotechnology 68: 1737-1745.
- Gorinstein S, Haruenkit R, Poovarodom S, Park YS, Vearasilp S, et al. (2009) The comparative characteristics of snake and kiwi fruits. Food Chem Toxicol 47: 1884-1891.
- Arie A, Vincent, Putranto A (2016) Activated carbons from KOH-activation of salacca peels as low cost potential adsorbents for dye removal. Adv Mater Lett 7: 226-229.
- M. Materials (2016) A Reviewâ√?¬?¯: Fundamental Aspects of Silicate.
- Ravikovitch PI, Neimark AV (2001) Characterization of nanoporous materials from adsorption.J Phys Chem 188: 11-21.
- Donohue MDU, Aranovich GL (1998) Classification of Gibbs adsorption isotherms. Adv Colloid Interface Sci 76: 137-152.
- Tseng RL,Wu FC, Juang RS (2010) Characteristics and applications of the Lagergren’s first-order equation for adsorption kinetics. J Taiwan Inst Chem Eng 41: 661-669.
- Ho YS, McKay G(1999) Pseudo-second order model for sorption processes.Process Biochem 34: 451-465.
- Thuan TV, Thi B, Quynh P, Duy T, Thanh VT (2016) Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surfaces and Interfaces. 0:1-9.
- Moreno-Piraján JC, Giraldo L (2010) Adsorption of copper from aqueous solution by activated carbons obtained by pyrolysis of cassava peel. J Anal Appl Pyrolysis 87: 188-193.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi